Mathematical Analysis of Malaria-Schistosomiasis Coinfection Model
Abstract
We formulated and analysed a mathematical model to explore the cointeraction between malaria and schistosomiasis. Qualitative and comprehensive mathematical techniques have been applied to analyse the model. The local stability of the disease-free and endemic equilibrium was analysed, respectively. However, the main theorem shows that if , then the disease-free equilibrium is locally asymptotically stable and the phase will vanish out of the host and if , a unique endemic equilibrium is also locally asymptotically stable and the disease persists at the endemic steady state. The impact of schistosomiasis and its treatment on malaria dynamics is also investigated. Numerical simulations using a set of reasonable parameter values show that the two epidemics coexist whenever their reproduction numbers exceed unity. Further, results of the full malaria-schistosomiasis model also suggest that an increase in the number of individuals infected with schistosomiasis in the presence of treatment results in a decrease in malaria cases. Sensitivity analysis was further carried out to investigate the influence of the model parameters on the transmission and spread of malaria-schistosomiasis coinfection. Numerical simulations were carried out to confirm our theoretical findings.
1. Introduction
Malaria is highly endemic in various parts of sub-Saharan Africa in which 85% of global malaria cases and 90% of malaria deaths occur [1]. Schistosoma mansoni (the causative agent of intestinal schistosomiasis) is also prevalent in many sub-Saharan African countries [2, 3], accounting for approximately one-third of the total cases of schistosomiasis in the region [4]. The disease is a major contributor to disease burden globally and affects low income countries with climates suitable for transmission seriously. It is a life-threatening disease caused by parasites that are transmitted to people by the bites of infected mosquitoes [4, 5]. The bites by mosquitoes have resulted in the death of a child from malaria every 30 secs according to the report by the World Health Organization (WHO) fact sheet (2009) [4, 5]. Plasmodium falciparum and Plasmodium vivax are the two common species and Plasmodium falciparum is the most deadly. Plasmodium falciparum malaria remains a major cause of mortality and morbidity in the tropics and subtropics areas of the globe [4, 6]. According to the 2009 world report, half of the world’s population is at risk of malaria, with an estimated 247 million cases that led to about 863,000 death in 2008 mostly among African children, a slight drop from 2006 statistics with the estimation that over 2000 young are lost every day across the globe [4]. This population made malaria the dominant parasitic disease of the tropics and one of the top three killer communicable diseases [4, 5, 7]. Malaria makes development to be very slow in several ways; it affects fertility, population growth, savings and investments, and worker productivity and causes absenteeism and premature mortality [4, 5, 7]. Malaria also affects fetal development during early stage of pregnancy in women due to loss of immunity. However, malaria is preventable and curable when treatment and prevention measures are sought early [4, 5, 7].
The disease, schistosomiasis, also known as bilharziasis or snail fever, is a parasitic disease that was first named bilharzia [8, 9] and it is prevalent in several regions of the developing world, predominantly Africa, South America, and Asia, with about 650 million people living in the endemic areas [10]. It is known that estimated 207 million people are infected, where 85 percent lives in underdeveloped areas of Africa [11], resulting in about 15,000 deaths annually presently [2]. Children below the age of 14 are the major victims of schistosomiasis infection in many parts of the world [10]. The basis of illness in victims is the eggs laid by the parasitic flat worms, that is, blood flukes of the genus Schistosoma [8]. The species of the water-borne flatworm or blood flukes known as schistosomes is the main type that initiates the human schistosomiasis, but Schistosoma mansoni, Schistosoma japonicum, and Schistosoma haematobium are the three major species that are found everywhere [8]. The urinary tract and kidneys as well as the reproductive systems are affected by the Schistosoma haematobium, and they are intense in Africa and the Middle East [8]. The most widely spread species is the Schistosoma mansoni while Schistosoma japonicum is chiefly found in Asia and these two cause chronic hepatic and intestinal fibrosis [8, 10]. When skin comes in contact with contaminated freshwater in which certain types of snails that carry the parasite are living then the infection can be established [8]. Whenever infected people urinate or defecate in the water, freshwater becomes contaminated by Schistosoma eggs [8]. The eggs hatch, and the parasites infect, mature, and reproduce inside the snails when the appropriate species of snails exist in the water [12]. The parasite eventually leaves the snail and go into the water where it can persist for about 48 hours [8, 12]. Schistosoma parasites, when wading, swimming, bathing, or washing, can enter the skin of anyone who comes in contact with contaminated freshwater [8, 12]. The parasites migrate through host tissue and develop into adult worms inside the blood vessels of the body for over numerous weeks [8, 12]. The worms mate and females produce eggs after maturity [8, 12]. Several of these eggs eventually travel to the bladder or intestine and are finally passed into the urine or stool [8, 12]. The schistosomiasis symptoms are caused by the body’s reaction to the eggs but not by the worms themselves [12, 13]. Eggs shed by the adult worms that do not pass out of the body can become lodged in the intestine or bladder, causing inflammation or scarring [12, 13]. Repeatedly infected children can acquire anemia, malnutrition, and learning difficulties [12, 13]. The parasite can as well damage the liver, intestine, spleen, lungs, and bladder even several years after infection [12, 13]. It is known at present that both malaria and intestinal schistosomiasis contribute to common epidemiological distributions and are currently posing a great task to public health and socioeconomic development throughout the tropical region [14]. The interactive pathology between malaria and S. mansoni has received increased investigation in the recent time, as a result of their coendemicities [1, 11, 15, 16]. It has been discovered that considerable S. mansoni infections are linked with a major increase in the incidence of malaria among school-age children [11]. In individuals infected with S. mansoni the technique responsible for the magnification of malaria is not yet fully understood [1, 9]. Thus, it is observed that the interface between the two diseases is perhaps set in motion by contradicting effects; the parasites possess the immunological cytokines; that is, the balance between Th1 and Th2 type immune responses which reduces immunological control of malaria may be altered by S. mansoni, while other methods are probable [1, 15, 17–19].
It is our view that this study represents the very first modeling work that presents a mathematical analysis of the qualitative dynamics of malaria-schistosomiasis coinfection. There are few studies done on the malaria-schistosomiasis coinfection model so far. In [20], a coepidemic model of malaria and S. mansoni transmission dynamics is established, where the model reports major epidemiological coupling between the two diseases in terms of aggravated malaria incidence among individuals with S. mansoni extreme egg output. Their model was factored for S. mansoni extreme-risk endemic areas, applying epidemiological and clinical data of the relationship between S. mansoni and malaria among children in sub-Saharan Africa. They also assessed the potential influence of the S. mansoni malaria interface and mass treatment of schistosomiasis on malaria prevalence in coendemic areas.
In this paper, we develop a mathematical model of the interplay between malaria and S. mansoni in which we have modeled the malaria transmission and the S. mansoni together as coendemic deterministic model. Our aim here is to study and analyse a mathematical model of malaria-schistosomiasis transmission model. Additionally, there are some important differences between the model in [16] and the one in this paper. This paper is organized as follows: we present a malaria-schistosomiasis coinfection transmission model formulation in Section 2, where the general mathematical framework, notations, and model equations were analysed with the basic properties of the models and their analysis. In Section 3, we present the existence of steady state solution. In Section 4, the basic reproduction number and stability were derived and carried out. Sensitivity analysis of the model was performed to determine the most important parameters that influence R0 in Section 5. In Section 6, we show our numerical simulation results while, in Section 7, we discussed our conclusions and recommendations.
2. Model Formulation
In this model, we denote the total human population by Nh and subdivide it into the following subclasses of individuals who are susceptible (SH), individuals with malaria symptoms only (i.e., who are already infected and infective with malaria parasite) (Im), individuals infected with schistosomiasis only (Ihs), individuals infected with both malaria and schistosomiasis (Vms), individuals who recovered from malaria only (Rm), individuals who recovered from schistosomiasis only (Rhs), and individuals who recovered from both malaria and schistosomiasis such that Nh = SH + Im + Ihs + Vms + Rm + Rhs + Rms. The total snail population is denoted by Ns, which comprises susceptible snails (Ss) and infected as well as infectious snails (Is). That is, Ns = Ss + Is. The total mosquito population is denoted by Nv, which comprises susceptible mosquitoes (Sv) and infected as well as infectious mosquitoes (Iv). That is, Nv = Sv + Iv.
2.1. The Full Schistosomiasis-Malaria Coinfection Model
We describe the associated model variables and parameters in the following list and Table 1.
| Parameter | Parameter values | Sensitivity to |
|---|---|---|
| θ | 0.05 | −0.00000000000186 |
| β1 | 0.8333 | +1.0 |
| β2 | 0.406 | +1.0 |
| β3 | 0.004 | +1.0 |
| β4 | 0.09 | +0.99 |
| bv | 1000 | +0.74 |
| dH | 0.00004 | −2.0 |
| ϵv | 0.2 | +1.0 |
| σ | 0.502 | +2.0 |
| ϵh | 0.2 | +1.0 |
| qH | 0.56 | −0.99 |
| ρS | 0.0004012 | −0.876 |
| ρH | 0.0039 | −0.0069 |
| dv | 0.1429 | −0.23 |
| bs | 200 | +1.0 |
| bH | 100 | +2.0 |
| ds | 0.0000569 | −0.11 |
| ω | 0.05 | −0.91 |
-
SH: susceptible human
-
Im: human infected with malaria only
-
Ihs: human infected with schistosomiasis only
-
Vms: human infected with malaria and schistosomiasis only
-
Rm: human recovered from malaria only
-
Rhs: human recovered from schistosomiasis only
-
Rms: human recovered from malaria and schistosomiasis only
-
Ss: susceptible snail
-
Is: infected snail
-
Sv: susceptible mosquito
-
Iv: infected mosquito
2.2. Qualitative Analysis of the Full Schistosomiasis-Malaria Coinfection Model
The schistosomiasis-malaria coinfection model (12) will be analysed in a biologically feasible region for both humans, snail, and mosquito populations. Hence, for it to be epidemiologically well posed, it is necessary to show that all its state variables are nonnegative for all time t > 0.
2.3. Positivity of the Solution
Theorem 1. If the initial value for SH ≥ 0, Im ≥ 0, Ihs ≥ 0, Vms ≥ 0, Rm ≥ 0, Rhs ≥ 0, Rms ≥ 0, Ss ≥ 0, Is ≥ 0, Sv ≥ 0, and Iv ≥ 0 then the solutions (SH(t), Im(t), Ihs(t), Rhs(t), Vms(t), Rm(t), Rhs(t), Rms(t), Ss(t), Is(t), Sv(t), Iv(t)) of the schistosomiasis only model (16) are nonnegative for all t > 0.
Proof. We let Γ = sup {t > 0 : SH(t) > 0, Im(t) > 0, Ihs(t) > 0, Vms(t) > 0, Rm(t) > 0, Rhs(t) > 0, Rms(t) > 0, Ss(t) > 0, Is(t) > 0, Sv(t) > 0, Iv(t) > 0}.
Since the variables SH(0) > 0, Im(0) > 0, Ihs(0) > 0, Vms(0) > 0, Rm(0) > 0, Rhs(0) > 0, Rms(0) > 0, Ss(0) > 0, Is(0) > 0, Sv(0) > 0, and Iv(0) > 0 then, Γ > 0. If Γ < ∞, then SH, Im, Ihs, Vms, Rm, Rhs, Rms, Ss, Is, Sv, Iv are equal to zero at Γ. It follows from the first equation of the system (12) that
And then
Now,
Furthermore,
Now,
Also
Therefore
Also,
Also,
Then,
Also,
Also,
2.4. Boundedness of the Solution
Theorem 2. All solutions (SH(t), Im(t), Ihs(t), Vms(t), Rm(t), Rhs(t), Rms(t), Ss(t), Is(t), Sv(t), Iv(t)) of the malaria-schistosomiasis coinfection model (12) are bounded. Therefore, from (12) if
Proof. For the proof of boundedness, we note that 0 < Im(t) ≤ Nh(t), 0 < Ihs(t) ≤ Nh(t), 0 < Vms(t) ≤ Nh(t), 0 < Is(t) ≤ Ns(t), and 0 < Iv(t) ≤ Nv(t). We add the first eight equations and the last two equations of the malaria-schistosomiasis coinfection model (12) and yield
2.5. Invariant Region
Theorem 3. The region is positively invariant for the malaria-schistosomiasis coinfection model (12) with nonnegative initial conditions in .
3. Existence of Steady States Solution
3.1. Existence of Disease-Free Equilibrium Point (DFE)
- (i)
the disease-free (dies out),
- (ii)
endemic.
When the malaria-schistosomiasis disease dies out naturally, the solution asymptotically tends to a disease-free equilibrium DMS of the form DMS = (SH = bH/dH, Im = 0, Ihs = 0, Vms = 0, Rhs = 0, Rm = 0, Rhs = 0, Rms = 0, Ss = bs/ds, Is = 0, Sv = bv/dv, Iv = 0). Hence, the threshold that determines the stability of this equilibrium is the reproduction number (which is the average number of secondary infections caused by a single infectious individual during their entire infectious lifetime).
4. The Basic Reproduction Number
Whenever , each individual releases on average less than one infected individual and therefore the disease (malaria-schistosomiasis only) dies out. Whenever , each individual release more than one new infected individual and therefore the disease is able to invade the susceptible population. This allows us to determine the effectiveness of control measures. Epidemiologically, the reproductive number tells us how many secondary cases will one infected individual or vector produce in an entirely susceptible population of hosts and vectors, in other words, to determine the number of infected people that are generated by the introduction of a single infected person into a susceptible population. We need the computation of the basic reproduction number to assess the stability of the disease-free equilibrium (DFE) and the endemic equilibrium point (EEP). The basic reproduction number for particular infections is dependent on the biological characteristics of the disease and on the behavioural pattern of the population. When there is a high transmission of the disease per unit time and durations of the infectious period, the basic reproduction number increases.
Proposition 4. The value of the basic reproduction number is .
Proof. The argument uses the approach of the next generation matrix (see [21]). Suppose
The product of matrix F and V−1 gives
Finding the eigenvalue of FV−1, we obtain for the malaria-schistosomiasis coinfection model (12) is given by
4.1. Local Stability of the Disease-Free Equilibrium of Malaria-Schistosomiasis Coinfection Model
The disease-free equilibrium for the malaria-schistosomiasis coinfection model given by = (bH/dH, 0,0, 0,0, 0,0, 0, bs/ds, 0, bv/dv, 0) exists for all values of dH > 0, ds > 0, and dv > 0. If the threshold value is , then the disease-free equilibrium is locally asymptotically stable and the disease cannot invade or spread in the population or community. The summary of the result is given by Theorem 5.
Theorem 5. The disease-free equilibrium point DMS is locally asymptotically stable if and unstable if .
4.2. Impact of Schistosomiasis on Malaria and Vice Versa
The impact of schistosomiasis on malaria and vice versa can be analysed by expressing R0m in terms of R0s. We solve dH to obtain the following.
If (90) is greater than zero, it means that an increase in the malaria accounts for an increase of schistosomiasis cases in the population. The impact of malaria treatment on schistosomiasis is evaluated by partially differentiating R0M with respect to ω. We obtain ∂R0M/∂ω = −1/(θ + ω + dH). Since R0M is a decreasing function of ω, the treatment of schistosomiasis will have a positive impact on the dynamics of malaria.
5. Sensitivity Analysis of the Malaria-Schistosomiasis Coinfection Model
Sensitivity analysis is the study of how the uncertainty in the output of a mathematical model or system can be distributed to different sources of uncertainty in its outputs. It is a scientific procedure used in determining how different values of an independent variable will affect a particular dependent variable under a given set of hypotheses. This method is applied within certain limits that will depend on more input variables. The sensitivity index is a measure of the relative change in a variable with respect to the relative change in its parameters. It is known that there are uncertainties which are associated with the estimation of certain parameter values. So, it will be useful to study the effect of these parameters on the basic reproduction number . The understanding from this study will help us to identify those parameters that cause the most increase/decrease in and by this we will be able to recognize the type of control measure(s) that is most suitable and effective in controlling the disease. Hence, we compute the normalized forward sensitivity index of with respect to the parameters in the model equation (12).
The normalized forward sensitivity index of a variable, u, that depends differentiably on index of a parameter, z, is defined as . As we have an implicit formula for we derive analytically for the sensitivity of as to each parameter involved in .
5.1. Interpretation of Sensitivity Indices
It is observed that the positive signs of sensitivity indices of the basic reproduction number to the model parameters reveal that an increase or reduction in the value of each of the parameter in this case will point to an increase or decrease in the basic reproduction number of the disease [22]. The case of the shows that increasing or reducing the mosquito biting rate by 10 percent increases or reduces the basic reproduction number, , by 10 percent. However, the negative signs of the sensitivity indices of the basic reproduction number to the model parameters show that an increase or reduction in the value of each of the parameter in this class indicates a corresponding reduction or increase in the basic reproduction number of the disease [22]. Hence, the sensitivity analysis of the malaria-schistosomiasis coinfection model (12) provides an effective means of knowing how the dynamics of the coinfection model respond to changes in parameters of the model [22]. More importantly, it helps as a guide to policy makers and public health authorities in focusing on an appropriate intervention strategy for preventing and controlling the spread of the disease. The sensitivity analysis is applied to study the relative importance of model parameters to malaria-schistosomiasis coinfection. The sensitivity analysis reveals how each parameters influence the malaria-schistosomiasis coinfection output. However, the analysis will be carried out with response to the most crucial and sensitive parameters to the malaria-schistosomiasis coinfection model.
6. Numerical Simulation and Graphical Illustration of the Malaria-Schistosomiasis Coinfection Model
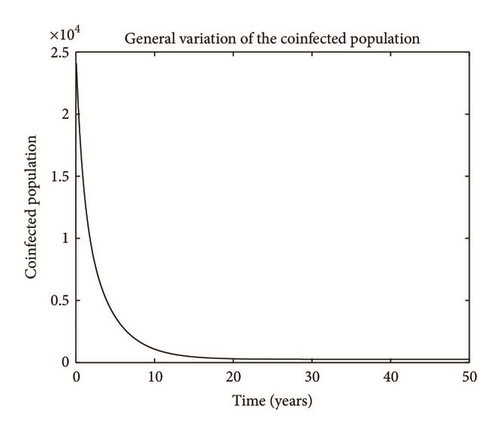
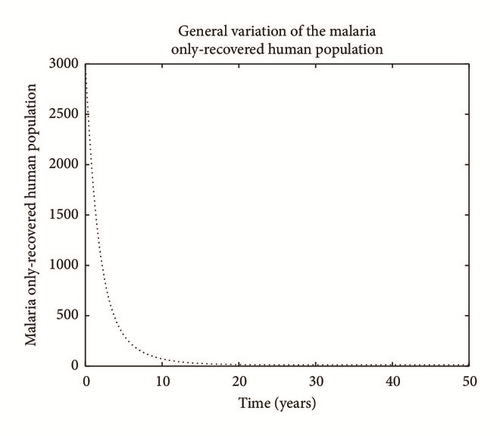
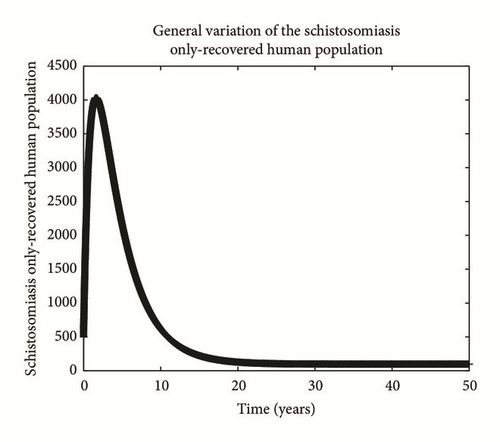
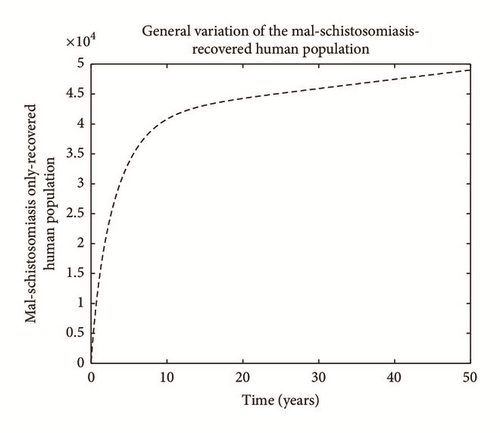
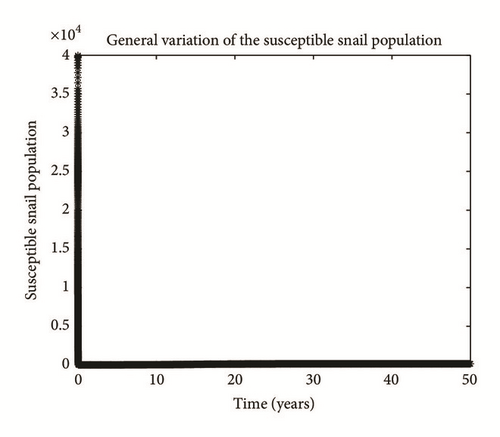
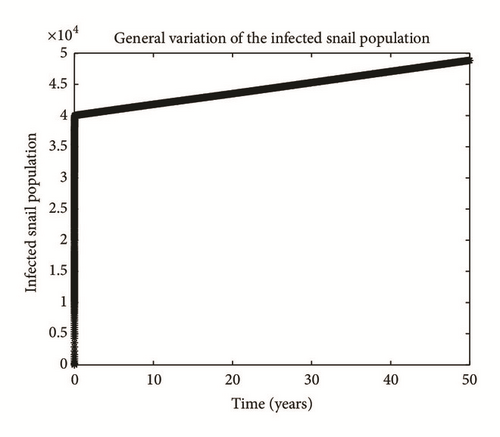
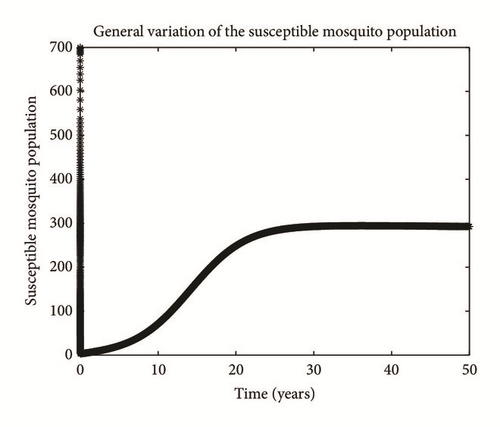
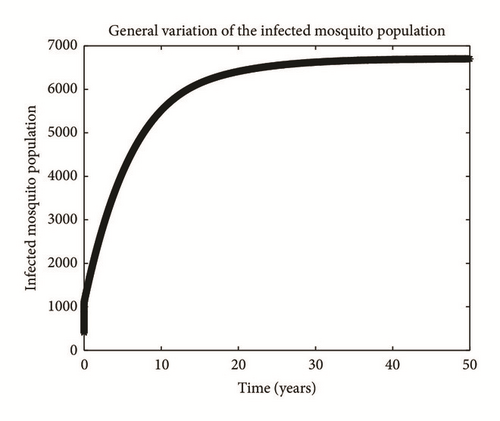
Numerical simulation and graphical illustrations are carried out in order to verify some of the analytical results. Different initial starts have been used from Table 2 to perform the computer simulations for different cases and displayed graphically in Figures 1–4. Figure 1 shows the variation of susceptible human (Sh), infected human with malaria only (Im), infected human with schistosomiasis only (Ihs), and humans infected with both malaria and schistosomiasis (Vms). It is observed that, in Figure 1(a), the susceptible human population decreases with time due to the malaria-schistosomiasis coinfections and in Figure 1(b), the population of malaria-infected human decreases with time due to treatment of the infected population. In Figure 1(c), the population of the schistosomiasis-infected human population also decreases due to the treatment while the population of the coinfected decreases to a particular level and then reaches its equilibrium point due to treatments. Figure 2 shows the variation of humans who recovered from malaria only (Rm), humans who recovered from schistosomiasis only (Rhs), and humans who recovered from both malaria and schistosomiasis (Rms). It is observed that, in Figure 2(a), the population of malaria-recovered human population decreases with time to a level and in Figure 2(b), the population of the schistosomiasis-only-recovered human population increases with time and then decreases with time until it reaches its equilibrium point while, in Figure 2(c), the population of malaria-schistosomiasis recovered human population increases with time and then reaches its equilibrium point. Figure 3 shows the variation of susceptible mosquito (Sv) and infected mosquito with malaria (Iv). It is observed that, in Figure 3(a), the susceptible snail population decreases with time due to infection with schistosomiasis while, in Figure 3(b), the population of the infected snail maintains a constant equilibrium and then increases with time. Figure 4 shows the variation of susceptible mosquito (Sv) and infected mosquito with malaria (Iv). It is observed that, in Figure 4(a), the population of susceptible mosquito decreases and then increases to maintain a steady state while the infected mosquito population increases with time because of the presence of the infection.
| Parameter | Symbol | Value | Source |
|---|---|---|---|
| Disease induced death rate due to malaria only | θ | 0.05 | [23] |
| Probability of human getting infected with malaria | β1 | 0.8333 | [24, 25] |
| Rate of acquiring schistosomiasis through contact with infected snails | β2 | 0.406 | [26] |
| Rate of acquiring schistosomiasis through contacts with infected humans | β3 | 0.0004 | [26] |
| Probability of mosquito getting infected by an infectious human | β4 | 0.09 | [24, 25] |
| Malaria schistosomiasis immunity waning rate | φ | 0.0005 | [24] |
| Schistosomiasis induced death rate | r | 0.02 | [assumed] |
| Malaria disease induced death rate | m | 0.06 | [assumed] |
| Coinfected population who recovered from malaria only | ϕ | 0.12 | [assumed] |
| Disease induced death rate of human due to schistosomiasis only | ρH | 0.0039 | [27] |
| Disease induced death rate of snails due to schistosomiasis only | ρs | 0.0004012 | [28] |
| Human spontaneous recovery | ω | 0.005 | [13] |
| Rate of loss of immunity to the schistosomiasis disease only | k | 0.7 | [assumed] |
| Rate of loss of immunity to malaria and schistosomiasis disease | φ | 0.0005 | [24, 25] |
| Human spontaneous recovery from schistosomiasis only | qH | 0.56 | [assumed] |
| Recovery rate of coinfected individual | α | 0.7 | [assumed] |
| Modification parameter | η1 | 1.3 | [assumed] |
| Modification parameter | η2 | 1.4 | [assumed] |
| Per capita birth rate of mosquitoes | bv | 1000 | [24, 29] |
| Per capita birth rate of snails | bs | 200 | [8] |
| Natural death rate of humans | dH | 0.00004 | [10] |
| Per capita biting rate of mosquitoes | ϵv | 0.2 | [30–32] |
| Contact rate of vector per human per unit time | σ | 0.502 | [33] |
| Per capita biting rate of humans | ϵh | 0.2 | [30, 34] |
| Natural death rate of mosquitoes | dv | 0.1429 | [24] |
| Natural death rate of snails | ds | 0.0000569 | [26] |
| Rate of loss of immunity to the malaria disease only | γ | 0.7902 | [24, 29, 35] |
| Per capita birth rate of humans | bh | 100 | [36] |
7. Concluding Remarks
In this paper, we developed and analysed a deterministic model for the transmission of malaria-schistosomiasis coinfection without intervention strategies. The model was rigorously analysed to gain insights into its qualitative dynamics. We obtained the following results: (i) the malaria-schistosomiasis coinfection model has a locally stable disease-free equilibrium whenever the associated reproduction number is less than unity. (ii) We observed from our analysis of the effect of schistosomiasis on malaria that schistosomiasis infection may be associated with an increased risk of malaria. (iii) We show that the interaction between schistosomiasis and malaria may reduce the effectiveness of malaria treatment for controlling malaria transmission. Sensitivity analysis of the reproduction number to the model parameter was performed to investigate the parameters that possess greater influence on the model. Sensitivity analysis also showed that mosquito biting rate ϵv and rate of acquiring schistosomiasis through contacts with infected snails β3 among other parameters contribute most significantly to the transmission and spread of the malaria disease. Hence the intervention strategy that inhibits the human-mosquito contact and human-snail contact should be encouraged in order to achieve a malaria-schistosomiasis-free population.
Competing Interests
The authors declare that they have no competing interests.




