Nonlinear Dynamics and Analysis of Intracranial Saccular Aneurysms with Growth and Remodeling
Abstract
A new mathematical model for the interaction of blood flow with the arterial wall surrounded by cerebral spinal fluid is developed with applications to intracranial saccular aneurysms. The blood pressure acting on the inner arterial wall is modeled via a Fourier series, the arterial wall is modeled as a spring-mass system incorporating growth and remodeling, and the surrounding cerebral spinal fluid is modeled via a simplified one-dimensional compressible Euler equation with inviscid flow and negligible nonlinear effects. The resulting nonlinear coupled fluid-structure interaction problem is analyzed and a perturbation technique is employed to derive the first-order approximation solution to the system. An analytical solution is also derived for the linearized version of the problem using Laplace transforms. The solutions are validated against related work from the literature and the results suggest the biological significance of the inclusion of the growth and remodeling effects on the rupture of intracranial aneurysms.
1. Introduction
Intracranial saccular aneurysm is a focal dilatation of the arterial wall that can be found in the circle of Willis which is a network of vessels at the base of the brain. The aneurysm which is a soft tissue interacts with a variety of flows including the blood as well as the cerebral spinal fluid. Based on the influence of various biomechanical factors, the growing aneurysm can potentially rupture that leads to either a neurological disorder or death. About 80% to 90% of ruptured aneurysms lead to death [1]. One of the invasive treatments for intracranial saccular aneurysms is to employ surgical procedures which may be associated with risk and death [2].
Over the last two decades, there have been several efforts to investigate the genesis of this disease and to develop a way for prediction of rupture through mathematical modeling [3–6]. Different groups of researchers had identified the elastodynamics of the arterial wall interaction with the blood flow to be the main reason for the rupture of an aneurysm [7–9]. A coupled fluid-structure model to understand the elastodynamics better was later studied more extensively [10–13]. These models introduced mathematical models of increasing complexity for intracranial saccular aneurysms that described the coupled interaction between blood, arterial wall, and cerebral spinal fluid (CSF). In [11], the CSF was modeled using simplified Navier-Stokes equations, whereas the arterial wall structure was modeled using a spring-mass system. A Fourier series was used to model the interaction between blood pressure and inner wall. While the model developed yielded good insight into understanding rupture, this model did not accommodate growth and remodeling effects of the soft-tissue wall.
There are three main constituents of the artery wall, namely, the elastin, the collagen, and the smooth muscle [14, 15]. The elastin is a stable protein and is considered the most load bearing element that functions as resistance to the formation of an aneurysm, whereas the collagen is the protein that is responsible for preventing rupture after formation of an aneurysm. The growth of the aneurysm is associated with deficiency of elastin and weakening of the artery wall [16]. Hence, elastin and collagen should be incorporated into the modeling of arterial wall in order to obtain an accurate biological model of the aneurysm that maybe can lead to better interpretation and prediction for this disease. This is one of the main contributions of this work. In the model we will consider in this paper we will assume elastin and collagen to be one-dimensional passive fibers and also, for simplicity, we will not consider the effects of the smooth muscle for this work.
In Section 2, we will describe the mathematical model that we will consider to solve a coupled fluid-structure problem. Section 3 discusses the nonlinear analysis and perturbation solution for the model. In Section 4, the linearization of the growth and remodeling model is considered and solved analytically using Laplace transformation. After that, some important computational results and comparisons are discussed in Section 5. Finally, we conclude by some biological interpretation of the results and suggestion for future work.
2. Models and Background
The mathematical modeling of intracranial saccular aneurysm builds on models developed in [11, 12] which included a simple mathematical model of a thin-walled, spherical intracranial aneurysm surrounded by cerebral spinal fluid which is referred to as CSF (see Figure 1).
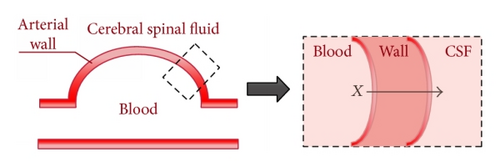
The model was based on three separate components: modeling the blood pressure acting on the inner wall of the arteries, modeling the CSF that is located outside the wall, and modeling the arterial wall itself. Combining the various components yields a coupled fluid-structure interaction model. This model assumed a simple spring-mass model for the wall and the focus of this paper is to enhance this wall model to incorporate realistic nonlinear soft-tissue behavior.
2.1. Model of the Blood Pressure
2.2. Model of the Arterial Wall
2.3. Model of the Cerebral Spinal Fluid
2.4. Governing Equation of Motion
3. Nonlinear Dynamics and Analysis
Nonlinear analysis and perturbation method techniques are powerful tools to study the behavior of complex nonlinear models and help to obtain an approximate solution to these models. In this work, the nonlinear phase analysis and perturbation technique for (13) will be presented. We will employ the following steps. First we will convert (13) which is an initial-boundary value partial differential equation system into an initial value ordinary differential equation system. Following this we will perform a phase analysis of the newly obtained initial value differential equation. We will then apply the perturbation techniques to the resulting equations. These steps are described next.
3.1. Converting the PDE System into Initial Value Differential Equation
Lemma 1. Nonlinear differential equation (24) can be rewritten as the following nonlinear second-order differential equation:
Proof. To prove this, we will use a change of variable. Let us define T = −ct and substitute this in differential equation (24) to obtain
3.2. Phase Analysis of the Initial Value Differential Equation
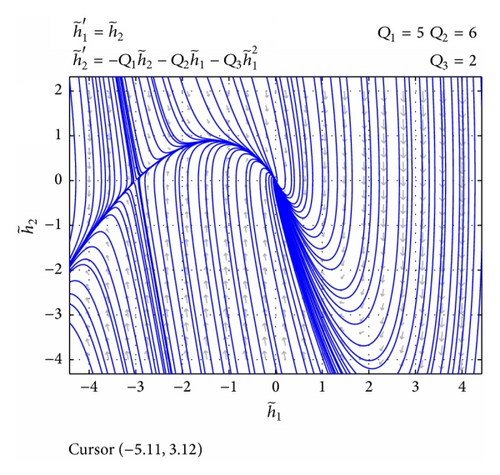
3.3. Existence of the Solution to Perturbed Nonlinear Nonhomogeneous System
3.4. Perturbation Technique to Find First-Order Approximation
4. Linearization of the Growth and Remodeling Model
5. Computational Experiments
5.1. Validation of the Perturbation Solution
First, we compare the first-order solution obtained in (65) using perturbation analysis against the linearized growth and remodeling model solution obtained analytically using Laplace transform in (76). In Figure 3, we plot these two solutions against the solution obtained from the linear model previously obtained by [11].

First, it is interesting to note that the solution to the linearized problem with growth and remodeling obtained via Laplace transform is close to the solution for nonlinear growth and remodeling problem obtained via perturbation method. The figure also illustrates that solution for the nonlinear problem obtained via the perturbation approach also has the same behavior but differs from the solution from [11]. This goes to show that the growth and remodeling effects are important and have a significant influence on the model. Moreover, the growth and remodeling model has the effect of reducing the displacement of the outer wall and preventing the aneurysm rupture consequently. In order to understand this further, we investigate the influence of elastin and collagen contributions in the growth and remodeling process on the potential of rupture. This is discussed next.
5.2. Influence of Collagen and Elastin Parameters
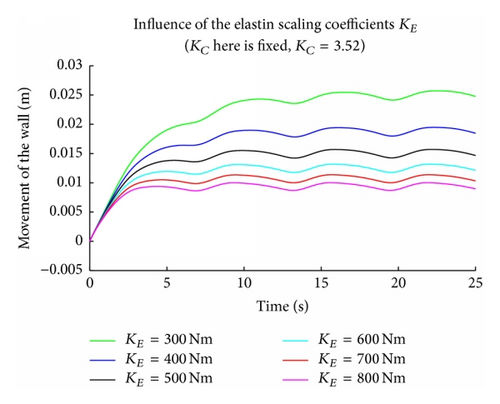
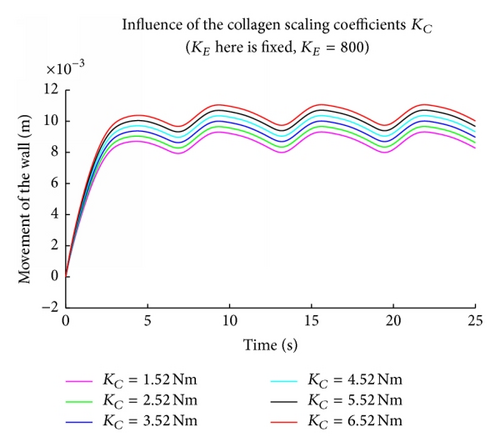
The elastic and collagen parameters (KE, KC) seem to play an important role in the modeling of the arterial wall since they are responsible for the elasticity and strength of wall tissue. It is known from the literature that a deficiency in elastin and an increase in collagen are often related to the rupture of an aneurysm. Figure 4 shows the solution of the nonlinear coupled problem using the perturbation solution for increasing values of KE starting from 800 N/M till 300 N/M. This corresponds to simulating the effect of deficiency in elastin and the figure clearly illustrates that as the KE value decreases the displacement of the outer wall increases which can lead to rupture. Figure 5 shows that the displacement of the outer wall increases in a steady periodic motion as KC increases which suggests biologically that an increase in collagen may lead to rupture. These findings are consistent with experimental findings which suggest the robustness of the one-dimensional model considered in this paper.
6. Conclusion and Future Work
In this work a new mathematical model was considered that couples the elastodynamics of the blood coupled with the arterial wall dynamics and the mechanics of cerebral spinal fluid. This geometrically nonlinear coupled fluid-structure problem for the first time considered the effects of growth and remodeling and a phase analysis of the model was presented. Since this nonlinear system does not admit an exact solution, the model was analyzed using perturbation techniques to obtain approximate solutions. Section 3 discusses the nonlinear analysis and perturbation solution for the model. The nonlinear coupled system was also linearized and a Laplace transform method was applied to derive the exact solution to the linearized problem. The models proposed in this paper compares well with similar models published for the linear case. Our results also seem to justify what has been experimentally observed by other research groups regarding the rupture of an aneurysm in relation to a deficiency in elastin and/or an increase in collagen. The novelty of the current paper is in the formulation, analysis, and simulation of the nonlinear coupled fluid-structure interaction model that incorporates growth and remodeling. The results from the computational experiments presented give encouragement to the authors to incorporate more features into the existing model such as incorporating viscoelasticity of the wall, adding nonlinear effects in the fluid model, and obtaining a fully implicit numerical approximation to the solution. These aspects will be considered in forthcoming papers.
Competing Interests
The authors declare that they have no competing interests.




