Ab Initio Theoretical Investigation on the Geometrical and Electronic Structures of Gallium Aurides: and (n = 1–4)
Abstract
This study presents a systematic investigation of the geometric and electronic properties of GaA and Ga2A (n = 1–4) clusters based on density functional theory and wave function theory. Detailed orbital analyses, adaptive natural density partitioning, and electron localization function analyses are performed and relevant results are discussed. GaA (n = 1–4) clusters with n-Au terminals and Ga2A (n = 1–4) clusters with bridged Au atoms possess geometric structures and bonding patterns similar to those of the corresponding gallium hydrides Ga and Ga2. Ga–Au interaction is predicted to occur through highly polar covalent bonds in monogallium aurides. In contrast to the highly symmetric ground states of C2v Ga2Au, C2v Ga2Au2, and D3h Ga2Au3, C3v Ga2Au4 is composed of strong interactions between a Ga+ cation and the face of a tetrahedral GaA anion. The adiabatic and vertical detachment energies of the anions under study are calculated to facilitate their experimental characterization. Geometric and electronic structural comparisons with the corresponding gallium hydrides are conducted to establish an isolobal analogy between gold and hydrogen atoms.
1. Introduction
Considering its strong relativistic effects, Au is highly different from other coinage metals (Cu and Ag); Au has the highest electron affinity (2.3086 eV) of any element other than the halogens as well as the highest electron negativity (2.4 in the Pauling scale) among all of the metals [1–4]. The H/Au isolobal is an extension of the most remarkable experimental discovery of the H/AuPR3 analogy thus far and has helped elucidate the structures and bonding types in various ligated Au compounds [5, 6]. The H/Au isolobal relationship in gas-phase Si–Au alloy clusters and B–Au alloy clusters, such as [7], [8, 9], Si3Au3, [10], [11], B10Au0/− [12], and closo-auro-branes [13], has been confirmed by joint photoelectron spectroscopy (PES) and density functional theory (DFT) investigations. Various compounds with 2c–2e N–Au and B–Au bonds [14–17], relativistic pseudopotential calculations on containing Au ligands (X=B–N, Al–S, n = 4–6) [18], and Au-bridged X ⋯ Au–Y Lewis acid-base pairs have also been reported [19].
The H/Au analogy has recently motivated our group to analyze the geometric and electronic structures of electron-deficient B–Au and Al–Au alloy small clusters, such as those of [20], [21], [22], [23], [24], , and , based on ab initio theories. These studies reveal a clear structural link between electron-deficient Au-containing clusters and the corresponding hydride molecules. Comparative studies on the properties of group IIIA element-Au alloy clusters have also attracted our interest. Boron, aluminum, and gallium hydrides, as well as their corresponding Au compounds, show several similarities and differences. To the best of our knowledge, no investigations on gallium aurides are yet available.
The present study describes a detailed ab initio investigation on the geometric and electronic structures of and (n = 1–4) based on DFT and wave function theory. Natural resonance theory (NRT) and electron localization function (ELF) [25, 26] are performed to characterize Ga–Au bonds in monogallium aurides. Natural localized molecular orbitals (NLMO) and adaptive natural density partitioning (AdNDP) [27] are performed to discuss the chemical bonding in digallium aurides. The adiabatic electron detachment energies (ADEs) and vertical electron detachment energies (VDEs) of and anions are calculated to aid their PES characterization. The results obtained in this work extend the concept of bridging Au interactions and enrich the chemistry of Au.
2. Theoretical Methods
Structural optimizations and frequency analyses were conducted on low-lying isomers using the hybrid B3LYP method [28, 29] and the second-order Møller-Plesset approach by frozen core approximation [MP2(FC)] [30, 31]. MP2 produces ground state structures and relative energy orders similar to B3LYP with slightly different bond parameters. Relative energies for the lowest-lying isomers were further refined using the coupled cluster method with triple excitations [CCSD(T)] [32] at B3LYP structures. Stuttgart quasi-relativistic pseudopotentials and basis sets augmented with two f-type polarization functions and one g-type polarization function (Stuttgart_rsc_1997_ecp+2f1g (α(f) = 0.498, α(f) = 1.464, and α(g) = 1.218)) [33] were employed for Au with 19 valence electrons. The augmented Dunning’s correlation consistent basis set of aug-cc-pvTZ [34] was used for Ga throughout this work. Bonding analyses were accomplished using NRT, NLMO, AdNDP [27], and ELF [25, 26]. The ADEs and VDEs of the anions were calculated as the energy differences between the anions and the corresponding neutrals at their ground state and anionic structures, respectively. All calculations in this work were performed using Gaussian 09 [35]. AdNDP and ELF analyses were performed with Multiwfn [36]. The NBO5.0 [37] program was used to calculate bond orders and atomic charges.
3. Results and Discussion
3.1. Geometric and Electronic Structures of GaAun and
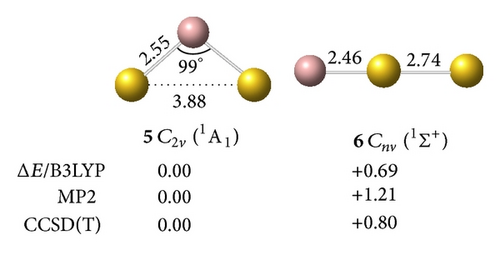
clusters with n–Au terminals possess geometric structures and bonding patterns similar to those of the corresponding gallium hydrides [38]. As shown in Figure 1, low-spin electronic states are consistently favored in . At all levels of theory, the ground structure GaAu− anion (1, 2Σ+) has a bond length of rGa–Au = 2.52 Å and is 1.76 eV more stable than its quartet isomer (2, 4Σ+) at the CCSD(T) level. The most stable GaAu neutral structure (3, 1Σ+) possesses a bond length of rGa–Au = 2.45 Å. For GaAu2, the V-shaped C2v (5, 1A1) with a bond length of rGa–Au = 2.55 Å is the ground state and is 0.80 eV more stable than the linear C∞v (6, 1Σ+) at the CCSD(T) level. V-shaped C2v GaAu2 (7, 2B2) is the most stable geometry on the potential surface of neutral GaAu2. A large geometric change may be observed upon electron detachment from the anion 4 to the neutral C2v GaAu2 7, although these molecules have the same symmetry: the Ga–Au bond length increases by 0.08 Å, the Au–Au distance decreases by 1.19 Å, and the Au–Ga–Au bond angle considerably decreases by 38° in the anion relative to the neutral molecule.
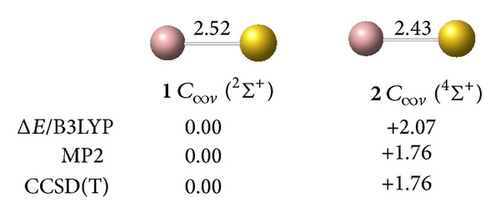
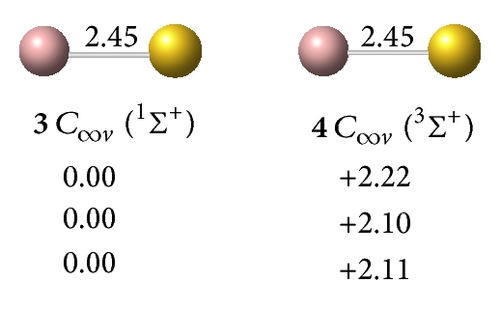
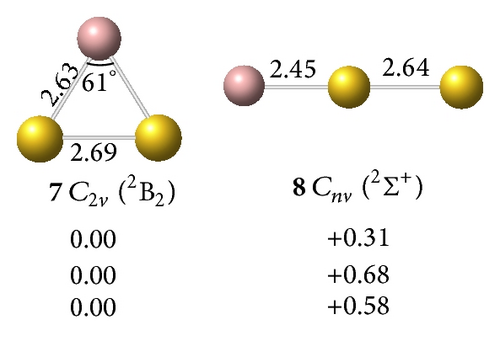
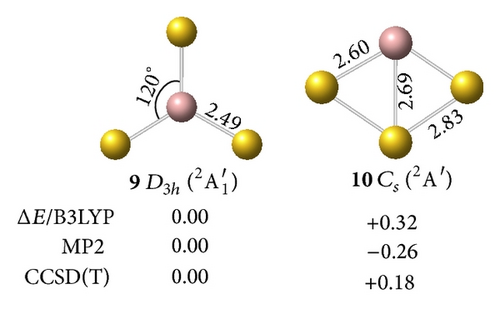
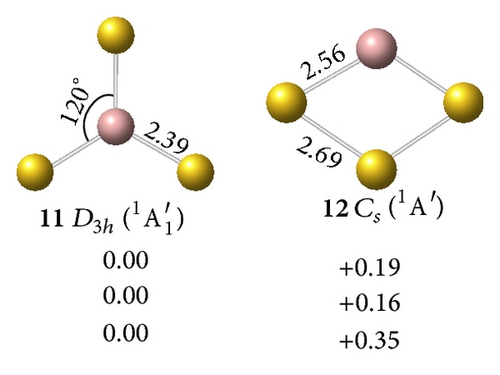
The perfect planar triangular structure has D3h symmetry (9,2A1′) with a bond length of rGa–Au = 2.49 Å and an Au–Ga–Au bond angle of AuGaAu = 120°. This structure is the ground state form and is 0.18 eV more stable than the off-plane Cs (10, 2A′) at the CCSD(T) level. Neutral GaAu3 (11,1A1′) with an sp2 hybridized Ga at the center of the molecule is a closed-shell singlet with D3h symmetry. Compared with the anion, the neutral molecule only exhibits slight shortening of the Ga–Au bond length (0.1 Å).
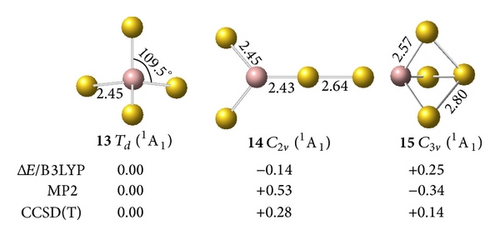
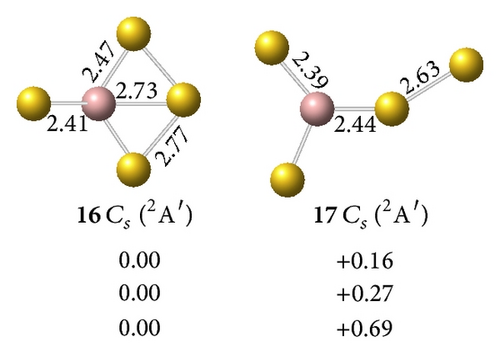
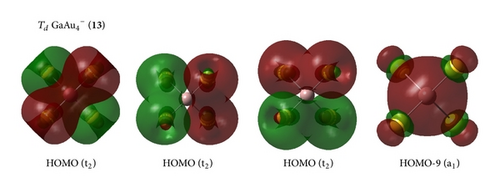
On , we calculated several isomers and found that the perfect tetrahedral (13, 1A1) has an sp3 hybridized Ga. This structure is the ground geometry; here, the four –Au terminals are singly σ-bound to the central Ga with a bond length of rGa–Au = 2.45 Å and a Wiberg bond index of WBIGa–Au = 0.93. Td (13) is separated by at least 0.14 eV from other 2D and 3D isomers at the CCSD(T) level, which suggests that an Ga– tetrahedral center is strongly favored in the anion. Interestingly, Td (13) has the shortest Ga–Au bond length and the largest HOMO-LUMO energy gap of ΔEgap = 2.89 eV in the GaAu− series. Detaching one electron from the perfect tetrahedral Td (13) involves a John-Teller process to produce the severely distorted global minimum of Cs GaAu4 (16, 2A′), which lies at least 0.22 eV higher than those of other low-lying isomers at the CCSD(T) level.
3.2. Bonding Consideration of GaAun and
NRT was used to calculate the bond orders and bond polarities of the molecules under study. As shown in Table 1, covalent contributions to the Ga–Au interactions continuously increase in the series from n = 1 to n = 4. The Ga–Au bonds in (13) have the highest percentage of covalence (78%). The Ga–Au bonds in (9), (5), and C∞v GaAu− (1) show covalent contributions of 54%, 44%, and 40%, respectively. This result indicates that Ga–Au interactions in the series render the characteristics of ionic structures, especially in and GaAu−. The characteristic of the Ga–Au bond in the series is also illustrated clearly by ELF analysis [27], which reflects the probability of finding an electron or a pair of pairs in specific basins (Figure 2). Contour line maps of C∞v GaAu− (1), (5), (9), and (13) reveal the presence of a weak electronic interaction between Ga and Au. This interaction is a highly polar covalent bond. NBO quantitatively reveals the Ga–Au bonding properties: the Ga–Au bond length of rGa–Au = 2.45–2.55 Å and the corresponding bond order of WBIGa–Au = 0.73–0.90 in the series. These properties further indicate that the interaction is covalent but with ionic characteristics.
| Isomers | Atom | Valency | Covalency | Electrovalency | Covalent percentage | q |
|---|---|---|---|---|---|---|
| 1 C∞v GaAu− | Ga | 1.02 | 0.41 | 0.61 | 0.40 | −0.44 |
| Au | 1.02 | 0.41 | 0.61 | 0.40 | −0.56 | |
| 5 C2v
|
Ga | 2.03 | 0.87 | 1.15 | 0.43 | 0.05 |
| Au | 1.01 | 0.44 | 0.58 | 0.44 | −0.53 | |
| 9 D3h
|
Ga | 2.85 | 1.53 | 1.32 | 0.54 | 0.08 |
| Au | 0.96 | 0.51 | 0.45 | 0.53 | −0.36 | |
| 11 Td
|
Ga | 3.77 | 2.95 | 0.82 | 0.78 | −0.34 |
| Au | 0.94 | 0.74 | 0.20 | 0.79 | −0.17 |
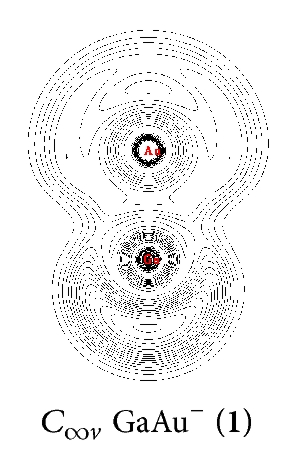
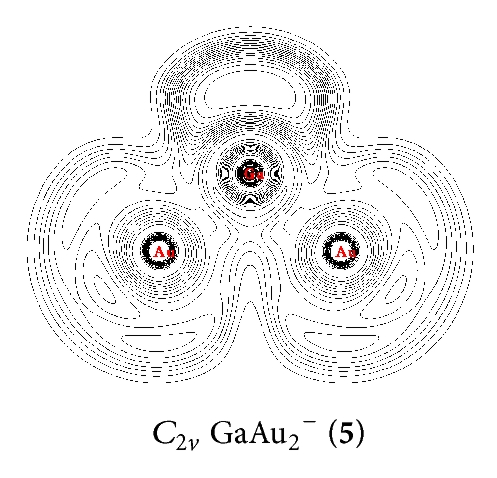
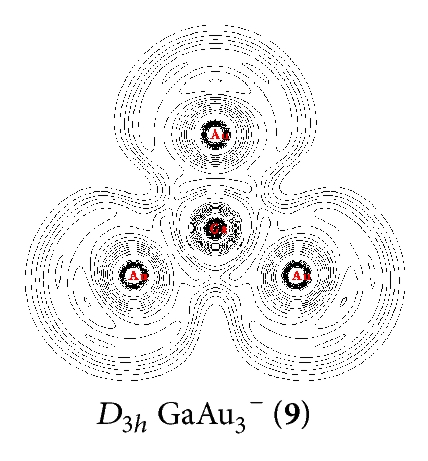
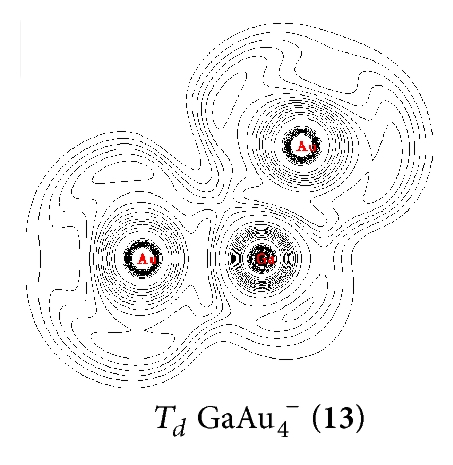
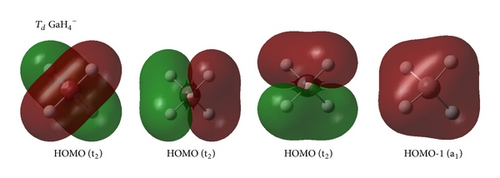
In the series, the perfect tetrahedral (13) is unique. Figure 3 shows the four valence molecular orbitals of the molecule, including a triply degenerate HOMO (t2) and a singlet HOMO-1 (a1). has a bonding pattern similar to that of , with an sp3 hybridized Ga center surrounded by four Au atoms to form four equivalent σ single bonds. The B–Au and B–H σ bonds in and show subtle differences in orbital composition because of obvious relative effects in Au. possesses the orbital combination of and the corresponding atomic contribution of 40%Ga + 60%Au, with Au 6s contributing 95.2% and Au 5d contributing 4.7% to the Au-based orbital. In , Au 5d contributes 8.5%–4.7% to the Au-based orbital, which is less than that in monoboron aurides.
3.3. Geometric and Electronic Structures of Ga2Aun and
All low-lying neutral and anion clusters of are summarized in Figure 4. clusters with bridged Au atoms possess geometric structures similar to those of the corresponding gallium hydrides [38]. As shown in Figures 4(a) and 4(b), the smallest digallium auride Ga2Au0/− contains a bridging Au atom. Anionic Ga2Au− exhibits three possible structures: triplet Au-bridged V-shaped (C2v, 18, 3B1), singlet Au-bridged V-shaped (C2v, 19, 1A1), and triplet linear (C∞v, 20, 3Σg−). At the CCSD(T) level, the triplet Au-bridged structure 18 with bond lengths of rGa–Au = 2.62 Å and rGa–Ga = 2.67 Å, respectively, lies 0.22 and 0.47 eV lower than the singlet Au-bridged 19 and triplet linear 20 structures. This result suggests that the triplet Au-bridged C2v Ga2Au− (3B1, 18) is the ground state of Ga2Au−. Similar to the V-shaped Ga2H (Ga(μ-H)Ga), the doublet Au-bridged C2v Ga2Au (21, 2B1) is a global minimum lying 0.72 eV lower than the linear C∞v Ga2Au (22) at the CCSD(T) level. Adding one Au atom to bridge two Ga atoms in C2v Ga2Au− (18) and C2v Ga2Au (21), respectively, produces the ground states of the off-plane di-Au-bridged C2v Ga2Au− ([Ga (μ-Au)2Ga]−) (23, 2A1) and C2v Ga2Au2 ([Ga (μ-Au)2Ga]) (26, 1A1), which are at least 0.43 and 0.67 eV, respectively, more stable than other isomers. Di-Au-bridged C2v Ga2Au2 (26) possesses the same geometry as di-H-bridged C2v Ga2H2. For (X=B, Al, Ga) systems, the global minima of and show similar V-shaped geometries. Both molecules differ from , which favors a linear structure containing a multiple-bonded BB core terminated by two Au atoms. This finding further demonstrates the presence of a strong chemical interaction between two B atoms in diboron aurides.
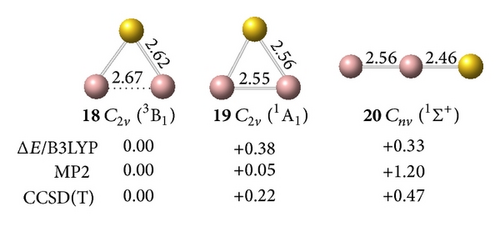
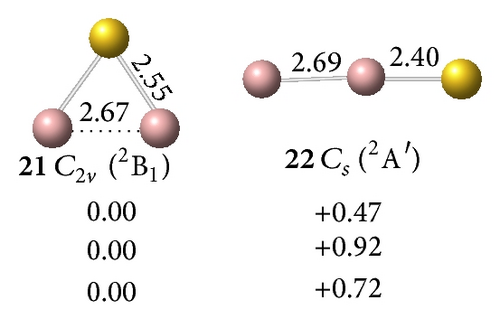
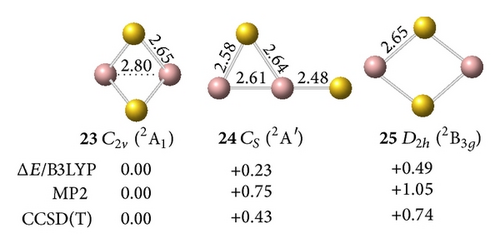
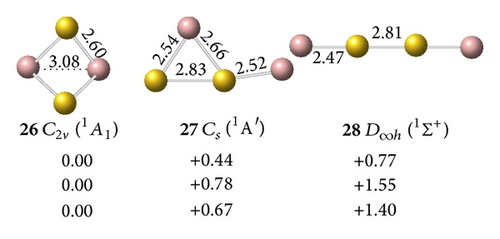
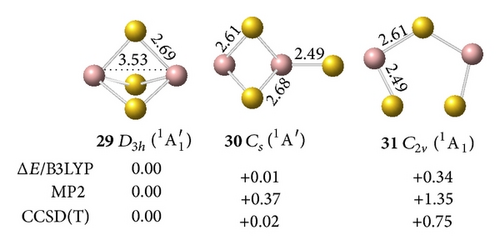
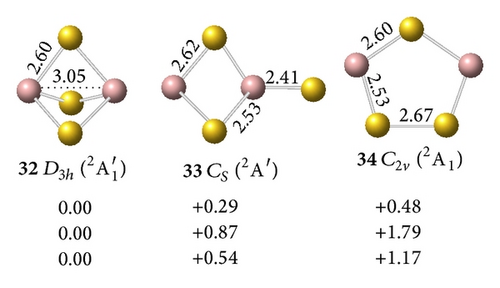
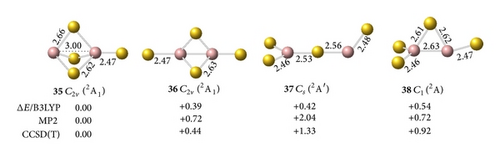
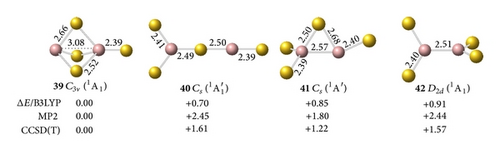
The interaction between two Ga atoms is so weak that two Au atoms prefer to bond with digallium auride isomers, such as D∞h Ga2Au2 (28). The anion prefers a tri-Au-bridged [Ga(μ-Au)3Ga]− form with a singlet electronic structure. The most stable geometry of the tri-Au-bridged (29, 1A1′) with a bond length of rGa–Au = 2.69 Å is at least 0.37 and 0.02 eV more stable than di-Au-bridged (30, 1A′) at the MP2 and CCSD(T) levels, respectively. Similar to Ga2H3 favoring a tri-H-bridged [Ga(μ-H)3Ga] structure, the global minimum of Ga2Au3 is the tri-Au-bridged D3h Ga2Au3 (2A1′, 32), which lies 0.54 and 1.17 eV lower than di-Au-bridged (33, 2A′) and planar (34, 2A1), respectively. The Ga–Ga distance decreases by 0.48 Å and the Ga–Au bond length decreases by 0.09 Å in anion 29 relative to the neutral molecule 32. This result indicates a large geometry change upon electron detachment from the anion to the neutral molecule, although they have the same symmetry.
Adding one Au atom terminally to a Ga in [Ga(μ-Au)3Ga]− (29, 1A1′) produces the ground state of tri-Au-bridged C3v [Ga(μ-Au)3Ga]Ga− (35, 2A1), which is 0.44, 1.33, and 0.92 eV more stable than di-Au-bridged (36, 2A1), distorted Y-shaped (37, 2A′), and mono-Au-bridged (38, 2A) at the CCSD(T) level, respectively. Ga2Au4 has the same high-symmetry ground state of tri-Au-bridged C3v Ga2Au4 Au+[Ga(μ-Au)3Ga]− (39, 1A1), which lies 1.61, 1.22, and 1.57 eV lower than Y-shaped Cs Ga2Au4 (40, 1A′), mono-Au-bridged Ga2Au4 (41, 1A′), and nonbridged perpendicular D2d Ga2Au4 (42, 1A1), respectively. Similar to the tri-H-bridged C3v Ga2H4 [39], tri-Au-bridged C3v Ga2Au4 (39) is composed of strong interactions between a Ga+ cation and the face of a tetrahedral anion. The global minima of Ga2Au4 and Al2Au4 have the same ionic conformer; both molecules differ from B2Au4, which has a di-Au-bridged covalent structure.
3.4. Bonding Consideration of Ga2Aun and
AdNDP analysis [27] is an effective tool for analyzing the bonding patterns of various organic and inorganic molecules. As shown in Figure 5, other bonds besides the lone pairs of the Au atom may be analyzed as follows. C2v Ga2Au (21) contains one localized Ga–Ga 2c–2e σ-bond with an occupation number of ON = 1.96 |e|, one localized Ga–Ga 2c–2e σ-anti-bond with an occupation number of ON = 1.96 |e|, and one delocalized Ga–Au–Ga 3c–2e bond with an occupation number of ON = 2.00 |e|. C2v Ga2Au2 (26) contains one localized Ga–Ga 2c–2e σ-bond with an occupation number of ON = 1.90 |e|, one localized Ga–Ga 2c–2e σ-anti-bond with an occupation number of ON = 1.90 |e|, and two delocalized Ga–Au–Ga 3c–2e bonds with an occupation number of ON = 1.97 |e|. D3h Ga2Au3 (32) contains three delocalized Ga–Au–Ga 3c–2e bonds with an occupation number of ON = 1.94 |e|. C3v Ga2Au4 (39) contains one localized Ga(2)–Au(t) 2c–2e σ-bond with an occupation number of ON = 1.95 |e| and three delocalized Ga–Au–Ga 3c–2e bonds with an occupation number of ON = 1.93 |e|. The same number of electrons occupies the bonding and antibonding orbitals in C2v Ga2Au (21) and C2v Ga2Au2 (26). Thus, no electronic effect is produced between two Ga atoms and the delocalized Ga–Au–Ga 3c–2e bond is the main interaction in high symmetric Ga2Aun (n = 1–4).
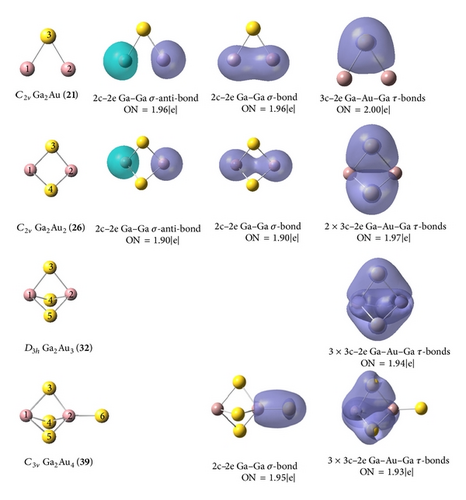
Detailed NLMO analyses quantitatively reveal the existence of bridging Ga–Au–Ga 3c–2e bonds in C2v Ga2Au (21), C2v Ga2Au2 (26), D3h Ga2Au3 (32), and C3v Ga2Au4 (39), as clearly shown in an image of their 3c–2e orbital and orbital combination (Figure 6). In C2v Ga2Au (21), the 3c–2e bond possesses the orbital combination of and the corresponding atomic contribution of 15%Ga + 70%Au + 15%Ga. In the Ga–Au–Ga 3c–2e bond, Au 6s contributes 97.8% and Au 5d contributes 1.64% to the Au-based orbital, whereas Ga 4p contributes 98.2% and Ga 4s contributes 0.76% to the Ga-based orbital. Obviously, Au 6s and Ga 4p provide the largest contributions to the Ga–Au–Ga 3c–2e bond, and it can be practically approximated as τGa–Au–Ga = 0.38(p)Ga + + 0.38(p)Ga. The orbital combinations of Ga–Au–Ga 3c–2e bond in C2v Ga2Au2 (26) [τGa–Au–Ga = 0.39(p)Ga + + 0.39(p)Ga] and D3h Ga2Au3 (32) [τGa–Au–Ga = 0.42(p)Ga + + 0.42(p)Ga] are surprisingly similar to that of C2v Ga2Au (21). However, the composition of the 3c–2e orbital in C3v Ga2Au4 (39) is obviously different from that in Ga2Aun (n = 1–3). Each 3c–2e bond has an orbital combination of and the corresponding atomic contribution of 16%Ga(1) + 50%Au + 35%Ga(2).
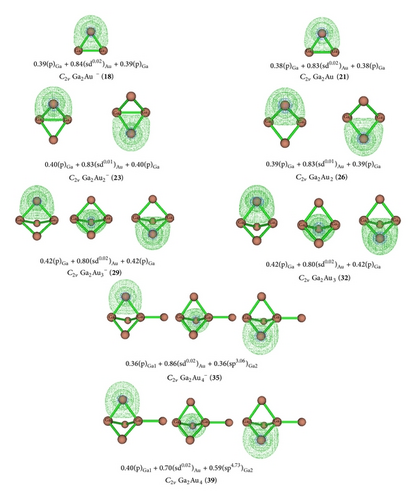
In the bridging Ga(1)–Au–Ga(2) 3c–2e bond, bridged Au 6s and 5d, respectively, contribute 80.7% and 1.4% to the Au-based orbital, Ga(1) 4s and 4p, respectively, contribute 0.7% and 98.6% to the Ga(1)-based orbital, and Ga(2) 4s and 4p, respectively, contribute 17.3% and 81.8% to the Ga(2)-based orbital. Obviously, the 17.3% contribution from Ga(2) is not negligible because of the Ga(2) atom of the unit. Au 6s and Ga(1) 4p provide the largest contributions to the Ga(1)–Au–Ga(2) bridging bond in C3v Ga2Au4 (39). This finding agrees with the ionic characteristic of [Ga(1)(μ-Au)3Ga(2)]− presented earlier. Thus, in contrast to the orbital combination in Ga2Aun (n = 1–3), the bridging bond of C3v Ga2Au4 (39) can be approximated as . Similar 3c–2e orbital combinations exist in the corresponding anions. Compared with the bridging Au 3c–2e bond observed in electron-deficient systems (B2Aun, Al2Aun, and Ga2Aun), we found that bridging Au provides greater contributions to dialuminum and digallium aurides (70%–68%) than to diboron aurides (50%–45%). Specifically, Au 5d contributes less than 2% to the Au-based orbital in dialuminum and digallium aurides.
3.5. Electron Detachment Energies
The ADE and VDE values of the anions were calculated in PES experiments. As shown in Table 2, the B3LYP and CCSD(T)//B3LYP levels produced consistent one-electron detachment energies for and anions. Except for C2v (5) and (13), (29), the calculated ADEs and VDEs at the CCSD(T) level lay at 0.30–1.87 eV. The small differences between ADE and VDE (0.03–0.32 eV) agree with the minor structural relaxation observed between the anion and the corresponding neutral molecule. At the same level, (5) shows ADE = 2.33 eV and VDE = 2.72 eV. The difference between the ADE and VDE (0.39 eV) shows considerable structure relaxation between the C2v anion (5) and the C2v neutral molecule (7). A similar result was observed in (29). (13) anion has the calculated one-electron detachment energies of ADE = 3.07 eV and VDE = 4.17 eV at the CCSD(T)//B3LYP level. B3LYP approaches produced close ADE and VDE values with CCSD(T). The extremely high electron detachment energies of indicate that GaAu4 neutrals lie considerably higher than anions in energy, while the big ADE-VDE differences (0.72–1.10 eV) agree with the considerable structural relaxation from (13) and its closely related Cs GaAu4 (16). The electron binding energies of these anions fall within the energy range of the conventional excitation laser (266 nm, 4.661 eV) in PES measurements.
| ADE | VDE | |||
|---|---|---|---|---|
| B3LYP | CCSD(T) | B3LYP | CCSD(T) | |
| 1 C∞v GaAu− ( 2Σ+) | 0.64 ( 1Σ+) | 0.30 ( 1Σ+) | 0.66 ( 1Σ+) | 0.60 ( 1Σ+) |
| 5 C2v
( 1A1) |
2.51 (2B2) | 2.33 (2B2) | 2.75 (2B2) | 2.72 (2B2) |
| 9 D3h
(
) |
1.99 (
) |
1.56 (
) |
2.10 (
) |
1.86 (
) |
| 13 Td
( 1A1) |
3.23 (
) |
3.07 (2T2) | 3.95 ( 2A′) | 4.17 (2T2) |
| 18 C2v Ga2Au− ( 3B1) | 1.47 (2B1) | 1.47 (2B1) | 1.49 (2B1) | 1.50 (2B1) |
| 23 C2v
( 2A1) |
1.45 (1A1) | 1.46 (1A1) | 1.50 (1A1) | 1.52 (1A1) |
| 29 D3h
(
) |
2.39 (
) |
2.13 (
) |
2.67 (
) |
2.60 (
) |
| 35 C3v
( 2A1) |
1.96 (1A1) | 1.55 (1A1) | 2.06 (1A1) | 1.87 (1A1) |
4. Summary
This study presents geometric and electronic structural analyses of and clusters based on DFT and wave function theory. The structure and bonding of a series of with one Ga atom at the center are characterized. NRT, ELF, and NBO analyses show that Ga–Au interactions in the aurogalliums are highly polar covalent bonds with ionic characteristics. is predicted to possess highly symmetric ground states of , , , and C3v . C3v Ga2Au4 present strong interactions between a Ga+ cation and the face of a tetrahedral anion. AdNDP and NLMO analyses demonstrate that a Ga–Au–Ga 3c–2e bond exists in these global minima. Detailed orbital analyses indicate that Au 6s and Ga 4p principally contribute to the Ga–Au–Ga bond in the Ga2Aun (n = 1–3) complex. In Ga+(GaAu4)− ionic conformers, besides Au 6s and cationic Ga 4p, tetrahedral Ga 4s and 4p also contribute significantly to the Ga–Au–Ga bond in Ga2Au4; here, the tetracoordinate unit has a greater influence than the cationic unit on the total 3c–2e orbital atomic contribution. The predicted ADE and VDE values of and may facilitate future PES experiments to confirm these species. Bridging Au interactions addressed in this work provide an interesting bonding mode for covalent and ionic deficient systems and will help design new materials and catalysis with highly dispersed Au atoms.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Acknowledgments
This work was financially supported by the North China University of Water Conservancy and Electric Power High-Level Experts Scientific Research Foundation (no. 201114) and the Science and Technology Research Project of Henan Provincial Education Department (no. 14A150024). The computational resources utilized in this research were provided by Shanghai Supercomputer Center.




