Isomers of Poly Aminophenol: Chemical Synthesis, Characterization, and Its Corrosion Protection Aspect on Mild Steel in 1 M HCl
Abstract
The oxidative chemical polymerizations of three isomers of aminophenol, ortho, meta, and para (PoAP, PmAP, and PpAP), were performed in aqueous HCl using ammonium persulfate as an oxidant at 0–3°C. The synthesized polymers were characterized by employing elemental analysis, GPC, UV-VIS-NIR, FT-IR, XRD, and TGA. The corrosion inhibition effect of these three polymers on mild steel in 1 M HCl solution was studied by using electrochemical techniques such as potentiodynamic polarization and electrochemical impedance spectroscopy. These measurements reveal that the inhibition efficiency obtained by these polymers increased by increasing their concentration. The inhibition efficiency follows the order PpAP > PoAP > PmAP. The results further revealed that PpAP at a concentration of 250 mg/L furnishes maximum inhibition efficiency (96.5%). Polarization studies indicated that these three polymers act as the mixed type corrosion inhibitors.
1. Introduction
Mild steel (MS) continues to be the prime construction material for a wide range of structures, as it has favorable properties and high speed of fabrication. However, its corrosion resistance is highly weakened when it is exposed to an acid environment [1–3]. Corrosion of MS will result in the loss of its mechanical properties and structural integrity [4]. At this juncture, it is necessary to protect it from corrosion when it is designed for long term service. A generic way to protect the material from corrosion is to apply protective coatings [5, 6]. Among the various types of protective coatings that are in use, organic polymers play important roles in corrosion prevention. These include organic inhibitors, generally rich in Π bonds, which adsorb onto a metal surface to reduce the number of active sites and barrier coatings which slow the transport of corrosive agents to the metal and removal of corrosion products [7]. The use of conducting polymers for the protection of metals against corrosion has been only recently investigated.
Unlike aniline and other substituted anilines, amino phenols present two oxidizable groups (NH2 and OH) providing more reactive sites. In principle, they could present electrochemical behavior resembling anilines and/or phenols, being an important condition for polymerization the relative position of the amino and hydroxyl groups in the aromatic ring [8]. The earlier studies [9–11] have shown that inhibitive properties of polyaniline and its derivatives on the corrosion of iron in acid solution are due to the presence of π electrons, quaternary nitrogen atom, and the large molecular size of them. The protection of metal surface against corrosion by aromatic diamine and aminophenol based polymers was reported on iron [12], copper [13], mild steel [14], and stainless steel [15]. In addition, it has been suggested that a derivative of aniline such as phenylenediamine and o-aminophenol polymers exhibits excellent anticorrosion properties [16, 17]. To the best of our knowledge, there was no report in the literature dealing with the corrosion protection behavior of isomers of poly aminophenols on MS and on other active metals synthesized by chemical oxidation method.
In this present work, isomers of aminophenol were synthesized by chemical oxidation method and characterized by different spectroscopic techniques. An attempt has been made to investigate the corrosion protection behaviour of chemically synthesised poly (ortho-, meta-, and para-aminophenol) over MS in acidic environment using potentiodynamic polarisation and electrochemical impedance spectroscopy (EIS) measurements.
2. Experimental Method
2.1. Synthesis of the Polymers
0.1 M monomers (ortho-, meta-, and para-aminophenol) and 1 M HCl were taken in separate beakers containing 50 mL toluene. Polymerization reactions were started by drop wise addition of aqueous solution of APS {0.1 M (22.8 g) APS in 100 mL distilled water} and the reactions were carried out for 12 h at 0–3°C with constant stirring. Polymerizations were stopped by addition of 50 mL methanol. The resulting precipitated polymers were filtrated and washed with excess amount of water, methanol, and acetone to remove excess of HCl and APS. The powder of PoAP, PmAP, and PpAP was then dried at 50°C for 24 h. Based on our observation as well as spectral and elemental analyses of the three synthesized polymers we have proposed polymerization reactions as shown in Schemes 1, 2, and 3.
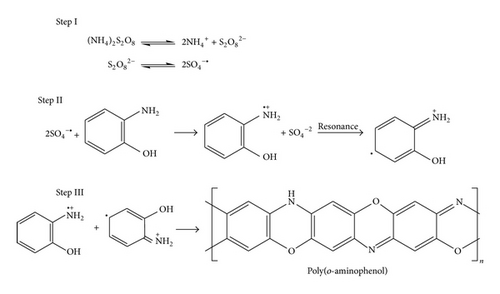
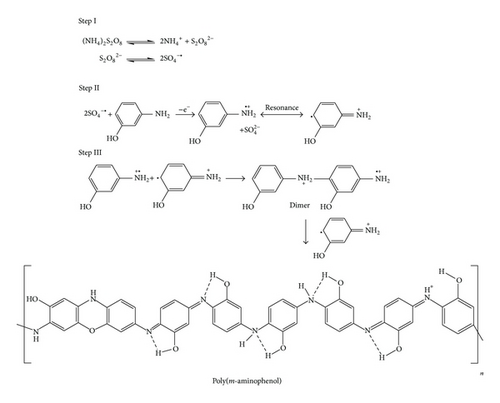
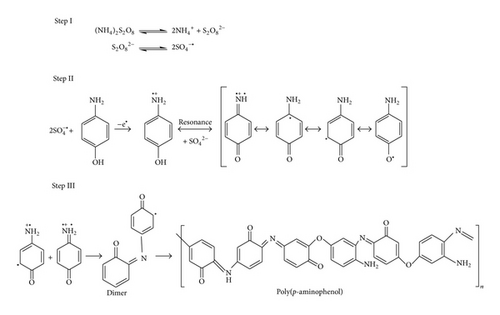
2.2. Characterization
Molecular weights of polymers were determined by gel permeation chromatography using Styragel columns and a refractive detector (Waters, model R 4000) with THF as the mobile phase. Carbon-hydrogen-nitrogen analyses of polymers were carried out by a microanalytical technique using an Elementar Vario EL 3 elemental analyzer. UV-VIS-NIR spectra of polymers dissolved in DMSO solvent were obtained using Varian, Cary-5000 spectrophotometer in the range of 200–2500 nm. The FT-IR spectrums of polymers were recorded by Thermo Nicolet, Avatar 370 spectrophotometer. The spectrum of the dry polymer powder in KBr pellet was recorded from 500 cm−1 to 4000 cm−1. X-ray diffraction (XRD) scan was done with Bruker AXS D8 advance diffractometer at room temperature using Cu Kα (λ = 1.5406 Å). Thermogravimetric analysis (TGA) was carried out in nitrogen atmosphere at a heating rate 10°C/min up to 750°C temperature by Perkin Elmer, Diamond TG/DTA analyzer.
Electrochemical measurements, including potentiodynamic polarization curves and electrochemical impedance spectroscopy (EIS), were performed in a conventional three electrodes cell using a computer-controlled potentiostat/galvanostat (Autolab PGSTAT 302N potentiostat from Eco-chemie, The Netherlands). Platinum electrode was used as the counter electrode, Ag/AgCl, 3 M KCl was used as the reference electrode, and the mild steel specimen was used as a working electrode. Specimen of dimension 1 × 1 × 0.1 cm was used for electrochemical studies. The specimens were embedded in epoxy resin leaving a working area of 1 cm2. The surface preparation of the mechanically abraded specimens was carried out by using different grades of silicon carbide emery paper (up to 1200 grit) and subsequent cleaning with acetone and rinsing with double distilled water were done before each experiment.
3. Results and Discussion
3.1. Elemental Analysis and Molecular Weight
The elemental analysis data of PoAP, PmAP, and PpAP are shown in Table 1. The PpAP contains higher H (4.4%) and N (10.2%) compared to other two polymers. The result indicates some sulfur incorporated in these three polymers due to the salt formation of liberated sulfuric acid from the reduction of oxidant APS with –N– present within the polymer chain [19].
| Polymers | Elemental analysis | Molecular weight | |||||
|---|---|---|---|---|---|---|---|
| C (%) | H (%) | N (%) | S (%) | Mn | Mw | PDI | |
| PoAP | 66 | 3.7 | 9.8 | 0.4 | 90000 | 297000 | 3.2 |
| PmAP | 53 | 4.3 | 8.2 | 0.7 | 57000 | 60500 | 1.1 |
| PpAP | 60 | 4.4 | 10.2 | 0.1 | 182000 | 297000 | 1.6 |
The molecular weights of the soluble portion of these polymers were determined by gel permeation chromatography performed in THF. The number-average molecular weight (Mn), weight-average molecular weight (Mw), and poly dispersity index (PDI = Mw/Mn) values were found and listed in Table 1. For PmAP and PpAP, the PDI values are found to be 1.1 and 1.6; these values demonstrated that the chromatograms were unimodal; no traces of monomers, oligomers, or high molecular masses were detected and no cross-linking or other by-products originating from parasitic reactions were present [20]. PoAP has the PDI value of 3.2 suggesting the presence of small amount of oligomers in the polymer sample. Among these three polymers PpAP has high Mn and Mw values; this result was supported as above from the elemental analysis result of PpAP.
3.2. UV-VIS-NIR Spectroscopy
The UV-VIS-NIR absorption spectrums of all the polymers dissolved in DMSO are shown in Figure 1. The spectrums of all the polymers consist of two major absorption peaks; the first peak at 285–305 nm (4.35–4.06 eV) is assigned to the π − π* transition of the phenyl rings which is related to the extent of conjugation between the phenyl rings in the polymer chain. The intensity of the π − π* absorption maxima is comparable for all the three polymers. The second absorption peak at 571–620 nm is assigned to n − π* transition between the HOMO orbital of the benzenoid ring and the LUMO orbital of the quinoid ring. It is sensitive to the overall oxidation state of the polymer [21]. The intensity of the peak around 850 nm is seen to be greater in the spectra of the PpAP compared to that of the other two polymers; this peak has been assigned to – species which is generated on doping or the polaronic transitions.
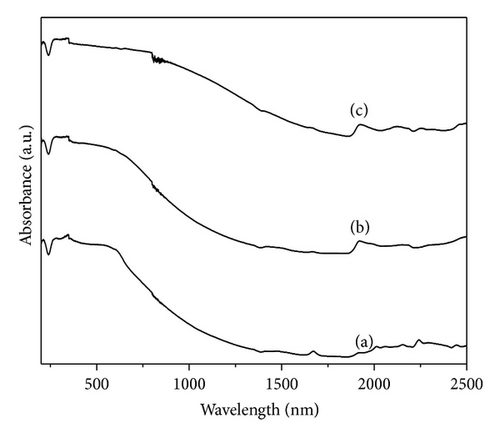
3.3. FT-IR Analysis
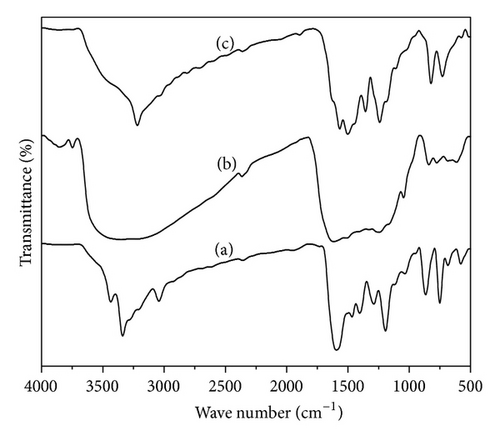
The representative FT-IR spectra of three polymers are given in Figure 2. In all the polymers a broad peak that appears in the region 3800–1800 cm−1 is due to the stretching of aromatic C–H, hydrogen bonded –OH, and –NH– groups. The –OH group is hydrogen bonded with nearest nitrogen of –NH group present in the polymer chain through the H2O molecule present in all the polymers. So –OH absorption peak appears at about 3338 (sharp), 3375 (broad), and 3270 cm−1 (sharp) for PoAP, PmAP, and PpAP, respectively. Two main peaks around 1600 and 1560 cm−1 in all the spectrums correspond, respectively, to the ring-stretching vibrations of the quinoid and benzenoid rings. The presence of these two rings clearly shows that the polymers are composed of the amine and imine units. The peak appears around 2362 cm−1 which is the characteristics stretching band for C=C=N or C=C=O [22]. The peak around 1270 cm−1 is assigned to the C–N stretching vibrations of the second aromatic amine, indicating the formation of a C–N–C structure in the polymers, whereas a peak at 2923 cm−1 in PoAP and PpAP is assigned to stretching vibrations of C=C in phenyl ring [23]. In three polymers the peaks around 1147 cm−1 are ascribed to the stretching of the C–O–C linkages [24] and further support that the aminophenol changed into poly aminophenol. The peaks between 900 and 600 cm−1 correspond to C–H bending of an aromatic ring substitution of the polymers.
3.4. XRD Analysis
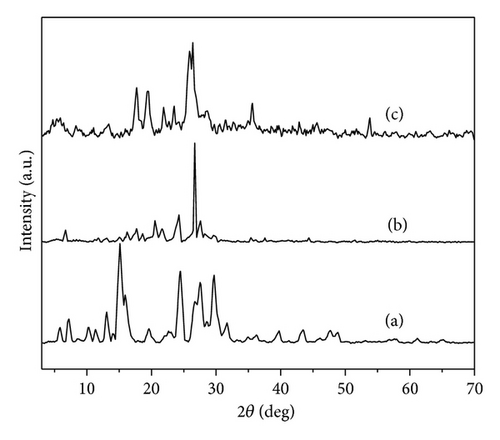
Figure 3 shows the X-ray diffraction patterns of three polymers. The diffraction patterns were typical of crystalline/amorphous polymers. The crystalline regions in the polymers are shown by the presence of relatively sharp peaks. The amorphous regions are visible by the broad low intensity halo; accordingly, the PoAP is more crystalline than PpAP and PmAP. The more amorphous nature of PmAP was inferred from their diffraction patterns. The position of the Bragg’s peaks (2θ) also gives information on the morphology of the polymers. All the polymers exhibit their strongest peaks at 17.6°, 19.4°, 24.2°, and 25.5°. 2θ = 25.5° is characteristics of the van der Waals distances between stacks of phenylene rings (poly aminophenol rings) [25]. These strongest peaks indicate crystalline domains in the amorphous structure of the polymers. The degree of crystalline or ordered structural pattern in PoAP and PpAP is due to the more intrachain hydrogen bonding or electrostatic interaction (through both amine and/or phenolic group) present in the polymer. The crystallinity and orientation of conducting polymers have been of much interest, because more highly ordered systems can display a metallic conductive state and may influence the anticorrosion performance [26].
3.5. Thermogravimetric Analysis
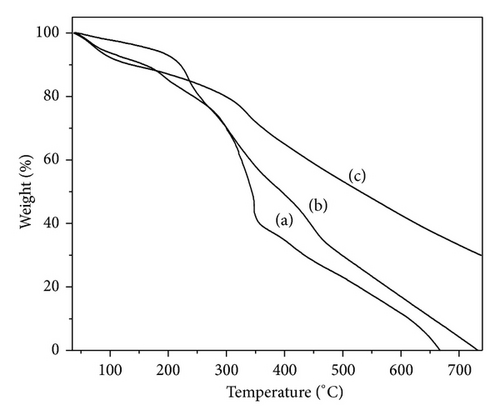
The thermal stability of the three polymers was evaluated using thermogravimetric analysis and shown in Figure 4. The thermal behaviour of the three polymers is similar and exhibits a three stage decomposition pattern. The first weight loss step starts from 60 to 110°C which corresponds to the loss of water molecules, adsorbed moisture, free acids, and volatile molecules in polymer matrix. The second step is in the TGA curves between 180 and 250°C because of loss of dopant, sublimation, and removal of low molecular weight polymer/oligomer from the polymer matrix. While the third weight loss step occurs between 265 and 360°C which is due to the complete degradation and decomposition of the polymer backbone. The degradation temperature of PpAP (480°C) is higher than that of PoAP (400°C) and PmAP (465°C).
3.6. Potentiodynamic Polarization Studies
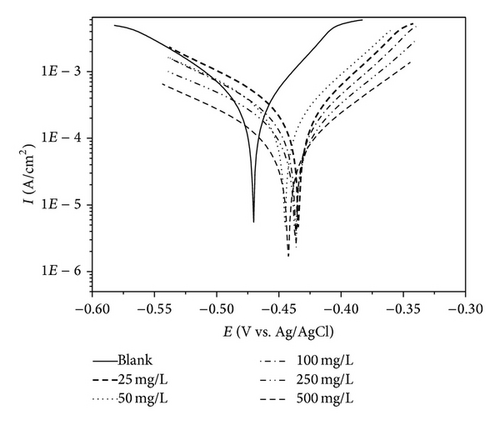
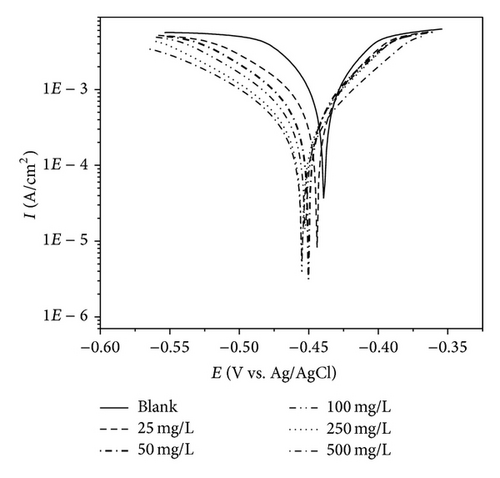
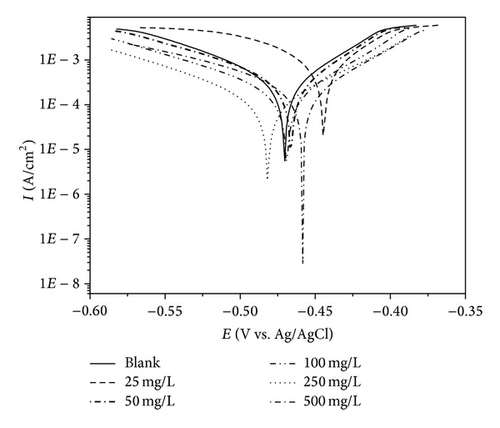
The potentiodynamic polarization curves of MS in 1 M HCl with the addition of various concentrations of PoAP, PmAP, and PpAP are shown in Figures 5(a), 5(b), and 5(c), respectively. The corrosion kinetic parameters such as corrosion current density Icorr, corrosion potential Ecorr, anodic Tafel slope ba, and cathodic Tafel slope bc, inhibition efficiency IE, and corrosion rate CR deduced from the curves are given in Table 2. The corrosion current density values decrease from 3710 μA cm−2 for the blank acid to 400, 164, and 131 μA cm−2 for PmAP, PoAP, and PpAP, respectively, for the addition of 250 mg L−1 three polymers resulting in 89.2, 95.6, and 96.5% of inhibition efficiency. It is clear that the Icorr values decrease with the presence of three polymers which indicated that polymers adsorbed on the metal surface and hence inhibition occurs. As the concentrations increase 25 to 500 mg L−1 for PoAP and PmAP the IE increases 92.5 to 97.2% and 47.7 to 90.2%, respectively. But for PpAP the IE reached maximum of 96.5% at 250 mg L−1 and further increase in concentration the IE value decreases. The Ecorr, ba, and bc values do not change appreciably with the addition of the inhibitors indicating that the inhibitors are not interfering with the anodic dissolution or cathodic hydrogen evolution reactions independently but acts as a mixed type of inhibitor [27, 28]. These results confirm that the isomers of poly aminophenol on MS act as a highly protective layer, which is mainly attributed to the presence of π electrons in aromatic ring coexisting with quaternary nitrogen atom and large molecular size [29]. Moreover, the outstanding corrosion protection offered by PpAP on MS may be due to the fact that the deposited polymer is strongly adherent and uniformly covers the entire electrode surface. Based on the corrosion protection mechanism put forth by several researchers [30–32], the polymer accepts electrons from the metal and gives them to oxygen. This reaction generates the formation of a passive layer at the polymer/metal interface which lowers the corrosion rate and shifts the corrosion potential to more positive values. Besides isomers of poly aminophenol is a ladder polymer having longer molecular structure with phenazine skeleton ensures greater adsorption on the MS surface and decreases the effective area for the corrosion reaction by blocking the reaction sites [33, 34].
Inhibitor concentration (mg/L) |
Ecorr (mV) | Icorr (μA/cm2) | ba (mV/decade) | bc (mV/decade) | IE (%) | Corrosion rate (mm/year) |
|---|---|---|---|---|---|---|
| PoAP | ||||||
| Blank | 471 | 3710 | 61 | 43 | — | 20.09 |
| 25 | 434 | 277 | 50 | 75 | 92.5 | 3.21 |
| 50 | 445 | 254 | 75 | 56 | 93.2 | 2.95 |
| 100 | 436 | 221 | 72 | 49 | 94 | 2.57 |
| 250 | 437 | 164 | 70 | 54 | 95.6 | 1.90 |
| 500 | 443 | 104 | 73 | 56 | 97.2 | 1.21 |
| PmAP | ||||||
| Blank | 471 | 3710 | 61 | 43 | — | 20.09 |
| 25 | 439 | 1938 | 77 | 42 | 47.7 | 12.08 |
| 50 | 452 | 1040 | 56 | 52 | 72 | 8.82 |
| 100 | 454 | 565 | 76 | 75 | 84.7 | 6.57 |
| 250 | 444 | 400 | 93 | 59 | 89.2 | 4.65 |
| 500 | 455 | 364 | 52 | 74 | 90.2 | 4.24 |
| PpAP | ||||||
| Blank | 471 | 3710 | 61 | 43 | — | 20.09 |
| 25 | 444 | 1041 | 58 | 41 | 72 | 8.81 |
| 50 | 466 | 283 | 44 | 75 | 92.4 | 3.98 |
| 100 | 469 | 196 | 81 | 50 | 94.7 | 2.27 |
| 250 | 481 | 131 | 71 | 58 | 96.5 | 1.52 |
| 500 | 458 | 244 | 40 | 51 | 93.4 | 2.84 |
3.7. Electrochemical Impedance Spectroscopy
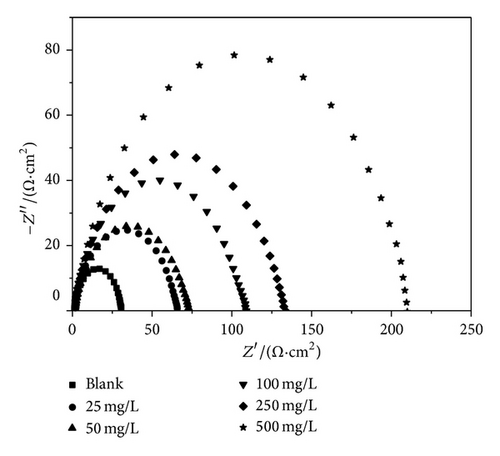
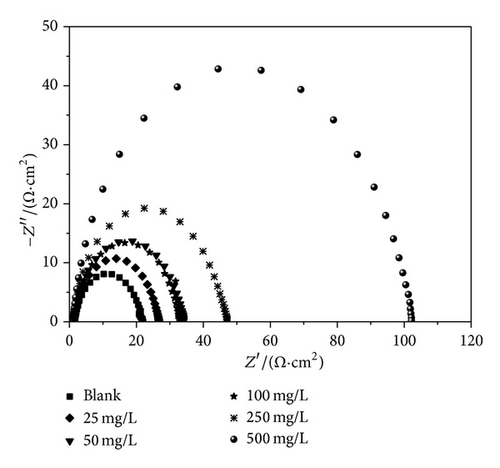
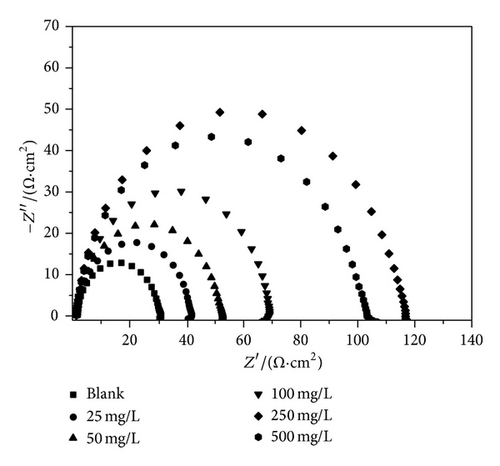
The Nyquist impedance plots were analyzed by fitting the experimental data to a simple equivalent circuit model. In this equivalent circuit, Rs is the solution resistance, Rct is the charge transfer resistance, and CPE is a constant phase element, which is placed in parallel to charge transfer resistance element. Thus, in these situations pure double layer capacitors (Cdl) are better described by a transfer function with constant phase elements to give a more accurate fit. The Nyquist plots of MS in 1 M HCl in the presence and absence of various concentrations of PoAP, PmAP, and PpAP are depicted in Figures 6(a), 6(b), and 6(c), respectively. The Nyquist spectra obtained for blank showed depressed semicircle, representing the corrosion process of the system, that is, the charge transfer resistance due to the metal corrosion and the double layer capacitance of the liquid/metal interface. However, the Nyquist spectra of isomers of aminophenol at various concentrations showed depressed semicircle. The depressed semicircles in the Nyquist plots were probably due to the surface heterogeneity or corrosion products of the metal substrate. The element at high frequency region is related with the charge transfer resistance of system; elements at low frequency region may represent adsorption of ions, for example, Cl− which is the initial step of anionic or solvent exchange between polymers and solution during equilibration in 1 M HCl [35–37]. The Nyquist spectra of blank and isomers of aminophenol revealed one-time constant behaviour, which is attributed to the charge transfer resistance of corrosion process [38]. The total impedance in the low frequency region for the polymers at higher concentrations seems to be almost one order of magnitude higher than that displayed by the blank MS. The higher impedance is probably due to an area effect where the inhibitor is blocking access of the aggressive electrolyte to the reactive metal surface [39]. The impedance values are given in Table 3. The charge transfer resistance is increased from 20.12 Ω cm2 for blank solution to 133.49, 45.65, and 138.95 Ω cm2 upon addition of 250 mg/L of PoAP, PmAP, and PpAP resulting in 85, 55.9, and 85.5% inhibition efficiency, respectively. The increase in Rct value is attributed to the formation of an insulating protective film at the metal/solution interface [40, 41]. The double layer capacitance decreases from 6.81 μF cm−2 to 4.31, 4.59, and 4.29 μF cm−2 in the presence of 250 mg/L of PoAP, PmAP, and PpAP, respectively. The initial decrease in Cdl value from blank solution to inhibitor containing electrolyte is due to a decrease in the local dielectric constant, while further decrease in Cdl with increasing concentrations of the inhibitor is due to increase in the thickness of the electrical double layer.
Inhibitor concentration (mg/L) |
Cdl (μF/cm2) |
Rct (Ω cm2) |
IE (%) |
|---|---|---|---|
| PoAP | |||
| Blank | 6.81 | 20.12 | — |
| 25 | 5.48 | 64.81 | 69 |
| 50 | 5.13 | 71.14 | 71.72 |
| 100 | 4.68 | 108.33 | 81.43 |
| 250 | 4.31 | 133.49 | 85 |
| 500 | 4.08 | 208.55 | 90.35 |
| PmAP | |||
| Blank | 6.81 | 20.12 | — |
| 25 | 6.22 | 25.20 | 20.17 |
| 50 | 5.22 | 31.43 | 35.98 |
| 100 | 4.83 | 32.52 | 38.13 |
| 250 | 4.59 | 45.65 | 55.93 |
| 500 | 4.38 | 100.29 | 79.94 |
| PpAP | |||
| Blank | 6.81 | 20.12 | — |
| 25 | 5.57 | 49 | 58.94 |
| 50 | 5.06 | 65 | 69.05 |
| 100 | 4.52 | 109.5 | 81.63 |
| 250 | 4.29 | 138.95 | 85.52 |
| 500 | 4.41 | 115 | 82.50 |
The enhanced corrosion protection of mild steel by isomers of aminophenol can be explained on the basis of molecular adsorption. The polymers inhibit corrosion by controlling both the anodic and cathodic reactions. In acidic solution, the polymer molecules exist as protonated species [42]. These protonated species adsorb on the cathodic sites of MS and decrease the evolution of hydrogen. The adsorption on anodic sites occurs through long π-electrons of aromatic rings (benzenoid and quinoid) and lone pair of electrons of nitrogen atoms, which decreases the anodic dissolution of mild steel [43]. It is well known that the species having high molecular weight and bulky structure may cover more area on the active electrode surfaces [44]. The high performance of the PpAP is attributed to the presence of long π-electrons conjugation, quaternary nitrogen atom, and the larger molecular size.
4. Conclusion
The isomers of aminophenol were successfully synthesized by chemical oxidative polymerization method and characterized by different spectroscopic techniques. UV-VIS-NIR and FT-IR suggest the formation of quinoid and benzenoid rings which confirmed the formation of polymer. The XRD studies reveal that the morphology of isomers of poly aminophenol is partially crystalline and amorphous nature. From thermal analysis it was found that all the polymers exhibit three-step decomposition patterns. The corrosion behaviour investigation, the percentage inhibition efficiency of these polymers obtained from potentiodynamic polarization, and EIS measurements are in good agreement and the corrosion inhibition efficiencies are in the order PpAP > PoAP > PmAP. Polarization curves demonstrated that the examined polymers behave as mixed type inhibitors. In these isomers of poly aminophenol the uniform increasing inhibition efficiency as the function of concentration deals with the adsorption phenomenon; the adsorption of inhibitors on the surface of MS is indicated by decrease in the double layer capacitance. The inhibition is due to the adsorption of the inhibitors on the steel surface and a resultant blocking of active sites. In addition, the π-electrons conjugation, quaternary nitrogen atom, and large size of the polymers also facilitate its strong adsorption on MS which leads to an efficient protection to the metal in highly corrosive medium. The results of this study clearly ascertain that isomers of aminophenol could be considered as a candidate for the protection of MS against corrosion in an acid environment.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.




