Beta2-Microglobulin as a Diagnostic Marker in Cerebrospinal Fluid: A Follow-Up Study
Abstract
Beta2-Microglobulin (β2-m) is a low molecular weight protein occurring in all body fluids. Its concentration increases in various pathologies. Increased values in cerebrospinal fluid (CSF) are ascribed to an activation of immune system. Using immunoturbidimetry, we examined concentrations of beta2-microglobulin in cerebrospinal fluid in a large group of 6274 patients with defined neurological diseases. Cell counts, total protein, albumin, glucose, lactic acid, immunoglobulins concentrations, and isofocusing (IEF) were also evaluated. We found substantial changes of CSF β2-m concentrations in purulent meningitis, leptomeningeal metastasis, viral meningitis/encephalitis, and neuroborreliosis, while in multiple sclerosis these changes were not significant. Intrathecal synthesis and immune activation were present in these clinical entities. A new normative study enables better understanding of beta2-microglobulin behavior in CSF.
1. Introduction
In 1968, Beggard and Bearn first isolated beta2-microglobulin (β2-m) from urine of patients with renal proximal tubule disorders. β2-m is a small membrane protein (11800 D) that is highly stable during evolution; it is encoded in the sixth chromosome. Beta2-microglobulin is composed of 99 amino acids; due to one peptidic bound it creates a loop. It belongs to the immunoglobulin superfamily, and its primary and secondary structure is strikingly similar to IgG structure; thus, it is suggested to arise from the same ancestral gene [1, 2].
Beta2-microglobulin constitutes the light chain of class I major histocompatibility complex proteins and is, therefore, present on the surface of all nucleated cells. Prevailing expression is on lymphocytes and macrophages, conversely none on erythrocytes [3, 4]. β2-m is noncovalently bound to the heavy chain, and during metabolism and degradation it is dissociated and released to all biological fluids. Concentration of beta2-microglobulin reflects a rate of cell membrane renovation and cellular turnover. Production and releasing of β2-m are constant and low in healthy people: 0.13 mg/h/kg b.w. β2-m is filtered through the glomerulal membrane due to its small size, but then it is nearly completely reabsorbed in the proximal tubules [5].
Serum and plasma beta2-microglobulin value increases in many pathologies. β2-m is a marker for an activation of the cellular immune system, as well as a tumor marker in certain hematologic malignancies (multiple myeloma, non-Hodgkin lymphoma, Hodgkin’s disease, and chronic lymphoblastic leukemia) [6–8]. Serum β2-m increases in patients with kidney diseases, because 99% is excreted by the kidneys. β2-m can form long protein chains deposited in joints and tissues (dialysis-associated amyloidosis) [9–11].
Increased urine beta2-microglobulin value indicates a damage of renal tubules because of the decrease of reabsorption. Measurement of concentrations in both serum and urine enables to differentiate a problem of cellular activation from a renal tubulointerstitial disorder [12–16].
Cerebrospinal fluid (CSF) reflects changes occurring in brain and spinal cord. Unlike blood analysis, examination of cerebrospinal fluid is not disturbed by other organ systems influence, and the CSF compartment is comprehensively autonomous. Assessment of beta2-microglobulin in cerebrospinal fluid is recommended within the enhanced level of examination [17]; β2-m concentrations in CSF have been proposed as a reliable marker in different inflammatory, autoimmune, or neoplastic central nervous system disorders. Increased CSF values are attributed to an immune activation and lymphoid cell turnover, a production of tumorous cells, and releasing in tissue destruction in CNS.
CSF values of beta2-microglobulin were studied in the context with neurological diseases by many authors. Simultaneous measurement of β2-m and ferritin in CSF was found as a useful instrument for differential diagnosis between viral meningitis and bacterial meningitis and for monitoring of ATB therapy effect [18–22]. Many studies confirm significantly elevated CSF β2-m values in neonates with cytomegalovirus infection, by reason of an intrathecal synthesis [23–25]. Findings in patients with HIV-1 infection raise a great interest. An elevation of CSF β2-m concentration correlates with a disease progression, and a decline of increased value reflects a successful therapy [26–31]. Assessment of specific proteins including beta2-microglobulin is the aim of many studies with dementias like Alzheimer’s disease and Parkinson’s disease [32–35].
A new study shows an antibacterial role of beta2-microglobulin in further human fluid: the amniotic fluid [36].
A large body of literature on monitoring β2-m levels in cerebrospinal fluid is currently available. While most groups of patients described are numerous enough, what is badly needed is a comparison of diagnostical subgroups of defined neurological entities in reasonably large groups. We examined a large CSF sample and follow-up values of beta2-microglobulin in patients with defined CNS diseases to clarify a biological behavior of this marker. We hypothesize that CSF values of β2-m would be increased particularly in patients with CNS inflammations and tissue disintegration. Our results support the great importance of β2-m examination for differential diagnosis and early therapy of central nervous system diseases, with concordance with the other CSF parameters, incl. CSF cytology, and biochemistry.
2. Materials and Methods
In the Laboratory for CSF and Neuroimmunology of the Homolka Hospital (the expert laboratory in CSF analysis in Czech Republic) we evaluated a total of 6274 CSF samples from patients aged from 3 to 86 years. In all the cases, the CSF samples were analysed only for diagnostic purposes, and they were completely investigated. We examinated firstly a CSF cell count (in the Fuchs-Rosenthal chamber) and basic biochemistry including total protein, glucose, lactic acid concentrations, and CEB (coefficient of energy balance) [37]. Secondly, we performed qualitative cytological examination (permanent cytological preparations). Thirdly, albumin CSF/S quotient, IgG, IgA, IgM CSF/S quotients, β2-m in CSF and serum, and the isoelectric isofocusing were evaluated within an enhanced CSF protocol [17]. With immunoglobulins, their intrathecal oligoclonal synthesis, if existent, was determined numerically by the formulae according to Reiber [38].
The functional state of blood-CSF barrier could have been expressed by Qalb only in the cases when albumin CSF and serum concentrations were measured simultaneously. Evaluation of a blood-CSF barrier permeability is not so important for β2-m as for other CSF proteins due to a small molecule of β2-m [39]. Nevertheless, physiological CSF concentration of β2-m is slightly lower than serum one and Qβ2-m is below 0.8 (see Table 1). Then a blood-CSF barrier impairment may cause an increase in CSF β2-m level and an equilibration of CSF and serum concentrations (whether any serum concentration), and consequently Qβ2-m elevated above 0.8. So we can say that only Qβ2-m increased above 1.0 points clearly to the intrathecal synthesis of beta2-microglobulin.
| Parameter | Range | Unit |
|---|---|---|
| GlucoseCSF | 2.20–4.20 | mmol/L |
| LactateCSF | 0.7–2.1 | mmol/L |
| CEB (coefficient of energy balance) | 28.0–38.0 | — |
| Total proteinCSF (TPCSF) | 0.2–0.4 | g/L |
|
to 7.4 | |
| Leukocyte count | 0–4 | /1 µL |
| β2-mCSF | 0.2–2.0 | mg/L |
| β2-mblood | 1.0–3.0 | |
|
0.41–0.79 | — |
In cytological examination, we used standard and special staining techniques in an effort to detect malignant elements (Toluidine blue stain, Papanicolaou) and the presence of CNS tissue destruction (Oil Red 0) by detecting lipophagic macrophages. Cytological samples were obtained from CSF using a gentle sedimentation technique. Concentrations of β2-m were established using immunoturbidimetry in CSF and corresponding sera in Synchron LX-20 analyser. Cytological CSF syndromes with a normal cell count (i.e., up to 10/3 of cells per chamber or up to 4/μL) were classified as normal or pathological oligocytosis (lymphocyte oligocytosis, monocyte oligocytosis, granulocyte oligocytosis, and tumorous oligocytosis) depending on the morphological and functional prevalence of individual cell lines in the cytological picture, whereas, with tumourous oligocytosis, the key factor was the detection of malignant elements in the cytological sample in general. In the case of pleocytosis, an elevated cell count in CSF, cytological syndromes were determined using similar criteria, that is, lymphocyte pleocytosis, monocyte pleocytosis, granulocyte pleocytosis, and tumorous pleocytosis, using classification acc. to Adam et al. [39]. From this point of view, using such a terminology may be often helpful in aetiological identification of the nosological entity involved.
Control group was declared as the group with normal cytological and biochemical findings (including total protein, albumin, immunoglobulins levels, glycorrhachia, and lactic acid concentration, CEB acc. to Bořecká et al. [37]), and after exclusion of the presence of inflammatory or organic neurological impairment. Our study used established laboratory norms—see Table 1. Although these criteria may be considered not to be fully convincing, there is virtually no other way of further differentiation (we do not perform lumbar puncture in healthy people). Samples with parameters not meeting the above criteria were automatically regarded as samples from patients with pathological CSF findings. This large pathological group was divided according to CSF findings into group with multiple sclerosis (n = 2674), neuroborreliosis (n = 215), brain tumours/malignant infiltrations (n = 95), viral meningitis/encephalitis (n = 641), bacterial meningitis/encephalitis (n = 312), scavenger reaction (n = 638), and polyneuritis/polyneuropathies (n = 1261). Malignant meningeal infiltration (MMI) or meningeal carcinomatosis is defined as a meningeal metastatic involvement without the presence of lokalized metastases (i.e., meninges are inhabited by malignant elements). This is relatively frequent severe complication of cancer, mostly in secondary, sometimes also in primary tumours of the nervous system. The phagocytosis of a specific substrate, that is, the scavenger reaction or reactions, is characterized by the presence of the phagocytosis of tissue destructive products and cellular detritus. This occurs in the presence of a morphological CNS and PNS impairment, for example, in brain ischemia or in CNS traumas [39].
The values were precisely statistically correlated, leading to substantial concrete findings both in the whole group of patients and after their division according to the presence of normal or pathological biochemical, and even cytological CSF findings. Descriptive methods, Kolmogorov-Smirnov test, box-and-whiskers plots, t-test, and one-way ANOVA with pairwise post hoc multiple comparisons were used for statistical analysis. As the β2-m distribution is not normal, we performed the logarithmic transformation of β2-m for one-way ANOVA testing.
3. Results and Discussion
Results are summarized in Tables 2–4 and Figures 1(a) and 1(b).
| Group | N | Beta2-microglobulin (mg/L) | Qβ2 | |
|---|---|---|---|---|
| Mean CSF | Mean serum | |||
| Control group | 438 | 1.6 | 2.5 | 0.64 |
| Pathological group | 5836 | 2.1 | 2.2 | 0.95 |
| Subgroup | N | Beta2-Microglobulin (mg/L) | ||||||
|---|---|---|---|---|---|---|---|---|
| Median | Minimum | Maximum | 1st quartile | 3rd quartile | 2.5th percentile | 97.5th percentile | ||
| Multiple sclerosis | 2674 | 1.7 | 0.0 | 9.5 | 1.3 | 2.2 | 0.8 | 4.1 |
| Neuroborreliosis | 215 | 2.0 | 0.4 | 15.4 | 1.5 | 2.8 | 0.8 | 6.8 |
| Brain tumours/malignant infiltrations | 95 | 2.5 | 0.2 | 16.7 | 1.7 | 3.5 | 0.4 | 10.1 |
| Bacterial meningitis/encephalitis | 312 | 3.2 | 0.0 | 21.1 | 2.1 | 4.9 | 0.6 | 10.6 |
| Viral meningitis/encephalitits | 641 | 2.2 | 0.0 | 17.5 | 1.4 | 3.4 | 0.2 | 7.4 |
| Scavenger reaction | 638 | 2.2 | 0.0 | 24.2 | 1.5 | 3.2 | 0.3 | 7.6 |
| Polyneuritis/polyneuropathies | 1261 | 1.5 | 0.0 | 14.8 | 1.0 | 2.1 | 0.0 | 4.5 |
| Control group | 438 | 1.5 | 0.0 | 5.8 | 1.0 | 2.0 | 0.2 | 3.4 |
| Compared groups | Level of Significance |
|---|---|
| Bacterial meningitis/encephalitis—Control group | P < 0.0001 |
| Bacterial meningitis/encephalitis—Viral meningitis/encephalitis | P < 0.0001 |
| Bacterial meningitis/encephaliti—Polyneuritis/polyneuropathies | P < 0.0001 |
| Bacterial meningitis/encephalitis—Neuroborreliosis | P < 0.0001 |
| Bacterial meningitis/encephalitis—Scavenger reaction | P < 0.0001 |
| Bacterial meningitis/encephalitis—Multiple sclerosis | P < 0.0001 |
| Bacterial meningitis/encephalitis—Brain tumours/Malignant infiltrations | P = 0.0066 |
| Brain tumours/Malignant infiltrations—Control group | P < 0.0001 |
| Brain tumours/Malignant infiltrations—Multiple sclerosis | P < 0.0001 |
| Brain tumours/Malignant infiltrations—Polyneuritis/polyneuropathies | P < 0.0001 |
| Viral meningitis/encephalitis—Control group | P < 0.0001 |
| Viral meningitis/encephalitis—Multiple sclerosis | P < 0.0001 |
| Viral meningitis/encephalitis—Polyneuritis/polyneuropathies | P < 0.0001 |
| Neuroborreliosis—Control group | P < 0.0001 |
| Neuroborreliosis—Multiple sclerosis | P < 0.0001 |
| Neuroborreliosis—Polyneuritis/polyneuropathies | P < 0.0001 |
| Scavenger reaction—Control group | P < 0.0001 |
| Scavenger reaction—Multiple sclerosis | P < 0.0001 |
| Scavenger reaction—Polyneuritis/polyneuropathies | P < 0.0001 |
| Multiple sclerosis—Control group | P < 0.0001 |
| Multiple sclerosis—Polyneuritis/polyneuropathies | P < 0.0001 |
| Other groups | No difference |
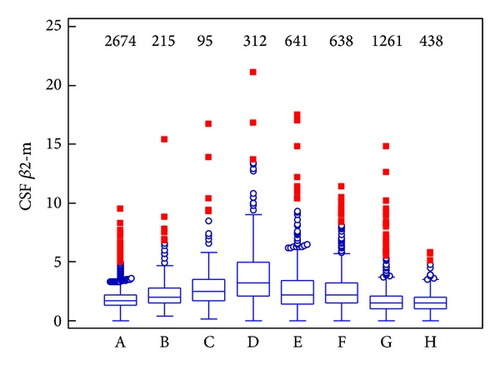
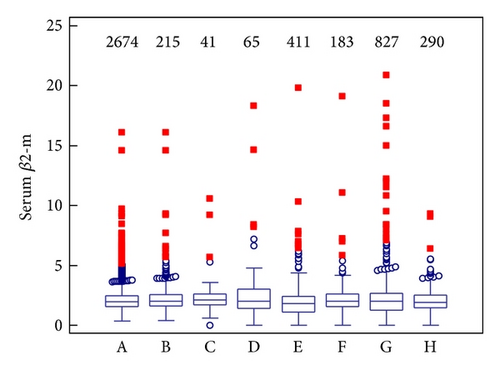
As can be seen from Table 2, higher values of β2-m in cerebrospinal fluid are present in the pathological group. We found a statistically significant difference between the control group and the pathological group (P < 0.001). Conversely, serum β2-m values are similar in pathological and control group. Although β2-m concentration was not certainly used for determination of the control group, in this group the mean of β2-m is in the reference range both in cerebrospinal fluid and in serum. In the pathological group, the mean of β2-m is slightly increased and Qβ2 is increased only in CSF.
Table 3, Figures 1(a) and 1(b) show values of β2-m in CSF and serum with individual diagnostic subgroups. We found the highest median of β2-m in cerebrospinal fluid in the group with bacterial meningitis/encephalitis, where increased β2-m values are surely caused by inflammatory response and an intrathecal synthesis (Qβ2 is 1.39 in this group, when Qβ2 > 1.0 means an intrathecal synthesis). The second group is the group with malignant infiltrations or brain tumours; highly increased values of β2-m are probably caused by tissue destruction, production of tumorous cells, and inflammatory response as well (Qβ2 = 1.32). The third ones are equal groups with viral meningitis/encephalitis and scavenger reaction. We found borderline values of β2-mCSF in group with neuroborreliosis. Our results confirm that β2-m behaves in cerebrospinal fluid as an inflammatory marker or a tumour marker. Elevated β2-mCSF values should lead to a suspicion of CNS inflammation or malignant infiltration, even if no tumorous cells are present in cerebrospinal fluid. On the other hand we demonstrated only slightly or no increased values of CSF β2-m and no intrathecal synthesis (evaluated according Qβ2) in groups with multiple sclerosis and polyneuritis/polyneuropathies. Concentrations of CSF β2-m probably reflect an activity and a progression of disease in patients with multiple sclerosis. In the control group, we found unique CSF and serum β2-m values greater than the upper reference limit (see “maximum” in Table 3); this is because dialysed patients were also included.
In serum, the upper reference limit (3.0 mg/L) was not exceeded by medians in any diagnostic subgroup. Serum β2-m values are similar in all groups (see Figure 1(b)).
As can be seen from Table 4, we found a statistically significant difference between the group with bacterial meningitis/encephalitis and the control group (P < 0.0001), as well as in comparison with all other groups. In other words, beta2-microglobulin values are the highest in the group with bacterial meningitis/encephalitis (see Table 3 and Figure 1(a)). The difference between groups with bacterial and viral meningitis/encephalitis is very important, because that means the CSF β2-m value allows distinguishing between these etiological diagnoses and contributes to early targeted therapy.
Group with the second highest CSF β2-m values (see Table 3 and Figure 1(a)), the group of brain tumours and malignant infiltrations, is statistically different from the control group and groups with multiple sclerosis, and polyneuritis/polyneuropathies. But there is no difference among this group and group with viral meningitis/encephalitis, neuroborreliosis, scavenger reaction, that is, values of CSF β2-m are similar in these groups.
No difference between the control group and group with polyneuritis/polyneuropathies must be again stressed, highly probably due to the reason, that this diagnostical group is not homogenous neither in clinical findings (concrete neurological peripheral syndromes) nor in laboratory evaluation. The etiology of these units is not usually completely clear, the majority of clinical case is autoimmune or metabolic one. An extensive inflammatory response is unusual in polyneuritis and polyneuropathies.
4. Conclusion
Today, laboratories determine, on a routine basis, many protein fractions in CSF and serum (or plasma). Terms like “CSF Proteinic Status” and “CSF proteinogram” appear. We examined a large CSF sample in patients with defined CNS diseases to clarify a biological behavior of beta2-microglobulin. The importance of our study is supported also by the fact that all measurements were performed in one laboratory by the same method of analysis. We found substantial elevation of β2-m values in groups with bacterial meningitis/encephalitis, malignant infiltrations or brain tumours, and viral meningitis/encephalitis. In these groups an immune activation or a tissue destruction is present. Our results confirm the role of beta2-microglobulin as an inflammatory or tumorous marker in cerebrospinal fluid. Further studies of beta2-microglobulin in children are needed to clarify a relation with a maturation of the blood-CSF barrier. And it is appropriate to deal with β2-m values in further clinical entities (e.g., hereditary degenerative diseases). Measurement of β2-m does not need any large costs; a requirement on the CSF amount is minimal. Our results support the great importance of β2-m examination for differential diagnosis, early therapy, and monitoring of therapeutic effect of central nervous system diseases.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.