Water Physicochemistry and Zooplankton Fauna of Aiba Reservoir Headwater Streams, Iwo, Nigeria
Abstract
The physicochemical water condition and zooplankton fauna of the two main inflows of Aiba Reservoir were assessed over an annual hydrological cycle (May 2013–March 2014). The concentrations of total solids (TS) and total suspended solids (TSS) in the two streams were unusually higher in the dry season for typical inland waters of Nigeria and showed a decrease from the upper reaches towards the lower reaches (reservoir’s inlet). Dissolved oxygen, , and recorded their highest concentrations at the reservoir’s inlet. A total of 37 species of zooplankton were recorded in the study, comprising 5 species of Protozoa, 14 species of Rotifera, 10 species of Copepoda, 4 species of Ostracoda, and 4 species of Insecta. Aiba stream recorded higher number of zooplankton species and abundance than Onikan stream. While number of zooplankton species and abundance showed an increase from the upper reaches to the lower reaches of both streams, species diversity and equitability generally showed a decrease. Correlation and regression analysis suggests that the concentration of TS and TSS played a major role in determining the zooplankton community structure of the streams. concentrations as well as the community structures of zooplankton faunas of the two streams were indicative of a polluted freshwater system with unstable habitat structure.
1. Introduction
Throughout human history, water has played a dual role as a life-giving liquid and as a resource for waste disposal. Without careful management, these two uses can conflict, potentially dangerously. In the developed world, water pollution reached crisis proportions around the mid-twentieth century but is now increasingly controlled. In developing countries however, poor water quality remains a major health threat. Also, while economic growth in developed countries has helped to bring about improvements in water quality, many developing countries are finding it difficult to follow this trend [1]. Ensuring that water quality in aquatic environments remains within natural ranges is essential for sustaining viable, abundant, and diverse communities of organisms. Degradation of water quality erodes the availability of water for humans and ecosystem and decreases species diversity and abundance of resident communities. The changes in environmental quality can be associated with changes in water quality parameters such as sediment load, nutrient concentration, temperature, dissolved oxygen levels, and pH [2].
Freshwater zooplankton in the tropics comprises predominantly the rotifers, cladocerans, copepods, and occasionally the ostracods and insects [3]. Crustacean plankton has been described as preferred fish food items by several authors (e.g., [4–6]). They are preferred by fishes to their Rotifera counterparts for several reasons. First, they are relatively bigger, and planktivorous fishes which practice size-selective predation often prefer them to the rotifers [6, 7]. Also, unlike the rotifers which are not easily spotted by predatory fishes as a result of their transparent lorica [8], crustaceans are easily spotted. Furthermore, crustaceans are more important than rotifers in the transfer of energy from autotrophic phytoplankton to fishes based on their ecological niche in freshwater systems. Rotifers are mostly herbivorous which could limit the amount of energy being harnessed from them by organisms at higher trophic levels, except there are intermediaries like crustaceans which feed on the rotifers. There is good evidence that rotifer species are differentially influenced by crustaceans via predation and competition [9]. Cyclopoid copepods have in particular been described as effective predators of rotifers [10], and so are some calanoid species which may include rotifers in their diets too [11, 12]. Aside their importance in fisheries, crustacean plankton (some cyclopoids) are also ecologically important by suppressing mosquito larvae. They are also known as intermediate hosts in the transmission of many parasites, especially worms. Some pathogenic human bacteria are carried and nurtured by Copepoda [13].
The vast majority of headwaters are rural streams with few urban influences. Their catchments are used primarily for farming. However, their rural context still leaves them vulnerable to broad spectrum of environmental stresses. These stresses may be exacerbated by the small size and low discharge of these streams, which limit the capacity to buffer either physical or chemical impacts [14]. The significance of headwater streams in freshwater systems cannot be overemphasized but is unfortunately often ignored in limnological studies. For instance, Aiba Reservoir has been copiously studied (e.g., [6, 15, 16]) but with little or no attention on its adjacent streams. Not until recently were studies carried out in the downstream section of the reservoir [17–19]. Previous zooplankton studies of the reservoir and its outflowing stream indicated a wide disparity in their compositions and community structures. While the reservoir was dominated by copepods [6], its receiving stream was dominated by rotifers [18]. Furthermore, while the previous physicochemical study of the reservoir indicated a healthy system [15], that of its outflowing stream indicated a stressed system [19]. Several studies have been carried out on the zooplankton fauna and water quality of Nigerian freshwaters, but such studies have always focused on reservoirs, lakes, and large rivers with no due attention on their headwater streams. There are very few studies which took cognizance of the significance of reservoirs’ headwater streams and their zooplankton communities and which attempted to relate such findings to the downstream lake/reservoir, for example, Opa Reservoir [20] and Lake Tiga [21, 22]. Since there have been several limnological studies on Aiba Reservoir with no documented findings on its headwater streams, this study was motivated by the need to bridge the information gap on the streams’ hydrology, water physicochemistry, and zooplankton fauna, since the health of a reservoir is intrinsic to its catchment area.
2. Materials and Methods
2.1. The Study Area
The study was carried out in the headwater streams (Aiba stream and Onikan stream) of Aiba Reservoir, Iwo, Osun State, Nigeria. Like most places in Southern Nigeria, the area is dominated by two major air masses (North East Trade Wind and South West Trade Wind) which shape the various seasons of the years. The North East Trade Wind which is dry and cold is predominant between November and February while the hot humid South West Trade Wind is predominant between April and October. The expected mean onset date of rainy season in the study area falls between March 13 and April 12 of a year, while the expected cessation date falls between October 19 and November 18. The annual regime of rainfall shows two peaks, one in July and the other in September [23]. One of the streams (Aiba stream) is characterized by Riparian forest with a heavy (70–90%) vegetation cover. Anthropogenic activities in and around the streams include washing of clothes, rearing of cattle, farming, and foot crossing of water course (especially in the upstream of Aiba stream).
2.2. Sampling Regime and Sample Collection
Samples were collected over an annual circle between May 2013 and March 2014 based on projected hydrological conditions of the streams, in every other month with a mind to capture the various seasons in the study area/duration. These were May 2013 (early rainy season), July 2013 (first peak of rainy season), September 2013 (second peak of rainy season), November 2013 (early dry/harmattan season), January (2014 (mid-dry/peak of harmattan season), and March 2014 (late dry season).
Two sampling stations were established on each of the streams, one at the upper reaches and the other at the lower reaches/inlet of the reservoir (Table 1). In situ water determinations included water temperature using a mercury-in-glass thermometer, water depth and Secchi disc depth (transparency), water channel width using a calibrated tape, flow velocity using a float displacement method, and water discharge (derived from the product of water depth, water channel width, and flow velocity). Others were pH using a calibrated pH meter (pHTestr2, Eutech Instruments, Malaysia) and electrical conductivity using a calibrated conductivity meter. Dissolved oxygen samples were also fixed in situ using Winkler’s reagents. Pelagic water samples were collected at the stations just below the surface for both physicochemical and zooplankton analyses in the laboratory. In the case of physicochemical analyses, samples were collected in 2 L plastic containers, first washed with liquid detergent, and rinsed with distilled water. They were then soaked in 10% nitric acid for 48 h. This was followed by vigorous rinsing three times with distilled water. The plastic bottles meant for sampling were further rinsed thrice on site with stream water to be sampled. The samples were transported to the laboratory and stored in refrigerators pending their analyses which were carried out within their holding time. Zooplankton samples were collected by towing a plankton net (64 μm mesh size) over a horizontal distance of 2 m. The samples were collected in a 100 mL bottle and preserved in 5% formalin solution.
| Lotic system | Grid coordinates | Site description | |
|---|---|---|---|
| Latitude | Longitude | ||
| Aiba stream (upper reaches/riffle) | 07°38′.579′′ N | 004°12′.572′′ E | Adjacent to herdsmen’s settlement; 90% vegetation cover; human activities include farming and cattle rearing |
| Aiba stream (lower reaches/reservoir’s inlet) | 07°38′.582′′ N | 004°12′.571′′ E | Closest to the reservoir; 50% vegetation cover; human activities include farming, bathing, and washing |
| Onikan upstream (upper reaches/riffle) | 07°38′.583′′ N | 004°12′.570′′ E | Opposite the Muslim praying ground; 10% vegetation cover; human activities include washing and encroachment. Site also traverses a major road |
| Onikan stream (lower reaches/reservoir’s inlet) | 07°38′.584′′ N | 004°12′.569′′ E | Closest to the reservoir; 40% vegetation cover; human activities include farming, palm oil processing, and logging |
2.3. Laboratory Analyses
2.3.1. Physicochemical Analysis
All chemicals used were AnalaR grade (BDH, England). Sulphate was determined by turbidimetric method [24]; phosphate was determined by colorimetric technique (APHA 1998); nitrate was determined by ultraviolet screening method [25]; total alkalinity, total hardness, and dissolved oxygen were also determined by methods described by Ademoroti [24]. The concentrations of suspended and dissolved solids were determined using the gravimetric method [25].
2.3.2. Quality Assurance and Control
To assess the precision and accuracy of results, replicate analysis of blanks, standard, and samples was carried out. The standard deviations were determined to find the precision of the analysis.
2.3.3. Zooplankton Analysis
In the laboratory, the 100 mL concentrate samples were further decanted using a hand pipette and reduced to 5 mL for microscopic viewing and identification under a calibrated compound light microscope (Max II 1202.4000) fitted with an ocular micrometer. Identification was aided with the use of several guides (e.g., [3, 26]) and observed morphometric features. The original volume of water filtered through the plankton net was estimated by using the formula V = ∧r2d, where V = volume of water filtered; r = the radius of the mouth of the net; d = length of the water column traversed by the net.
The actual abundance of zooplankton in each sample was estimated from the count records of the final concentrate volume in relation to the original volume of water filtered by the plankton net.
2.3.4. Data Analyses
The data were subjected to various statistical analyses (e.g., descriptive analysis, Mann-Whitney U test, and correlation analysis) and diversity analyses (e.g., species diversity and equitability) using the PAleontological STatistics (PAST) software.
3. Results
3.1. Physicochemical Water Parameters
Descriptive statistics and seasonal/temporal variations of physicochemical parameters of water in the two streams are presented in Tables 2 and 3. In terms of hydrological parameters, higher values of water depth, channel width, and water discharge were recorded in Aiba stream, while a higher value of flow velocity was recorded in Onikan stream. Temporally, higher values of hydrological variables were recorded in the rainy season than in the dry season, although none was significant (P > 0.05). With the exception of total suspended solids (TSS) in Onikan stream which recorded higher values in the rainy season (P > 0.05), the concentrations of solids—total solids (TS in both streams), total dissolved solids (TDS in both streams), and TSS (in Aiba stream)—were higher in the dry season than in the rainy season. Of these solids, only TDS showed significant seasonal variation (P < 0.05). Higher value of pH and lower value of electrical conductivity were recorded in Aiba stream in comparison with the Onikan stream. In both streams, pH was lower (P > 0.05) and electrical conductivity was higher (P > 0.05) in the dry season than in the rainy season. Total alkalinity was significantly higher (P < 0.05) in the rainy season than in the dry season in the Onikan lotic system. Higher values of nutrient parameters ( and ) and lower concentration of dissolved oxygen (DO) were recorded in Aiba stream than in Onikan stream. Higher concentrations of DO, , and were recorded in the dry season in both streams but with no significant difference (P > 0.05).
| Parameter | Descriptive statistics | Seasonal variation | Mann-Whitney U test | ||||
|---|---|---|---|---|---|---|---|
| Minimum | Maximum | Mean ± S.D. | Rainy season | Dry season | U | P | |
| Water temperature (°C) | 24.5 | 29.0 | 26.4 ± 1.6 | 26.0 ± 1.3 | 26.9 ± 2.0 | 12.5 | 0.4237 |
| Water depth (m) | 0.6 | 1.8 | 1.0 ± 0.4 | 1.0 ± 0.4 | 1.0 ± 0.4 | 17 | 0.9362 |
| Secchi depth (m) | 0.10 | 0.90 | 0.41 ± 0.22 | 0.35 ± 0.21 | 0.47 ± 0.24 | 13.5 | 0.5222 |
| Channel width (m) | 10.0 | 18.0 | 14.7 ± 2.7 | 14.7 ± 3.2 | 14.7 ± 2.5 | 17 | 0.9362 |
| Flow velocity (m/s) | 0 | 0.05 | 0.016 ± 0.017 | 0.02 ± 0.02 | 0.011 ± 0.008 | 18 | 0.9362 |
| Discharge (m3/s) | 0 | 1.440 | 0.334 ± 0.491 | 0.178 ± 0.127 | 0.165 ± 0.164 | 17 | 0.9362 |
| TS (mg/L) | 86 | 773 | 191 ± 188 | 132 ± 62 | 251 ± 257 | 8 | 0.1282 |
| TSS (mg/L) | 16 | 643 | 127 ± 169 | 96 ± 58 | 157 ± 240 | 16 | 0.8102 |
| TDS (mg/L) | 30 | 130 | 64 ± 31 | 36 ± 5 | 86 ± 25 | 0 | 0.0051 |
| pH | 6.65 | 8.30 | 7.24 ± 0.55 | 7.50 ± 0.66 | 6.97 ± 0.26 | 8 | 0.1285 |
| Electrical conductivity (µS/cm) | 90.0 | 186.0 | 121.7 ± 29.8 | 116.7 ± 19.7 | 126.7 ± 38.8 | 17 | 0.9362 |
| Total alkalinity (mgCaCO3/L) | 16.0 | 62.0 | 36.3 ± 14.6 | 39.4 ± 15.1 | 33.3 ± 14.7 | 14 | 0.5752 |
| (mg/L) | 0 | 2.09 | 0.75 ± 0.65 | 0.45 ± 0.35 | 1.05 ± 0.76 | 10 | 0.2298 |
| (mg/L) | 0 | 1.25 | 0.57 ± 0.42 | 0.43 ± 0.43 | 0.71 ± 0.37 | 11.5 | 0.3367 |
| Dissolved oxygen (mg/L) | 0.7 | 7.5 | 4.3 ± 2.2 | 3.7 ± 1.6 | 4.9 ± 2.7 | 14 | 0.5752 |
- n = 12, critical value of U = 5 at P < 0.05.
| Parameter | Descriptive statistics | Seasonal variation | Mann-Whitney U test | ||||
|---|---|---|---|---|---|---|---|
| Minimum | Maximum | Mean ± S.D. | Rainy season | Dry season | U value | P value | |
| Water temperature (°C) | 24.8 | 29.0 | 26.4 ± 1.5 | 25.3 ± 0.6 | 27.6 ± 1.3 | 6.5 | 0.0784 |
| Water depth (m) | 0.2 | 1.2 | 0.7 ± 0.4 | 0.8 ± 0.5 | 0.6 ± 0.4 | 15 | 0.6892 |
| Secchi depth (m) | 0.20 | 0.70 | 0.40 ± 0.21 | 0.36 ± 0.21 | 0.46 ± 0.22 | 16.5 | 0.8729 |
| Channel width (m) | 1.0 | 5.0 | 3.1 ± 1.4 | 3.6 ± 1.7 | 2.7 ± 1.0 | 15.5 | 0.7488 |
| Flow velocity (m/s) | 0 | 0.33 | 0.092 ± 0.129 | 0.113 ± 0.141 | 0.067 ± 0.124 | 8 | 0.1282 |
| Discharge (m3/s) | 0 | 0.300 | 0.102 ± 0.126 | 0.113 ± 0.131 | 0.089 ± 0.133 | 9.5 | 0.2002 |
| TS (mg/L) | 62 | 198 | 146 ± 47 | 146 ± 36 | 147 ± 63 | 17 | 0.9362 |
| TSS (mg/L) | 12 | 130 | 78 ± 42 | 99 ± 23 | 53 ± 48 | 5.5 | 0.0547 |
| TDS (mg/L) | 34 | 144 | 68 ± 37 | 47 ± 18 | 94 ± 39 | 9 | 0.1735 |
| pH | 6.70 | 8.00 | 7.16 ± 0.46 | 7.17 ± 0.48 | 7.14 ± 0.49 | 14.5 | 0.6312 |
| Electrical conductivity (µS/cm) | 85.0 | 300.0 | 175.2 ± 72.6 | 127.4 ± 67.4 | 190.0 ± 79.5 | 10 | 0.2298 |
| Total alkalinity (mgCaCO3/L) | 14 | 82 | 39.0 ± 18.6 | 48.7 ± 17.8 | 27.3 ± 12.4 | 4.5 | 0.0374 |
| (mg/L) | 0 | 0.98 | 0.56 ± 0.39 | 0.50 ± 0.41 | 0.64 ± 0.39 | 18 | 0.9362 |
| (mg/L) | 0 | 1.4 | 0.51 ± 0.42 | 0.42 ± 0.52 | 0.63 ± 0.27 | 13 | 0.4712 |
| Dissolved oxygen (mg/L) | 4.5 | 7.8 | 5.9 ± 1.2 | 5.1 ± 0.6 | 6.8 ± 1.1 | 8.5 | 0.1495 |
- n = 12, critical value of U = 5 at P < 0.05.
Spatially (Table 4), hydrological variables, save the flow velocity in both streams, showed an increase from the upper reaches of both systems downstream towards the reservoir inlet. Significant spatial variations (P < 0.05) in the environmental variables of Aiba stream included water depth, Secchi depth, TS, TSS, and DO, while those of Onikan stream were water depth, Secchi depth, and channel width. The concentrations of solids also showed a downward trend from the upper reaches of both streams towards the reservoir inlet, though not all were significant. pH and electrical conductivity both showed a nonsignificant decrease (P > 0.05) in the two streams towards the reservoir inlet. In both streams, , , and DO generally showed an increase towards the reservoir inlet, although a significant variation (P < 0.05) was only recorded for DO in the case of Aiba stream.
| Parameter | Upper reaches | Lower reaches | Mann-Whitney U test | |
|---|---|---|---|---|
| U value | P value | |||
| Aiba stream | ||||
| Water temperature (°C) | 25.6 ± 1.3 | 27.3 ± 1.6 | 7 | 0.0927 |
| Water depth (m) | 0.8 ± 0.1 | 1.3 ± 0.4 | 2.5 | 0.0163 |
| Secchi depth (m) | 0.26 ± 0.14 | 0.56 ± 0.18 | 3 | 0.0202 |
| Channel width (m) | 14.3 ± 2.3 | 15.0 ± 3.3 | 15 | 0.6889 |
| Flow velocity (m/s) | 0.022 ± 0.022 | 0.009 ± 0.009 | 11 | 0.298 |
| Discharge (m3/s) | 0.252 ± 0.116 | 0.473 ± 0.635 | 10 | 0.2298 |
| TS (mg/L) | 269 ± 251 | 114 ± 26 | 3 | 0.0202 |
| TSS (mg/L) | 200 ± 223 | 53±19 | 2 | 0.0131 |
| TDS (mg/L) | 69 ± 36 | 61 ± 28 | 16.5 | 0.8728 |
| pH | 7.27 ± 0.76 | 7.20 ± 0.29 | 12 | 0.3785 |
| Electrical conductivity (µS/cm) | 126.0 ± 32.4 | 117.3 ± 29.3 | 13.5 | 0.5218 |
| Total alkalinity (mgCaCO3/L) | 36.1 ± 16.0 | 36.6 ± 14.5 | 17 | 0.9362 |
| (mg/L) | 0.65 ± 0.77 | 0.86 ± 0.55 | 10.5 | 0.2623 |
| (mg/L) | 0.57 ± 0.41 | 0.57 ± 0.45 | 16.5 | 0.8728 |
| Dissolved oxygen (mg/L) | 2.6 ± 1.4 | 5.9 ± 1.6 | 2 | 0.0131 |
| Onikan stream | ||||
| Water temperature (°C) | 25.9 ± 1.5 | 26.7 ± 1.5 | 10.5 | 0.2623 |
| Water depth (m) | 0.32 ± 0.09 | 0.98 ± 0.32 | 2.5 | 0.0163 |
| Secchi depth (m) | 0.24 ± 0.07 | 0.54 ± 0.18 | 3.5 | 0.0250 |
| Channel width (m) | 1.9 ± 0.7 | 4.1 ± 0.9 | 1 | 0.0082 |
| Flow velocity (m/s) | 0.187 ± 0.144 | 0.014 ± 0.019 | 10 | 0.2298 |
| Discharge (m3/s) | 0.146 ± 0.136 | 0.065 ± 0.117 | 15.5 | 0.7488 |
| TS (mg/L) | 184 ± 20 | 115 ± 40 | 8 | 0.1282 |
| TSS (mg/L) | 106 ± 31 | 55 ± 37 | 11 | 0.298 |
| TDS (mg/L) | 78 ± 39 | 60 ± 36 | 15 | 0.6889 |
| pH | 7.38 ± 0.63 | 6.97 ± 0.13 | 17.5 | 1 |
| Electrical conductivity (µS/cm) | 194.0 ± 62.3 | 159.5 ± 82.3 | 15.5 | 0.7488 |
| Total alkalinity (mgCaCO3/L) | 45.5 ± 23.5 | 33.6 ± 13.2 | 17 | 0.9362 |
| (mg/L) | 0.43 ± 0.39 | 0.68 ± 0.38 | 10 | 0.2298 |
| (mg/L) | 0.36 ± 0.28 | 0.64 ± 0.49 | 9 | 0.1735 |
| Dissolved oxygen (mg/L) | 5.8 ± 1.4 | 6.0 ± 1.1 | 13 | 0.4712 |
- n = 12, critical value of U = 5 at P < 0.05.
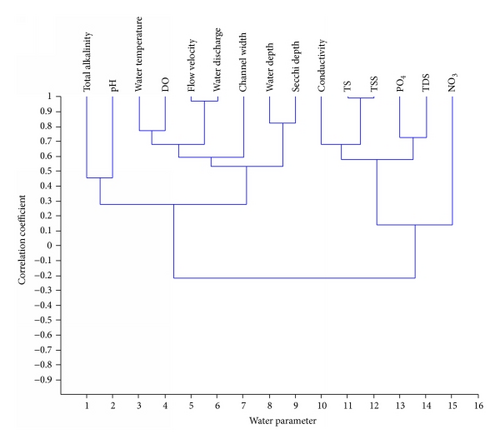
The interrelationships among the physicochemical parameters in the two streams are shown in Figures 1 and 2. Three major clusters were observed among the parameters in Aiba stream at P < 0.05. The first cluster was among water temperature, DO, flow velocity, and water discharge while the second was among water depth, Secchi depth, flow velocity, channel width, and water discharge. The third cluster in Aiba stream was among conductivity, TS, TDS and TSS, and . Three major clusters were also observed in Onikan stream at P < 0.05. Water temperature, pH, and DO formed the first cluster while the second was among water depth, Secchi depth, channel width, , and . Total alkalinity, flow velocity, water discharge, TS, and TSS formed the last major cluster in Onikan stream.

3.2. Zooplankton Fauna, Community Structure, and Their Relationship with Water Physicochemical Parameters
A total of 37 species of zooplankton comprise 5 species of Protozoa, 14 species of Rotifera, 10 species of Copepoda, 4 species of Ostracoda, and 4 species of Insecta (Table 5). The comparative compositions and community structures of zooplankton in the two streams are shown in Table 6. Aiba stream recorded more species and higher abundance than Onikan stream. Although number of species and abundance showed an increase towards the reservoir in both streams, significant spatial variation (P < 0.05) in number of species was only recorded in Onikan stream while significant spatial variation (P < 0.05) in abundance was recorded in both streams. Only four taxa (Mesocyclops ogunnus, Microcyclops varicans, nauplius larva, and chironomid sp.) were recorded in all the stations in this study. Species with very rare occurrences were categorised as those that occurred in only one station each and had less than 50 ind·m−3 abundance, and there were 15 species in this category. A total of 18,988 ind·m−3 zooplankton abundance was recorded in this study, comprising 2.54% Protozoa, 3.71% Rotifera, 85.86% Copepoda, 3.59% Ostracoda, and 4.29% Insecta. The most abundant individual taxon was nauplius larva which accounted for 63.61% of the total abundance. Microcyclops varicans and Mesocyclops ogunnus also accounted for relatively high abundance with 5.45% and 5.11%, respectively.
| Taxon | Aiba stream | Onikan stream | Total abundance (ind·m−3) | ||
|---|---|---|---|---|---|
| Upper reaches’ abundance (ind·m−3) | Lower reaches’ abundance (ind·m−3) | Upper reaches’ abundance (ind·m−3) | Lower reaches’ abundance (ind·m−3) | ||
| Protozoa | |||||
| Actinophrys sp. | 22 | — | — | — | 22 |
| Amoeba radiata | 22 | — | — | 22 | 44 |
| Arcella sp. | — | 286 | — | 22 | 308 |
| Centropyxis sp. | — | — | — | 22 | 22 |
| Loxodes magnus | — | — | — | 88 | 88 |
| Rotifera | |||||
| Anuraeopsis fissa | — | — | — | 44 | 44 |
| Anuraeopsis racenensis | 44 | — | — | 22 | 66 |
| Ascomorpha ovalis | 22 | — | — | — | 22 |
| Asplanchna brightwelli | — | 22 | — | — | 22 |
| Asplanchna priodonta | — | 198 | 22 | 44 | 264 |
| Brachionus angularis | — | 22 | — | — | 22 |
| Brachionus quadridentatus | — | 22 | — | 22 | 44 |
| Euchlanis triquetra | 22 | — | — | — | 22 |
| Keratella lenzi | — | — | — | 22 | 22 |
| Lepadella patella biloba | 22 | — | — | — | 22 |
| Lepadella patella similis | — | 44 | — | 22 | 66 |
| Mytilina ventralis | — | — | — | 44 | 44 |
| Testudinella patina | — | — | — | 22 | 22 |
| Trichocerca sp. | — | — | — | 22 | 22 |
| Copepoda | |||||
| Bryocamptus minutus | 22 | — | — | — | 22 |
| Cleptocampus sp. | 66 | 88 | — | — | 154 |
| Eucyclops macrurus | 44 | 506 | — | 132 | 682 |
| Halicyclops korodiensis | — | — | — | 88 | 88 |
| Halicyclops troglodytes | — | — | — | 88 | 88 |
| Mesocyclops ogunnus | 154 | 244 | 44 | 528 | 970 |
| Microcyclops varicans | 44 | 528 | 22 | 440 | 1034 |
| Nauplius larva | 506 | 7216 | 44 | 4312 | 12078 |
| Thermocyclops crassus | — | 308 | — | 176 | 484 |
| Thermocyclops neglectus | 22 | 396 | — | 286 | 704 |
| Ostracoda | |||||
| Chrissia humilis | — | 66 | — | — | 66 |
| Hemicypris ovata | — | 22 | 22 | — | 44 |
| Heterocypris makua | — | 308 | — | 110 | 418 |
| Stenocypris derupta | 44 | 110 | — | — | 154 |
| Insecta | |||||
| Chaoborus sp. | 110 | — | — | 44 | 154 |
| Ceratopogonid larva | — | 22 | — | — | 22 |
| Chironomid sp. | 220 | 198 | 44 | 154 | 616 |
| Coenagrion pulchellum | 22 | — | — | — | 22 |
| Number of species | 17 | 19 | 6 | 24 | 37 |
| Total abundance (ind·m−3) | 1408 | 10606 | 198 | 6776 | 18988 |
| Diversity index | Overall | Upper reaches | Lower reaches | Mann-Whitney U test | |
|---|---|---|---|---|---|
| U value | P value | ||||
| Aiba stream | |||||
| Number of species | 28 | 17 | 19 | 9 | 0.1735 |
| Total abundance | 12014 | 1408 | 10606 | 3 | 0.0202 |
| Shannon, H′ | 1.624 | 2.196 | 1.423 | 14 | 0.5752 |
| Simpson, 1-D | 0.5763 | 0.8203 | 0.5271 | 14 | 0.5752 |
| Margalef | 2.874 | 2.207 | 1.942 | 12 | 0.3785 |
| Menhinick | 0.2555 | 0.4531 | 0.1845 | 18 | 0.9362 |
| Equitability, J | 0.4874 | 0.7749 | 0.4834 | 14 | 0.5752 |
| Onikan stream | |||||
| Number of species | 25 | 6 | 24 | 2 | 0.0131 |
| Total abundance | 6974 | 198 | 6776 | 1 | 0.0082 |
| Shannon, H′ | 1.632 | 1.735 | 1.59 | 4 | 0.0306 |
| Simpson, 1-D | 0.5942 | 0.8148 | 0.5804 | 4 | 0.0306 |
| Margalef | 2.712 | 0.9455 | 2.607 | 3 | 0.0202 |
| Menhinick | 0.2994 | 0.4264 | 0.2916 | 12 | 0.3785 |
| Equitability, J | 0.5069 | 0.9684 | 0.5002 | 6 | 0.0656 |
- n = 12, critical value of U = 5 at P < 0.0.
All the diversity indices employed save Margalef index recorded higher values in Onikan stream than in Aiba stream. Equitability of species also followed the same trend as the diversity indices by recording a higher value in Onikan stream. With the exception of Margalef index in Onikan stream, all other indices showed a decrease from the upper reaches towards the reservoir. Table 7 shows that number of species and Shannon-Weiner species diversity were higher in the rainy season than in the dry season (P > 0.05 and P < 0.05, resp.). Generally, higher values of zooplankton abundance were recorded in the dry season than in the rainy season, with the exception of Onikan upstream where the reverse was the case.
| Lotic system | Number of species | Abundance | Shannon-Weiner diversity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Season | Mann-Whitney U test | Season | Mann-Whitney U test | Season | Mann-Whitney U test | |||||||
| RS | DS | U | P | RS | DS | U | P | RS | DS | U | P | |
| Aiba stream | ||||||||||||
| Upper reaches | 13 | 5 | 550 | 858 | 2.32 | 1.104 | ||||||
| Lower reaches | 13 | 12 | 2288 | 8318 | 2.13 | 0.7511 | ||||||
| Overall | 20 | 14 | 2838 | 9176 | 2.42 | 0.8509 | ||||||
| Onikan stream | 53 | 0.2855 | 65 | 0.7075 | 30.5 | 0.0179 | ||||||
| Upper reaches | 5 | 1 | 176 | 22 | 1.56 | 0 | ||||||
| Lower reaches | 16 | 12 | 2266 | 4510 | 2.289 | 0.6293 | ||||||
| Overall | 17 | 13 | 2442 | 4532 | 2.324 | 0.6569 | ||||||
- RS: rainy season, DS: dry season; n = 12; critical value of U = 37 at P < 0.05.
Relationships between zooplankton fauna and water physicochemical parameters are presented in Table 8. Number of species showed a significant positive correlation (P < 0.05) with Secchi depth and dissolved oxygen (DO) and a significant negative correlation with total solids (P < 0.01) and total suspended solids (P < 0.05). Abundance showed a significant positive correlation with water temperature (P < 0.01) and DO (P < 0.001), while TS and TSS both showed a significant negative correlation with abundance. pH showed a significant positive correlation (P < 0.01) with Shannon-Weiner species diversity, while a significant negative correlation (P < 0.05) was recorded between total dissolved solids and Shannon-Weiner species diversity.
| Parameter | Pearson’s correlation with number of species | Pearson’s correlation with abundance | Pearson’s correlation with species diversity, H′ | |||
|---|---|---|---|---|---|---|
| Aiba stream (n = 12) | Onikan stream (n = 12) | Aiba stream (n = 12) | Onikan stream (n = 12) | Aiba stream (n = 12) | Onikan stream (n = 12) | |
| Water temperature (°C) | 0.3247 | 0.0767 | 0.7052 ** | 0.4206 | 0.0927 | −0.3132 |
| Water depth (m) | 0.2341 | 0.4530 | 0.4322 | 0.4717 | 0.2251 | 0.3366 |
| Secchi depth (m) | 0.5967 * | 0.3613 | 0.4162 | 0.4703 | 0.4331 | 0.1469 |
| Channel width (m) | 0.1029 | 0.4597 | 0.0530 | 0.4528 | 0.3319 | 0.2586 |
| Flow velocity (m/s) | −0.0627 | −0.4299 | 0.4801 | −0.4065 | 0.0795 | −0.2110 |
| Discharge (m3/s) | −0.0876 | −0.3910 | 0.5273 | −0.4080 | −0.0170 | −0.0283 |
| TS (mg/L) | −0.5998 * | −0.7316 ** | −0.3196 | −0.6635 ** | −0.5276 | −0.3429 |
| TSS (mg/L) | −0.6013 * | −0.5421 * | −0.4144 | −0.6810 ** | −0.4700 | −0.0211 |
| TDS (mg/L) | −0.3528 | −0.3216 | 0.2980 | −0.0739 | −0.6570 * | −0.4187 |
| pH | 0.5288 | −0.1543 | −0.1446 | −0.2174 | 0.7457 ** | 0.0242 |
| Electrical conductivity (µS/cm) | −0.1503 | −0.1138 | −0.1056 | 0.0408 | −0.3848 | −0.0356 |
| Total alkalinity | 0.1440 | −0.1706 | −0.2277 | −0.5160 | 0.3882 | 0.3406 |
| (mg/L) | 0.0909 | −0.0139 | 0.2387 | 0.2556 | −0.0894 | −0.1299 |
| (mg/L) | 0.2873 | −0.0028 | 0.3064 | 0.2801 | −0.0918 | −0.1353 |
| Dissolved oxygen (mg/L) | 0.5436 * | −0.0024 | 0.8219 *** | 0.3985 | 0.2962 | −0.4751 |
- n = 12; r = 0.5324, P < 0.05; r = 0.6614, P < 0.01; r = 0.7800, P< 0.001.
- *Significant; **highly significant; ***very highly significant.
4. Discussion
Aiba stream seemed to make much more hydrological contribution than Onikan stream based on variables such as water depth, channel width, and water discharge. It could therefore be regarded as the main catchment of the reservoir, hence the name Aiba Reservoir. As a stream winds its way from its source to its mouth, the volume (discharge) of water passing down increases and the speed at which the water moves (flow velocity or current) decreases [27], as indicated by spatial variation of discharge in Aiba stream and that of flow velocity in Onikan stream. Since the volume or discharge capacity of a basin is dependent on water depth, water channel width, and the flow velocity of water [28], natural and human factors could go a long way in controlling these variables. Aiba stream, for instance, is relatively undisturbed as it meanders down the reservoir through a Riparian forest, while human interference and encroachment (as a result of expanding population) in and along the basin of Onikan stream has drastically reduced its discharge capacity. Onikan stream currently passes through the heart of the town and is greatly threatened by humans through competition for space. The negative impact of reduced discharge of a stream/river on a downstream reservoir cannot be overemphasized. Discharge influences the susceptibility of a stream to pollution. Large, swiftly flowing rivers or streams can receive pollution discharges and, through dilution, be little affected. In contrast, small, slow-flowing streams have a reduced capacity to attenuate and degrade wastes [2].
Stream velocity affects the amount of silt and sediment carried by the stream [2], and this could most likely justify the spatial trend in the concentrations of solids in the two streams. Sediment in slow-moving streams will settle to the stream bottom, whereas sediment in fast-moving waters will remain suspended in the water column, eventually settling in lakes and reservoirs [2]. The seasonal trend in the concentrations of solids (TS, TSS, and TDS) in both streams was at variance with the usual pattern for most inland waters in Nigeria (e.g., [29, 30]). Except in a rare case of resuspension of autochthonous sediments in dry season due to human disturbances as the case was in this study, Nigerian streams and rivers usually record the highest concentrations of TS and TSS in the rainy season as a result of allochthonous runoff from the surrounding terrestrial environments. The relatively high concentrations of TS and TSS recorded in the streams’ riffles may be explained in the light of Voshell [31] who divided lotic habitats into two zones, that is, erosional or riffle zone and depositional or pool zone. The riffle is the zone where streams at normal flow (not just during floods) have sufficient power to pick up fine sediments, keep them in suspension, and transport them downstream. The pool is the area where the stream does not have sufficient power at normal flow to keep fine sediments in suspension, so the small particles in transport settle out on the bottom. concentration in the two streams was far above the maximum limit (0.1 mg/L) for aquatic life [32, 33], thus indicating a polluted system.
The interrelationships among the physicochemical parameters can be summarized into two groups. The first was among electrical conductivity, nutrient parameters ( and ), and solids (TS, TSS, and TDS), while the second was among hydrological variables, solids, and nutrient parameters. These relationships go a long way to further establish the direct relationship of conductivity and TDS [25] and the importance of sediments (autochthonous or allochthonous) in the eutrophication of inland waters [34–36].
This study recorded more species of zooplankton than the previous zooplankton studies of the reservoir (17 taxa) and its outflowing stream (36 taxa). Furthermore, this study was uniquely different as Copepoda quantitatively dominated the zooplankton fauna of the headwater streams and there was no record of cladoceran zooplankton, in contrast with Rotifera dominance and cladoceran presence in comparable studies. Although Rotifera dominated the fauna qualitatively, their quantitative contribution was much lower than that of Copepoda, a greater percentage of which was recorded between November and March when the flow velocity was greatly reduced. The highest abundance of Copepoda was recorded between January and March when the streams were reduced to base flow (data not shown). It is expected that, during this period, zooplankton with longer generation times (e.g., Copepoda, Cladocera) will have sufficient time to grow in populations owing to the little or no flow velocity of their environments. The relatively low abundance of Rotifera in comparison with Copepoda may not also be unconnected with the predatory nature of the latter on the former [10–12]. Very striking in this study was the absence of Cladocera fauna, a taxon which rarely occurred in the reservoir and its outflowing stream, based on previous zooplankton studies. Only one taxon of Cladocera (Daphnia sp.) was reported in the previous zooplankton study of the reservoir [6], while six taxa (Allonia affinis, Camptocercus rectirostris, Bosmina longirostris, Macrothrix laticornis, Moina micrura, and Moinodaphnia macleayi) were recorded in its outflowing stream [18]. Zooplanktivorous fishes have been reported to show preference for cladocerans above other groups [7]. The rare occurrence of cladocerans in these headwater streams could therefore affect the food preference and feeding of zooplanktivorous fishes in the reservoir. In terms of species composition and abundance, Aiba stream again recorded higher values than Onikan stream, underscoring its relevance to the hydrology and water quality of the reservoir. The various diversity indices recorded in this study were a clear indication of polluted and unstable habitats, that is, Margalef indices ranging from 1.0 to 3.0 and Shannon-Weiner indices <3.0 [37, 38].
There was a direct relationship between pH and species diversity, most likely because the recorded pH values fell within the WHO recommendation (6.0–8.5) for aquatic life [28]. This underscores the fact that neither an acidic medium nor an extremely alkaline medium is favourable for aquatic life. Secchi depth (water transparency), DO, and water temperature favoured the composition and abundance of zooplankton in this study as indicated by their significant direct relationships, while the concentrations of TS and TSS could be considered limiting owing to the inverse relationships they showed with species composition and abundance. These relationships could also explain the spatial trend in species composition and abundance of zooplankton in the two streams. Generally, higher number of species and abundance were recorded at the reservoir’s inlet in both streams, and these incidentally recorded lower concentrations of solids as well as higher values of water transparency and DO. The spatial trend in these variables could have affected the amount of incident solar radiation required to stimulate phytoplankton growth at the base of the trophic levels in the two systems. In addition, the upper reaches were more of running waters than the reservoir’s inlet stations, and these running waters have been identified as an inhospitable environment for the development of plankton, particularly microcrustaceans [36, 37]. The transition of the streams from lotic to lentic status at the mouth of the reservoir could have therefore enhanced the occurrence and population growth of zooplankton therein.
In conclusion, Aiba Reservoir’s headwater streams could be considered threatened and negatively impacted, as indicated by their hydrology, physicochemical water quality, and zooplankton community structure. Onikan stream in particular could be highly susceptible to pollution stress due to its very low discharge, and this could negatively affect the reservoir’s health downstream. The polluted and unstable habitat nature of these streams could negatively affect the physicochemical and biotic nature of the downstream reservoir. The absence of Cladocera in this study may have justified its rarity in the previous zooplankton study of the reservoir, and this could negatively affect the food preference and nutrition of zooplanktivorous fishes therein.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.




