Pattern Formation in a Diffusive Ratio-Dependent Holling-Tanner Predator-Prey Model with Smith Growth
Abstract
The spatiotemporal dynamics of a diffusive ratio-dependent Holling-Tanner predator-prey model with Smith growth subject to zero-flux boundary condition are investigated analytically and numerically. The asymptotic stability of the positive equilibrium and the existence of Hopf bifurcation around the positive equilibrium are shown; the conditions of Turing instability are obtained. And with the help of numerical simulations, it is found that the model exhibits complex pattern replication: stripes, spots-stripes mixtures, and spots Turing patterns.
1. Introduction
The problem of pattern formation is, perhaps, the most challenging in modern ecology, biology, chemistry, and many other fields of science [1]. Patterns generated in abiotically homogeneous environments are particularly interesting because they require an explanation based on the individual behavior of organisms. They are commonly called “emergent patterns,” because they emerge from interactions in spatial scales that are much larger than the characteristic scale of individuals [2].
Turing [3] showed how the coupling of reaction and diffusion can induce instability and pattern formation. Turing’s revolutionary idea was that the passive diffusion could interact with chemical reaction in such a way that even if the reaction by itself has no symmetry-breaking capabilities, diffusion can destabilize the symmetry so that the system with diffusion can have them. Segel and Jackson [4] first used reaction-diffusion system to explain pattern formation in ecological context based upon the seminal work by Turing [3]. Since then, a lot of studies have been devoted to spatiotemporal patterns which were produced by reaction-diffusion predator-prey, models with either a prey-dependent or a ratio-dependent predator functional response, for example, [1, 2, 5–20] and references cited therein.
Recently, there is a growing explicit biological and physiological evidence [21–23] that in many situations, especially, when the predator has to search for food (and therefore has to share or compete for food), a more suitable general predator-prey theory should be based on the so-called ratio-dependent function which can be roughly stated as that the per capital predator growth rate should be a function of the ratio of prey to predator abundance, and so would be the so-called predator functional responses [24]. This is supported by numerous fields and laboratory experiments and observations [25, 26]. In [24], the authors investigated the effect of time delays on the stability of the model and discussed the local asymptotic stability and the Hopf bifurcation. Liang and Pan [27] have studied the local and global asymptotic stability of the coexisting equilibrium point and obtained the conditions for Poincare-Andronov-Hopf-bifurcating periodic solution. M. Banerjee and S. Banerjee [28] have studied the local asymptotic stability of the equilibrium point and obtained the conditions for the occurrence of Turing-Hopf instability for reaction-diffusion model. It is shown that prey and predator populations exhibit spatiotemporal patterns resulting from temporal oscillation of both the population and spatial instability.
Besides, in [29], Smith has shown that the logistic equation is not realistic for a food-limited population under the effects of environmental toxicants and established a new growth function—Smith growth function. And it has been proposed by several authors [29–34] for the dynamics of a population where the growth limitations are based on the proportion of available resources not utilized. However, pattern formation in the case of Holling-Tanner type predator-prey models with ratio-dependent functional response and Smith growth still remains an interesting area of research.
In this present work, we will focus on the ratio-dependent Holling-Tanner model with Smith growth for predator-prey interaction where random movement of both species is taken into account. The rest of the paper is organized as follows. In Section 2, we establish the ratio-dependent Holling-Tanner predator-prey model with Smith growth and study the local asymptotic stability of the positive equilibrium, existence of Hopf bifurcation around the positive equilibrium, and the conditions for the occurrence of Turing instability. In Section 3, we present and discuss the results of pattern formation via numerical simulation, which is followed by the last section, that is, conclusions and discussions.
2. The Model and the Linear Stability Analysis
2.1. The Model
2.2. The Stability of the Nonspatial Model (1)
Theorem 1. (i) The positive equilibrium E* = (u*, v*) is locally asymptotically stable if and only if
(ii) The positive equilibrium E* = (u*, v*) is unstable if and only if
(iii) The model enters into a Hopf-bifurcation around E* = (u*, v*) at s = s*, where s* satisfies the equality
Proof. (i) If m(ar + cm + 2hr − acr) < r(a + h) 2(r + s(1 + c)), then tr (J*) < 0. Thus, the equilibrium point E* is locally asymptotically stable, similar to the proof of (ii).
(iii) A Hopf bifurcation occurs if and only if there exists a s = s* such that
Substituting λ = α + iβ into the equation λ2 − tr (J*)λ + det (J*) = 0 and separating real and imaginary parts we obtain
In Figure 1, we show the phase portraits of (1) with r = 1, m = 1, a = 0.4, K = 4, h = 0.7, c = 0.8, and s = 0.55. The horizontal axis is the prey population u, and the vertical axis is the predator population v. The dashed curve is the u-nullcline, and the dotted vertical line is the v-nullcline. It is easy to see that the equilibrium E0 = (4,0) is a saddle and E* = (0.21053,0.30075) is locally asymptotically stable.
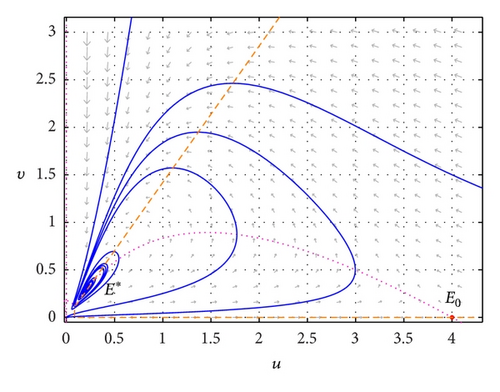
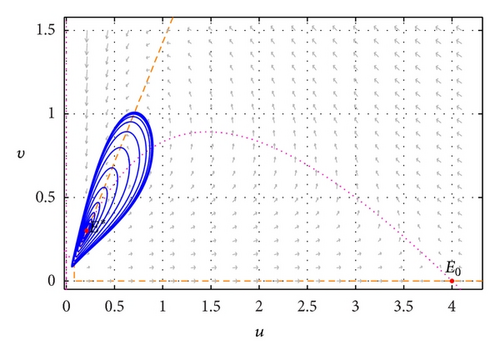
Figure 2 illustrates a Hopf-bifurcation situation of the model around E* = (0.21053,0.30075) for s = s* = 0.4912764003. In this case, limit cycle arising through Hopf bifurcation is a stable limit cycle which attracts all trajectories starting from a point in the interior of first quadrant.
2.3. The Stability of the Spatial Model (4)
In this subsection, we will focus on the effect of diffusion on the model system about the positive equilibrium.
Mathematically speaking, a positive equilibrium E* of model (4) is Turing unstable, which means that it is an asymptotically stable steady-state solution of the model (1) without diffusion but is unstable with respect to the solutions of the model (4) with diffusion.
Summarizing the previous calculation, we conclude the following theorem.
Theorem 2. Assume that
- (A1)
m(ar + cm + 2hr − acr) < r(a + h) 2(r + s + cs),
- (A2)
r(a + h) 2(d1s(1 + c) + d2r) + 2 <d2m(ar + cm + 2hr − acr).
Then the positive equilibrium E* of model (4) is Turing unstable.
From Theorem 2, we can know that there is Turing instability in model (4) if conditions (A1) and (A2) hold. In this situation, the solutions to model (4) may be unstable and Turing patterns can emerge in the model.
3. Turing Pattern Formation
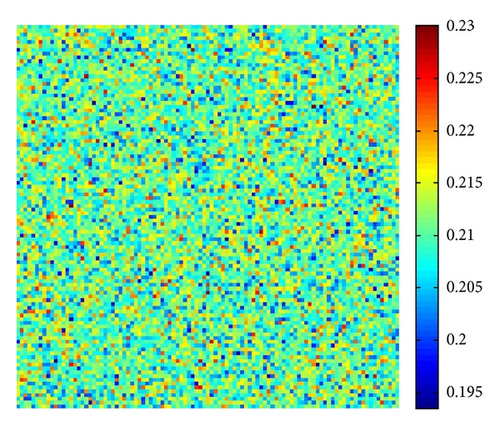
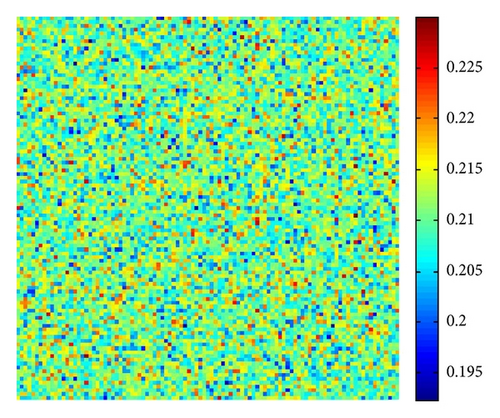
The numerical integration of model (4) is performed by using a finite difference approximation for the spatial derivatives and an explicit Euler method for the time integration [35] with a time step size of 1/100. The initial condition is always a small amplitude random perturbation around the positive constant steady-state solution E* = (u*, v*). After the initial period during which the perturbation spread, the model either goes to a time-dependent state or to an essentially steady-state solution (time independent).
In the numerical simulations, different types of dynamics are observed and it is found that the distributions of predator and prey are always of the same type. Consequently, we can restrict our analysis of pattern formation to one distribution. In this section, we show the distribution of prey u, for instance. We have taken some snapshots with red (blue) corresponding to the high (low) value of prey u.
Now, we show the Turing patterns for the different values of the control parameter s. Via numerical simulations, one can see that the model dynamics exhibits spatiotemporal complexity of pattern formation, including stripes, stripes-spots mixtures, and spots Turing patterns.
In Figure 3, with s = 1, starting with a homogeneous state E* = (0.21,0.3) (cf. Figure 3(a)), the random perturbations lead to the formation to stripes spots (cf. Figure 3(c)), and the latter random perturbations make these spots decay, ending with the time-independent stripes pattern (cf. Figure 3(d)).
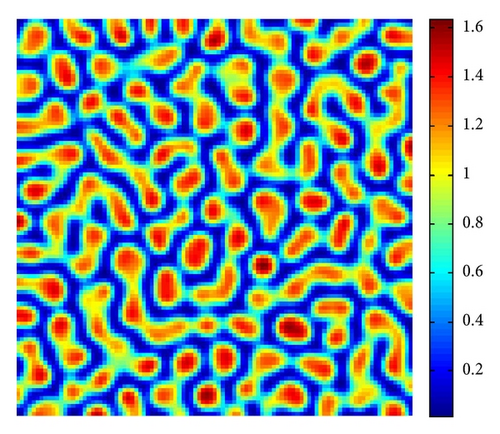
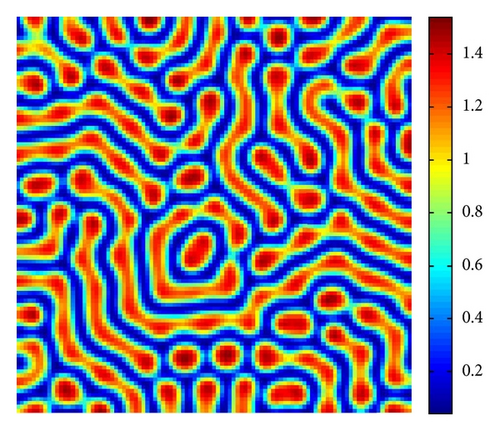
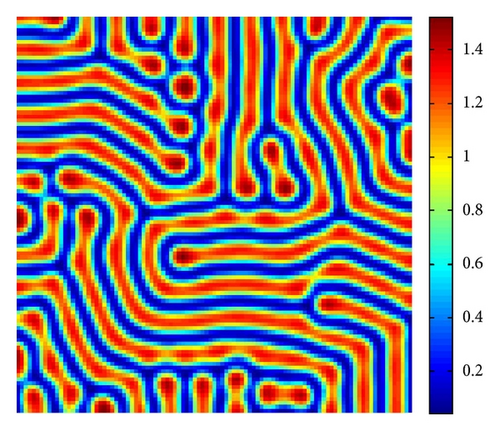
In Figure 4, with s = 1.375, we show the stripes-spots mixtures pattern for model (4).
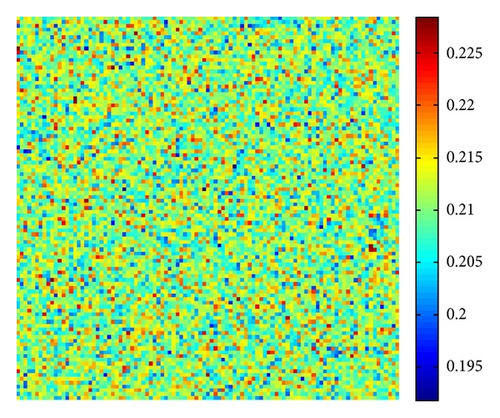
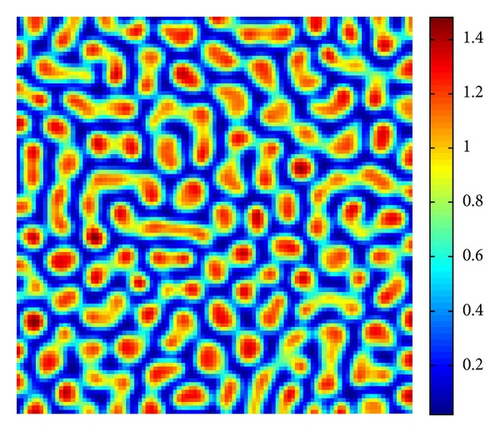
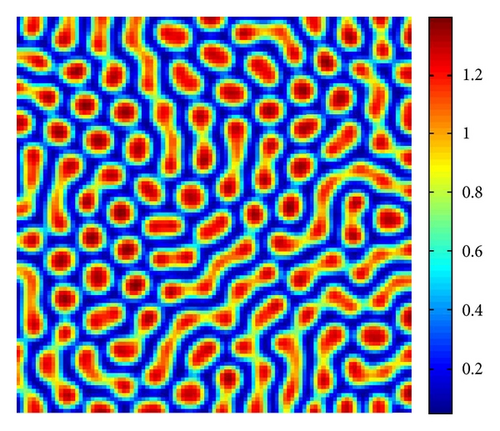
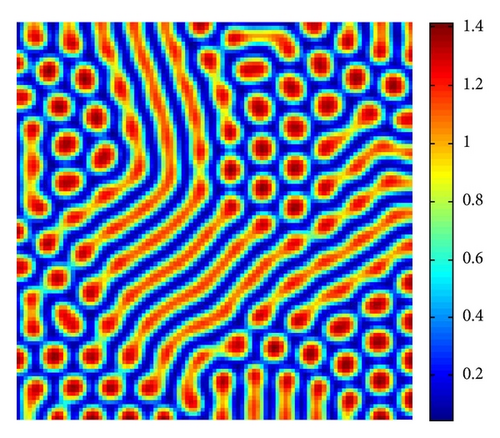
Figure 5 shows the time process of spots pattern formation of prey u for s = 2.5. In this case, the pattern takes a long time to settle down, starting with a homogeneous state E* = (0.21,0.3) (cf. Figure 5(a)), and the random perturbations lead to the formation of stripes and spots (cf. Figure 5(b)), ending with spots only (cf. Figure 5(d))—the prey u is isolated zones with high population density, and the remainder region is of low density.
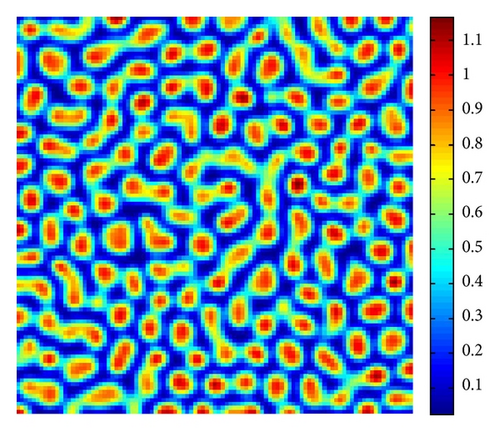
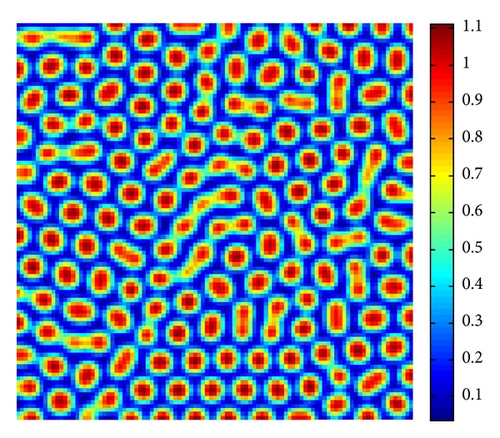
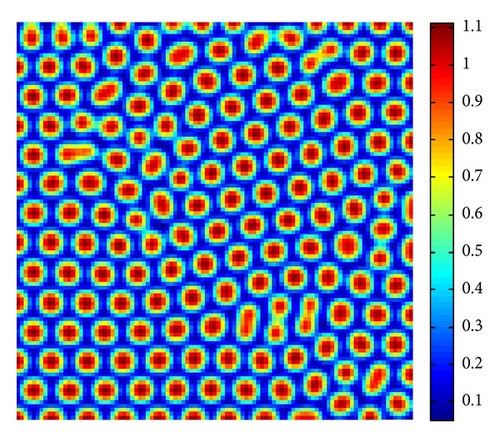
From Figure 3 to Figure 5, we can see that, on increasing the control parameter s from 1 to 2.5, the pattern sequence “stripe → stripes-spots mixtures → spots” can be observed.
4. Conclusions and Remarks
In summary, in this paper, we have investigated the spatiotemporal dynamics of a diffusive predator-prey model where the interaction between prey and predator follows Holling-Tanner formulation with ratio-dependent functional response and Smith growth. The value of this study is threefold. First, it presents the conditions for the stability of the equilibrium and the existence of Hopf bifurcation for the nonspatial model. Second, it rigorously proves Turing instability by linear stability analysis for the spatial model. Third, it illustrates the Turing pattern formation via numerical simulations, which shows that the spatial model dynamics exhibits complex pattern replication.
By a series of numerical simulations, we find that the spatial model (4) has rich Turing pattern replications, such as stripes, stripes-spots mixtures, and spots patterns. In the viewpoint of population ecology, in the case of stripe pattern (cf. Figure 3), the prey u is the isolated “stripes-like region” with high density, and the remainder stripes-like region is of low density. And in the case of spots pattern (cf. Figure 5), the prey u is the isolated “cycle region” with high density, and the remainder region is of low density, which is larger than the “spots” region.




