Porous and Cross-Linked Cellulose Beads for Toxic Metal Ion Removal: Hg(II) Ions
Abstract
Mercury is a highly toxic and hazardous pollutant even in trace quantity which poses a major threat to the ecosystem on deposition in the environment. Removal of mercury from aqueous systems has been a subject of immense interest for researchers. The synthesis of highly cross-linked cellulose beads embedded with ferric oxide for removal of Hg(II) ions from aqueous systems has been investigated. The beads were synthesized by solution polymerization technique. The impact of solution pH, ferric oxide content, and initial concentration of Hg(II) ions on the uptake of Hg(II) ions revealed that maximum adsorption occurs at pH 6.0 with beads having 10 wt% Fe2O3 content. Equilibrium adsorption of Hg(II) ions followed the Langmuir isotherm model. Adsorption was observed to follow pseudo second-order kinetic model and intraparticle diffusion model.
1. Introduction
Heavy metals such as mercury are considered as toxic and their accumulation over the period of time in human bodies causes serious health hazards, possessing major threats to human health. Heavy metal pollution can arise from various sources but is highly common from purification of metals, for example, smelting of copper and preparation of nuclear fuels. Unlike organic pollutants, heavy metals do not biodegrade which is the largest problem associated with the persistence of heavy metals which has the potential of bioaccumulation and biomagnification entering the food chain causing heavier exposure and hence biological disorders. Mercury affects the central nervous system, kidneys [1], and liver. Compared to other heavy metals, mercury even at low concentrations is highly neurotoxic [2].
Various conventional methods such as chemical precipitation, membrane filtration, ion exchange, lime softening, coagulation-flocculation, photoreduction [1, 3, 4], and electrochemical treatment have been employed for removal of mercury from aqueous systems. Adsorption is the most effective technique for removing heavy metal ions from aqueous solutions. The important aspect of the adsorption process is easy regeneration ability and less operational cost, simple design, easy operation, and free or less generation of toxic substances [5]. Adsorption techniques have proven successful in removing colored organic species and the choice of the adsorbent is one of the key factors determining the effectiveness of any adsorption process. The adsorption process at solid/liquid interface has been extensively employed for several reasons, mainly due to its efficiency and economy [6]. Physical adsorption because of its low cost, high efficiency, easy handling, wide variety of adsorbents and high stabilities toward the adsorbents has become the most widely used method for the elimination of heavy metal ions from wastewater. Adsorption of mercury ions onto various natural substrates like saw dust, banana stalk, coconut coir, and so forth, has been extensively studied [7–9]. Modified cellulose substrates used for adsorption of mercury. Porous cellulose modified with polyethyleneimine for removal of mercury from wastewater was reported by Navarro et al. [10]. Poly(glycidylmethacrylate-methylmethacrylate-ethyleneglycoldimethacrylate) magnetic beads were used for the removal of mercury(II) ions from aqueous solutions by Bayramoǧlu and Arica [11].
In this study, we report a simple, cost-effective technique for removal of Hg(II) ions from aqueous systems. The adsorbate was synthesized using cellulose acetate beads embedded with Fe2O3. The impact of solution pH, Fe2O3 content in the beads, and initial metal ion concentration was studied and optimized experimental parameters were obtained. Further experiments were performed under these optimized parameters and the obtained data was used to validate the adsorption isotherm models and adsorption kinetics. The thermodynamic aspect of the adsorption was studied to understand the feasibility of the process.
2. Experimental
2.1. Materials and Methods
Cellulose acetate, ferric oxide, epichlorohydrin, sodium chloride (NaCl), sodium hydroxide (NaOH), hydrochloric acid (HCl), dimethylsulphoxide (DMSO), mercury chloride (HgCl2), potassium iodide (KI), rhodamine B, and sulphuric acid (H2SO4) were all purchased from Merck Inc., India, and used as received. Deionized water was used for all experimental work.
2.2. Synthesis of Ferric Oxide Embedded Cellulose Acetate Beads
The ferric oxide loaded cellulose acetate beads were synthesized by solution polymerization technique [12, 13]. Briefly, varying concentrations of ferric oxide (1–20 wt%) were sonicated in DMSO for 15 min and calculated amount of cellulose acetate, epichlorohydrin, and NaHCO3 was added to this solution and stirred till the solution became homogeneous. This mixture was precipitated in an acid coaggulation bath containing 0.1 M HCl. The beads obtained were filtered, washed with deionized water, hydrolyzed in 0.1 M NaOH, finally washed with deionised water, and air-dried at room temperature to constant weight.
2.3. Adsorption Studies
All the experiments were repeated thrice to validate the repeatability of the results. The results presented are the average of all the three experiments conducted.
3. Results and Discussion
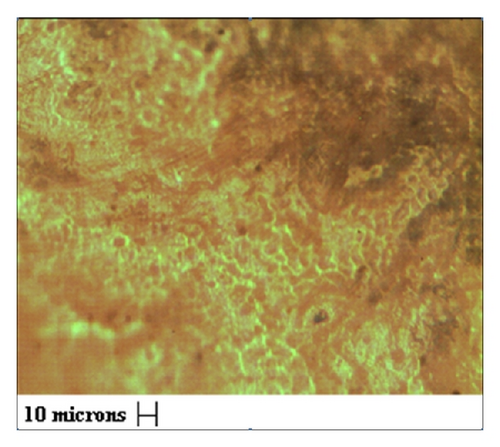
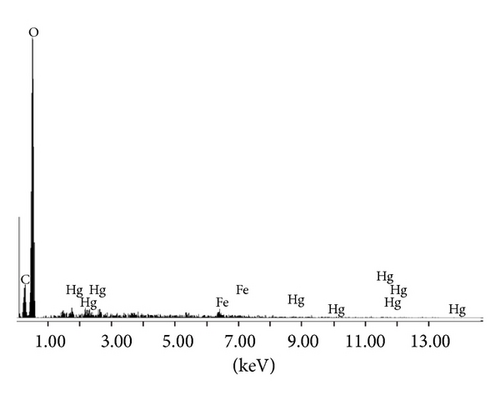
The Fe2O3 loaded cellulose acetate beads of uniform diameter 0.5 cm (Figure 1(a)) were synthesized during the precipitation polymerization method. The optical image analyzer and scanning electron microscopy (Jeol JSM-840 A) were used to examine the surface morphology of the beads. The optical image of beads shows the presence of porous surface structure (Figure 1(b)) which is supported by the SEM images confirming the existence of highly micro/macro structure. The SEM micrographs also revealed the presence of large number of micropores (1–25 μm), voids, and well-defined flow lines as can be observed from Figure 1(c) indicating presence of capillary action. The presence of mercury in the beads after adsorption was confirmed from the EDX pattern (Figure 1(d)). The surface porosity calculated as from the SEM images per ASTM B 276 standard [15] was found to be 13.1%.


3.1. Effect of pH
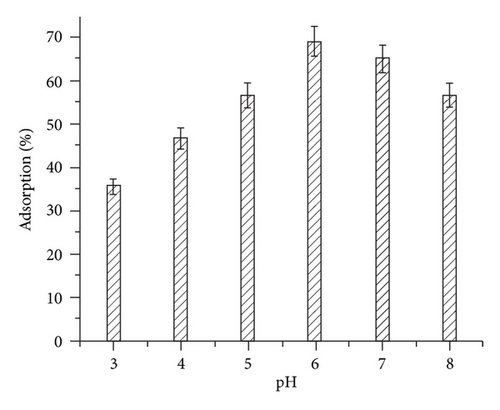
The adsorption of Hg(II) ions onto the beads was studied in the pH range 3–8 at a constant volume. Maximum adsorption was observed at pH 6. The variation in adsorption with pH of the solution is depicted in Figure 2(a). According to the stability constant calculations, in the presence of Cl− ions, the predominant species at pH < 4 is HgCl2. This prevents the binding of Hg(II) ions onto the sorbent as chloride ions tend to form more stable complexes with Hg(II) such as HgCl2, (HgCl3)−, and (HgCl4)2− [16]. Also at low pH, the excess H+ ions present in the solution compete with Hg(II) ions for active sites present on the beads decreasing the adsorption of Hg(II) ions. However, at pH 6.0 the predominant species is Hg(OH)2 in the medium and the protonation of the beads is minimum leading to the increased adsorption of Hg(II) ions. It was observed that at higher pH values, a slight decrease in the adsorption was noted since the metal ions tend to form insoluble hydroxides.
3.2. Effect of Varying Fe2O3 Content in the Beads
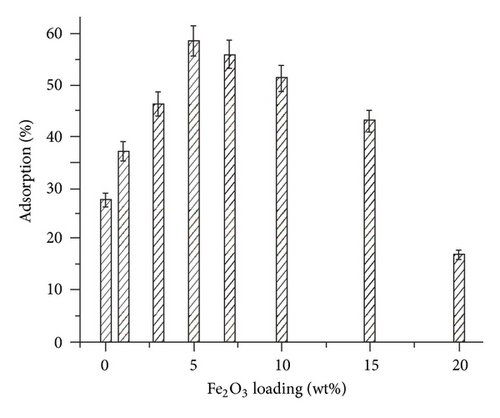
The effect of varying the concentration of Fe2O3 in beads on adsorption of Hg(II) ions was studied by varying the Fe2O3 content from 0 to 20 wt%. Results indicate that adsorption increases with increasing the Fe2O3 content up to 10 wt% which is correlated to the degree of dispersion of Fe2O3 in the polymer matrix. Further increase in Fe2O3 content results in agglomeration of the particles which causes steric hindrance for adsorption of Hg(II) ions.
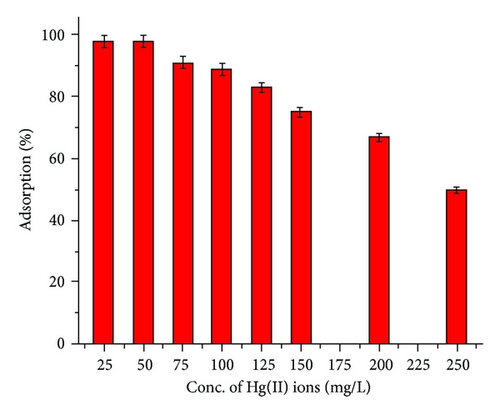
3.3. Effect of Hg(II) Ion Concentration
The graph of adsorption as a function of initial Hg(II) ion concentration (Figure 2(c)) reveals that the extent of adsorption decreases with increasing the Hg(II) concentration. As a rule, increasing the initial metal ion concentration results in an increase in the adsorption capacity since it provides a driving force to overcome all mass transfer resistances of the metal ions between the aqueous and solid phase. However, the sorption efficiency decreases since the adsorbent has a limited number of active sites, which saturates after a certain concentration. This indicates that the adsorption capacity will increase with the increase of initial concentration mainly due to the rise in the mass transfer from the concentration gradient. However, the concentration will inversely impact the adsorption efficiency because of the limited adsorption sites available for uptake of metal ions [17].
3.4. Equilibrium Adsorption Isotherms
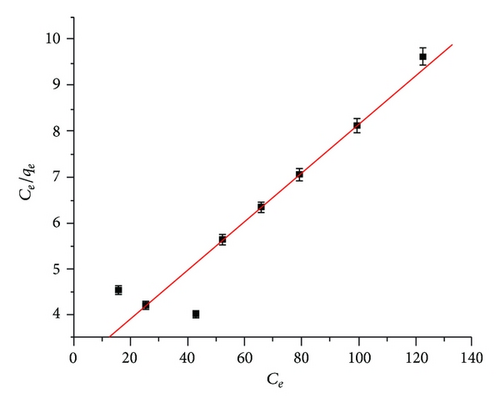
Adsorption isotherm studies are important to determine the efficiency of adsorption. These isotherms indicate the distribution of adsorbate between liquid and solid phase when equilibrium state was achieved. Analysis of the isotherm data by fitting them to different isotherm models is an important step to find the suitable model that can be used for design purpose [17]. The isotherm constants were obtained by linear regression methods and are presented in Table 1.
| Adsorption isotherm models | Different isotherm parameters | Values of isotherm parameters |
|---|---|---|
| Langmuir | qmax (mg g−1) | 18.92 |
| KL (L mg−1) | 0.018 | |
| RL | 0.32 | |
| R | 0.9454 | |
| Freundlich | KF | 0.72 |
| n | 1.59 | |
| R | 0.9160 | |
| Dubinin-Radushkevich (D-R) | qmax (molg−1) | 12.94 |
| KD (mol2J−2) | −5.5 × 10−5 | |
| ε | 77.67 | |
| R | 0.9999 | |
3.4.1. Langmuir Isotherm Model
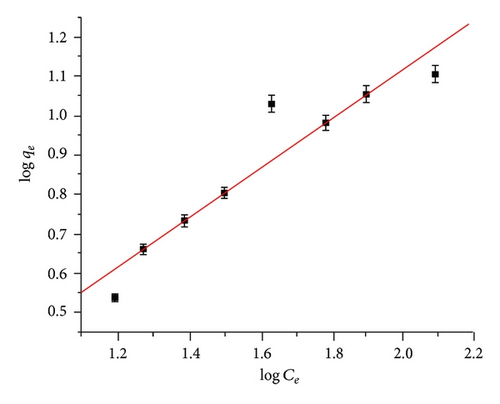
3.4.2. Freundlich Isotherm Model
3.4.3. Dubinin-Radushkevich (D-R) Isotherm
The plot for D-R model yields high correlation coefficient values (Figure 3(c)) confirming the applicability of this model.
According to the above results, it is predicted that the initial monolayer adsorption occurs (as confirmed from Langmuir model) leading to further multilayered chemical interactions between the Hg(II) ions and the beads as explained by the Freundlich isotherm. The effect of porosity of the adsorbent on the process of adsorption is explained by the Dubinin-Radushkevich isotherm model.
3.5. Kinetic Studies
Adsorption kinetics have been thoroughly investigated to predict the adsorption mechanism and rate determining step. The knowledge of adsorption mechanism and rate limiting steps is applicable in selecting optimum conditions for large scale applications.
3.5.1. Pseudo Second-Order Model
The linear plots for t/qt versus t with high correlation coefficient values indicates pseudo second-order kinetics for adsorption of Hg(II) ions as shown in Figure 4(a).
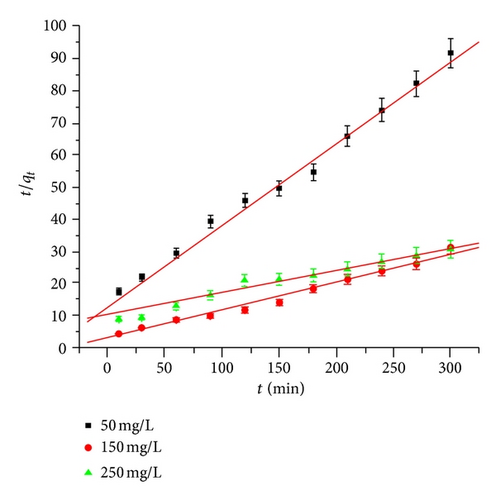
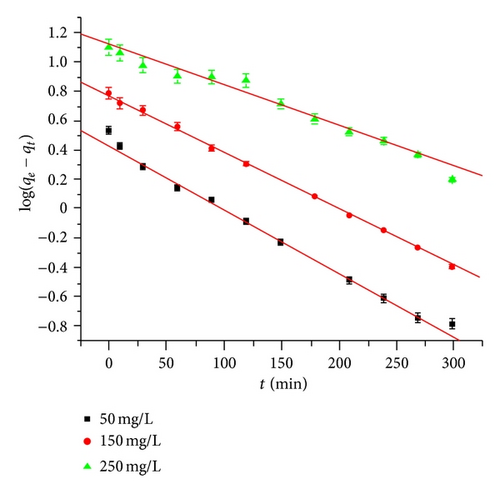
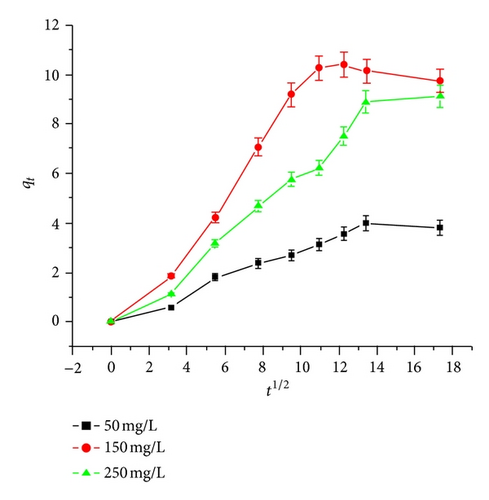
3.5.2. Pseudo First-Order Model
The straight line plot with negative slope suggests the applicability of pseudo first-order kinetic model as evident from Figure 4(b). However, low R2 values indicate poor fit of this model to the adsorption Hg(II) ions.
3.5.3. Intraparticle Diffusion Model
The plot of qt versus t1/2 does not pass through the origin and shows multilinearity indicating that but not only intraparticle diffusion, but some other processes are also involved in the adsorption process. The first sharper portion indicates instantaneous adsorption of Hg(II) ions on the external surface of the beads from the bulk of the solution which is due to boundary layer diffusion. Value of I indicates thickness of the boundary layer. The second portion is the gradual adsorption stage with controlled intraparticle or pore diffusion where Hg(II) ions move into the interior of the beads. The third step indicates the attainment of equilibrium stage where intraparticle diffusion starts to slow down due to very low concentration of Hg(II) ions in the solution [17]. The intraparticle diffusion rate is obtained from the slope of the gentle-sloped portion of the graph. The graph presents a three-stage adsorption process as can be observed from Figure 4(c).
The kinetic results suggest that the adsorption data of Hg(II) ions from aqueous solution using these cellulose acetate beads gives satisfactory fit for pseudo second-order and intraparticle diffusion models. High R2 values for these kinetic models suggest that the adsorption of Hg(II) ions can be well explained by these models. The pseudo second-order rate equation suggests the presence of numerous active sites on adsorbent surface. The multilinear graphs obtained for the intraparticle diffusion model confirm that initially there will be an instantaneous adsorption of Hg(II) ions onto the surface of the adsorbent system through surface diffusion. With increase in surface coverage there is a gradual adsorption through controlled intraparticle or pore diffusion where Hg(II) ions move into the interior of the beads and at a later stage when the equilibrium is reached the intraparticle diffusion starts to slow down. Various kinetic parameters calculated from different kinetic models are summarized in Table 2.
| Conc. (mg/L) | Pseudo second-order | Pseudo second-order | Intraparticle diffusion | ||
|---|---|---|---|---|---|
| kp2 × 10−4 | R2 | kp1 × 10−3 | R2 | Kint | |
| 50 | 38.93 | 0.9698 | −8.71 | 0.8496 | 0.25 |
| 150 | 5.93 | 0.9151 | −20.13 | 0.9328 | 0.95 |
| 250 | 5.89 | 0.9989 | −0.69 | 0.7730 | 0.21 |
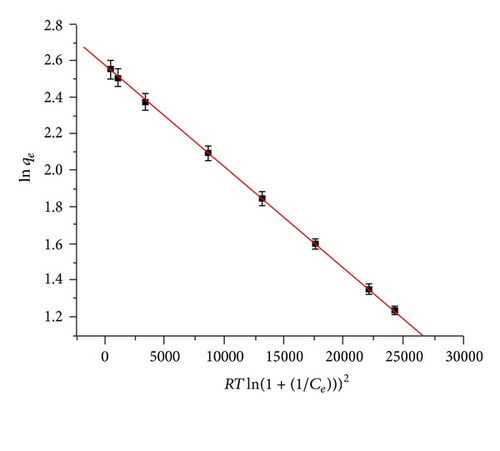
3.6. Thermodynamic Studies
4. Conclusion
The naturally abundant material, cellulose, is used to synthesize polymer beads using a simple, easy, and less energy consuming procedure using dimethylsulphoxide as solvent. The adsorption capacity of the beads was enhanced by embedded Fe2O3 and used to remove Hg(II) ions from aqueous systems. Maximum adsorption occurred at pH 6.0, and using beads loaded with 10 wt% of Fe2O3. The experimental adsorption data fitted well with Langmuir, Freundlich, and D-R equilibrium isotherm models indicating that initial monolayer adsorption occurs leading to further multilayered chemical interactions between the Hg(II) ions and the beads. The kinetic studies suggested pseudo second-order and intraparticle diffusion playing a key role in the adsorption process. The key advantage of the system is that it exhibits equal potential when used in batch adsorption process as well as continuous flow process. The adsorbents can be shaped in various forms such as thin films, membranes, and beads, which provides additional application oriented advantage. This adsorbents can find good applications in industrial effluent treatment processes. The cost effectiveness of this technique makes it more feasible for the large scale use.
Acknowledgments
The authors would like to thank Dr. Prahlada, Vice Chancellor, DIAT(DU), for his support and encouragement in this research activity. The authors would also like to thank Dr. Partha Ghosal, Sc’E’, DMRL Hyderabad, for SEM images.




