Data Reconstruction for a Disturbed Soil-Column Experiment Using an Optimal Perturbation Regularization Algorithm
Abstract
This paper deals with data reconstruction problem for a real disturbed soil-column experiment using an optimal perturbation regularization algorithm. A purpose of doing the experiment is to simulate and study transport behaviors of Ca2+, Na+, Mg2+, K+, , , , and Cl− when they vertically penetrating through sandy soils. By data analysis to breakthrough data of the eight kinds of solute ions, two kinds of models describing their transport behaviors in the column are given. One is the advection-dispersion equation with time-dependent reaction terms suitable for three ions of , , and K+, the other is the ordinary advection-dispersion equation suitable for the rest ions. Furthermore, all the unknowns in each model are determined by utilizing the optimal perturbation regularization algorithm, respectively, and then the breakthrough data for each considered ion are reconstructed successfully. The inversion results show that the advection-dispersion model with suitable time-dependent reaction terms can be utilized to describe the experimental process and reconstruct the experimental data.
1. Introduction
Soil and groundwater pollution has become a serious threat to sustainable development throughout the world. It is important to characterize physical/chemical reactions quantitatively for a solute transport process in the soils and groundwater (see [1–4], e.g.). To understand transport behaviors of the soil in the presence of infiltrating contaminants, soil column experiments are often performed in the laboratory. There are disturbed and undisturbed soil-column infiltrating experiments, and the soil-column in a disturbed experiment is often loaded uniformly and orderly with a lucite tube. The primary purpose of doing a disturbed soil-column experiment is to simulate and study transport behavior of the considered ion when it vertically penetrating through the column with artificial chemical liquid.
On the research of soil-column experiments in the known literatures we have, 1D case is often concerned with. In early studies, it is a main task to construct mathematical models for soil-column experiments. The typical works could belong to Nielsen and van Genuchten and their researching groups. Nielsen et al. (see [5], e.g.) gave a general advection-dispersion equation describing solute transport in unsaturated soils, and van Genuchten and Wagenet (see [6], e.g.) discussed solute transport models of two sites/two regions in the soils. With development of computational tools and techniques, numerical methods and software packages based on convection-dispersion equations are widely utilized in the research of soil-column infiltrating experiments (see [7–13], e.g.). Recently, the authors have ever considered an undisturbed soil-column experiment using a linear model (see [14]) and a nonlinear model (see [15]), respectively, and gave successful numerical inversions by utilizing the optimal perturbation algorithm based on the measured breakthrough data.
As we know, for a disturbed soil-column infiltrating experiment, there always has no more complicated physical/chemical reactions in the solute transport process, and then a mathematical model seems to be easy to establish due to linearity of the transportation. However, it is still a trouble on how to uniquely determine transport parameters in the model which can not be measured directly by the experiment. Another thing is how to work out the unknown parameters with high precision as much as possible. Thus, inverse problem and inversion analysis methods are often encountered with for solutes transport problems in soil-column infiltrating experiments.
In this paper, we will investigate a disturbed soil-column experiment carried out in Zibo, Shandong Province, China, and study transport behaviors of eight kinds of ions of Ca2+, Na+, Mg2+, K+, , , , and Cl− by the inversion method. On the basis of advection-dispersion mechanism and hydrochemical analysis to the experimental data, two kinds of models describing their transport behaviors in the column are discussed respectively. By applying the optimal perturbation regularization algorithm, all unknown transport parameters for each ion are determined, and then the measured breakthrough data are reconstructed successfully. The inversion results show that the reconstruction data basically coincide with the measured breakthrough data, and the advection dispersion equation with suitable time-dependent reaction terms can be utilized to describe the experimental process and reconstruct the experimental data. The paper is arranged as follows.
Section 2 gives the soil-column experiment, breakthrough curves of the eight ions and preliminary analysis of their transport behaviors. In Section 3, two kinds of transport models are given respectively, one is suitable for , , and K+, and the other is for the rest. With the help of the models, the parameter identification problem for each ion is set forth based on the breakthrough data, and an optimal perturbation regularization algorithm is introduced. In Section 4, all the unknowns for each considered ion are determined by the inversion algorithm, and the breakthrough data are reconstructed. Finally, conclusions and discussions on the real problem here are presented.
2. Experimental Analysis
The disturbed soil-column experiment was carried out in a simple apparatus in a lab in Shandong University of Technology. The device is installed with three systems which are infiltrating system, soil-column system, and sample collector system, respectively. The soil column is composed by fine sands with diameter of 2 [mm], the diameter of the lucite tube loading the column is 18.6 [cm], and the height of the column is about 62 [cm]. After infiltrating the column with distilled water for 24 hours, the experiment was performed by infiltrating into the column with artificial coalmine water at the temperature of 20 centigrade.
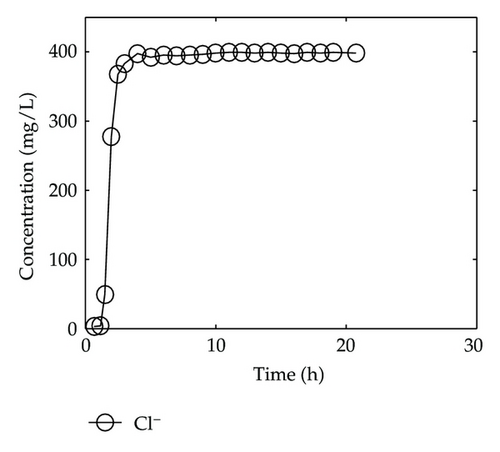
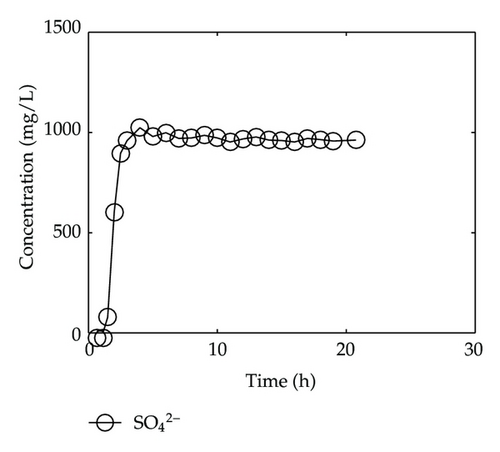
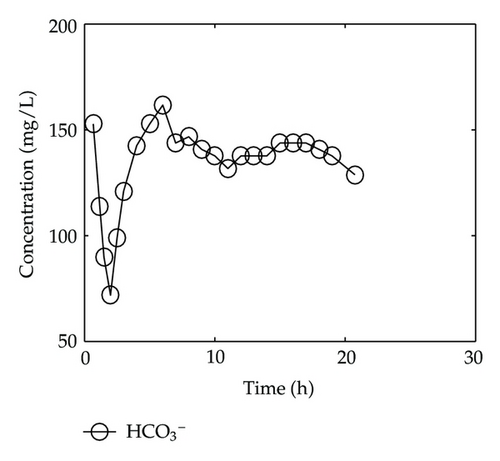
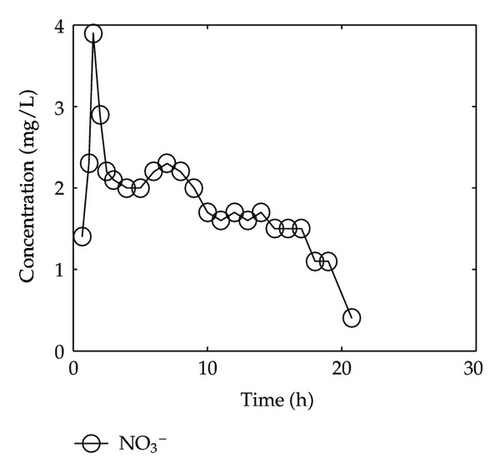
By collecting water samples at the bottom of the column, 23 samples were collected, and all of them were immediately sent to tested and analyzed in Shandong General Inspecting Station of Geology and Environment. Thus, we get the so-called breakthrough data of positive ions of Ca2+, Na+, K+, and Mg2+ and negative ions of Cl−, , , and , which are plotted in Figure 1 and Figure 2, respectively, where the transverse axis denotes the experimental time [h], and the vertical axis represents concentration of the solute ion at the bottom of the column [mg/L].
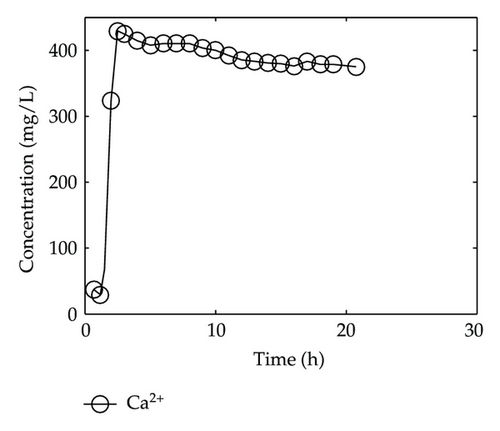
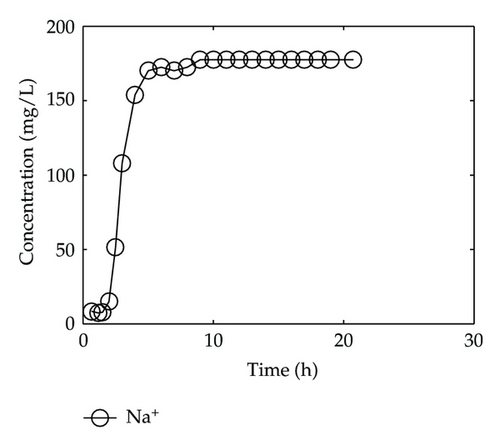
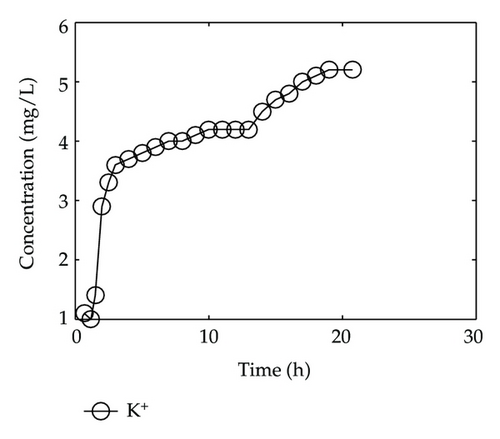
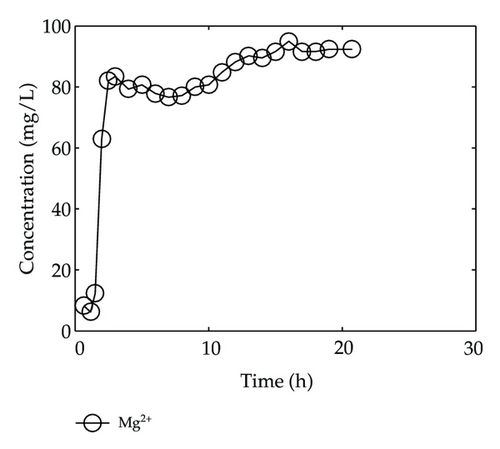
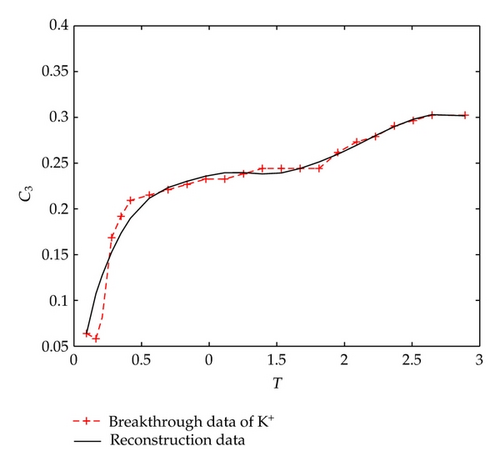
In Figure 1, we find that Ca2+, Na+, and Mg2+ in the liquid phase almost have same behaviors which go up quickly at the initial stage and then tend to steady situations gradually after 2-3 hours; however, there is a different performance for K+. Although K+ goes up rapidly during the initial stage of 3 hours, it is still in an increasing trend at a slow speed after t = 3 [h].
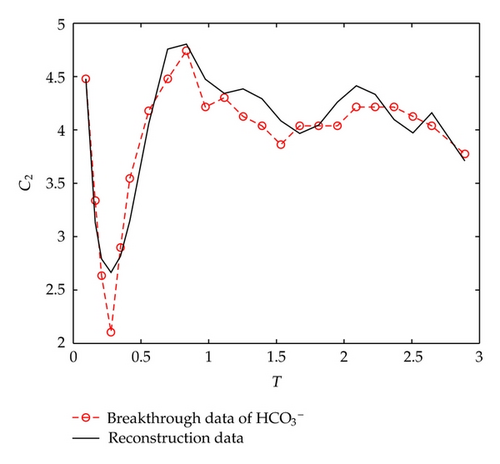
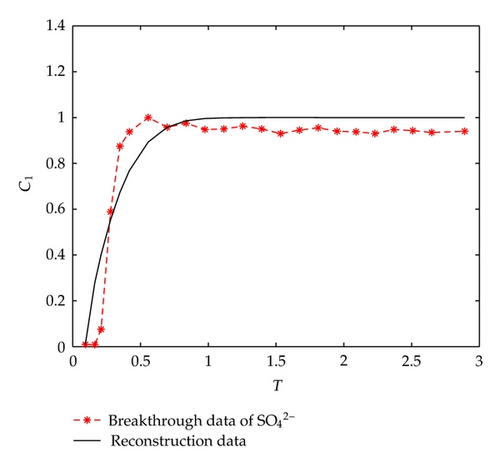
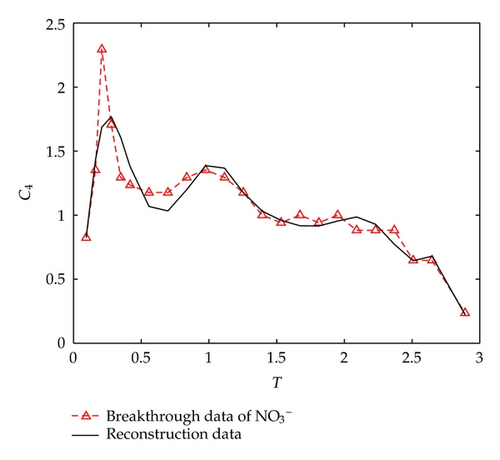
On the other hand, let us investigate behaviors of negative ions plotted in Figure 2. Cl− and have the same tendency with Ca2+, Na+, and Mg2+, but the situations are complicated for and . It is noticeable that the measured initial concentration of in the inflow is very small, and the first sample (at t = 2/3 [h]) gives a high concentration which is 152.7 [mg/L]. The reason may be ascribed to the actions of free CO2 in the inflow. From t = 2/3 [h] to t = 2 [h], the concentration goes down rapidly, it goes up quickly from t = 2 [h] to t = 6 [h], and approaches to an equilibrium after t = 6 [h]. As for , its first breakthrough concentration at t = 2/3 [h] is 1.4 [mg/L], which is lower than that of 1.7 [mg/L] in the inflow, and after t = 2/3 [h], it goes up rapidly during t ∈ (2/3,3/2) [h], and then goes down rapidly until t = 3 [h]. It is surprising that it still goes down after t = 3 [h] but with small oscillations.
In the following, on the basis of the above analysis, two kinds of mathematical models describing the solutes transport behaviors in the column will be given respectively, and an optimal perturbation algorithm will be introduced to determine the unknown parameters in the models.
3. Mathematical Model and Inversion Algorithm
3.1. The Mathematical Models
It is wellknown that, under suitable hypotheses, solute transportation in homogeneous porous media can be characterized using 1D advection-dispersion equation. For the disturbed soil-column experiment discussed in Section 2, physical/chemical reactions between the liquid and solid phases can be ignored since the column is composed by clean silver sands, and all of the ions transport in the column could be dominated by hydrologic dispersion and chemical reactions/ions exchanges among the solutes ions in the liquid phase. On the other hand, on the basis of the analysis in Section 2, we can deduce that transport behaviors of the ions of Ca2+, Na+, Mg2+, and Cl− with could be described by the ordinary advection-dispersion equation, and transport model for the ions of , K+, and could be different, but can be described by the advection-dispersion equation with different time-dependent reaction terms representing possible chemical reactions/ions exchanges depending on the experimental time.
Then, two kinds of mathematical models describing solutes transport behaviors in the soil-column are given by (3.1) and (3.2) together with the initial boundary value conditions (3.3) and (3.4), respectively. Since the initial indexes in condition (3.3) and the reaction coefficient functions in (3.2) are all unknown, a suitable inversion algorithm must be applied to determine them numerically. Furthermore, thanks to the independence of (3.1) with (3.2), we can see in the next section that each ion can be reconstructed independently of the others.
In the following, we will take (c2) as example and introduce an optimal perturbation regularization algorithm (see [14–16]), e.g.) to determine the unknown parameters based on the measured breakthrough data. For convenience of computation, we will transform the model to a dimensionless form.
As a result for , an inverse problem of determining the time-dependent reaction function and the initial index is formulated by (3.5), the initial boundary condition (3.6)-(3.7), and the over-posed condition (3.8).
In addition, the initial concentration of (c1), (c2), K+(c3), and (c4) are given as c10 = 1023.2 [mg/L], c20 = 34.1 [mg/L], c30 = 17.2 [mg/L], and c40 = 1.7 [mg/L], respectively. By the experiment, we know that the length of the column is l = 0.62 [m], the average flow velocity is v = 2.4 × 10−5 [m/s], and the total infiltrating time is Ttol = 21 [h]. Finally, noting that the soil medium is composed by sand with 2 [mm] diameter, the longitudinal dispersivity aL = 8.8 × 10−3 [m], which is estimated by known experimental results (see [1], e.g.).
3.2. The Optimal Perturbation Regularization Algorithm
τ = (τ0, τ1, …, τN+1) is numerical differential steps vector.
4. Inversion Results
Following the methods as used in paper [14, 15], we will implement the above optimal perturbation regularization algorithm to determine the unknown vector r given by (3.10) numerically, and then reconstruct the measured breakthrough data for the ions of , , K+, and , respectively. The reaction coefficient can be determined in different approximate space, and one needs to choose a suitable approximate dimension N so as to reconstruct the measured data with high accuracy. For the four kinds of ions, we will realize the inversion with different dimension. All computations are performed in a PC of Dell Dimension 9200.
4.1. Data Reconstruction for
4.2. Data Reconstruction for
Let us consider the solute ion of . As for (3.1) with corresponding initial boundary conditions, there is only one initial parameter m1 to be determined. Therefore, the optimal perturbation algorithm can be simplified as done in paper [14]. By utilizing the optimal perturbation algorithm without regularization term (choosing regularization parameter α = 0), and taking initial iteration value as 1, numerical differential step as τ = 1e − 1, the initial index can be determined by 7 iterations costing CPU time 1.7 [s] which is m1 = 3.7588, and the relative inversion error is Err = 0.0277. Figure 4 plots the measured breakthrough data and the reconstructed data of , respectively.
4.3. Data Reconstruction for
Now let us deal with data reconstruction for in which case the situation seems to be a little complicated. If still following the above method, there are no inversion results we need to appear. So, we perform the algorithm in a little different way as compared with the above computations by choosing a fixed initial index prescribed. Moreover, if setting an initial index in advance, then we only need to determine the time-dependent reaction coefficient.
5. Discussions and Conclusions
5.1. Discussions
5.1.1. On Reaction Coefficient
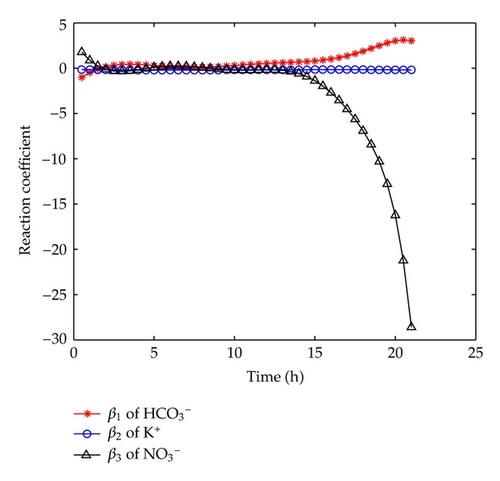
From Figure 7, we can find that the reaction to K+ almost has no change throughout the experiment; and the reaction to goes up at the initial stage, and then keeps a little increase during t ∈ (5,15)[h]. As for the reaction to , it decreases during the initial stage, and then keeps unchanging during t ∈ (2,15)[h], and after t > 15 [h], it becomes decreasing quickly.
It is noticeable that the above arguments basically coincide with those hydrochemical analysis given in Section 2 except for in the case of t > 15 [h]. In addition, we also find that β2(t) and β3(t) both have similar asymptotic properties as β1(t), that is, the expansion coefficients of the reaction term go to zero as N becomes large.
5.1.2. On Space Distribution at the First Outflow
Now, look at the following figure which plots space distributions at the first outflow time of T = T0 for the four kinds of solute ions.
From Figure 8, we can see that at the first outflow time of t = 2/3 [h], , , and K+ are in similar situations, and has a special behavior.
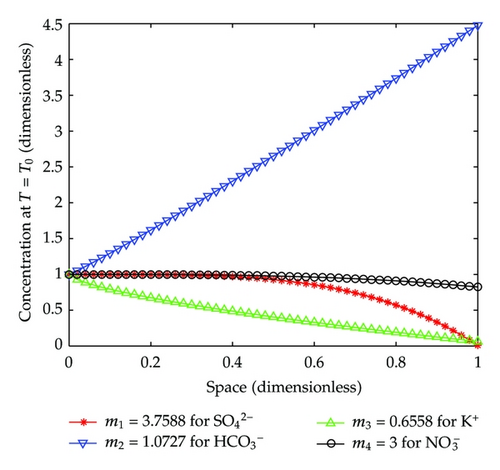
For , its concentration varies from 1 (t = 0) to 1.4/1.7 ≈ 0.8235 (t = 2/3 [h]), and goes down almost along with a straight line very slowly.
For , its concentration varies from 1 (t = 0) to 10.0/1023.2 ≈ 0.0098 (t = 2/3 [h]), and goes down with convexity but faster than .
For K+, its concentration varies from 1 (t = 0) to 1.1/17.2 ≈ 0.0640 (t = 2/3 [h]), and decreases also faster than , but with concavity.
As for , its concentration changes from 1 (t = 0) to 152.7/34.1 ≈ 4.4780 (t = 2/3 [h]), and goes up quickly with almost linearity.
In summary, during this interval of t ∈ (0,2/3)[h], hydrodynamical advection dispersion actions may be play dominating roles in the solutes transport through the column. However, there are some chemical reactions occurring in except for mechanical advection and dispersion. For example, the free CO2 in the inflow can produce when penetrating through the column, and the amount of reaches a maximum at the bottom of the column at t = 2/3 [h].
5.2. Conclusions
- (1)
By the model and data reconstruction results, we can see that the main transport behaviors of solutes ions in the soil-column are dominated by hydrodynamical advection and dispersion mechanisms. There are no complicated physical/chemical reactions between solid and liquid phases, especially for the solutes ions of , Cl−, Ca2+, Na+, and Mg2+, whose transport behaviors through the column can be described by purely advection-dispersion equation (3.1) with suitable initial conditions.
- (2)
As for , K+, and , their transport behaviors in the column could be described by the advection-dispersion equation (3.2) with suitable time-dependent reaction terms and initial conditions. It is noticeable that K+ has some properties similar to those five ions; but for and , they have some different properties as compared with the other ions maybe resulting from chemical reactions or ions exchanges in the liquid phase.
- (3)
As stated in Section 5.1.2, at the initial interval of t ∈ (0,2/3) [h], it is still a trouble to describe the solute transport behaviors in mathematics due to lack of measured data. Maybe better experimental devices, and other mathematical tools are needed. In addition, noting that nonlinear processes could occur quickly during this short time, perhaps it is feasible and prospective that utilizing the model in a smaller time scale of second instead of hour during this interval.
- (4)
By performing the inversion algorithm, we find that a numerical uniqueness on the inverse problem can be obtained in the sense of ignoring higher-order terms. Let us take the inversion for β2(t) as example. For the reason of seeking for regularity, we perform the algorithm utilizing the initial index of m3 = 0.6558 determined in Section 4.3, and keeping the same inversion error. Table 1 gives the inversion results for β2(t) taking on higher-order polynomials including the case of N = 3.
| N | β2(t) |
|---|---|
| 3 | (−0.1410, −0.0260, 2.9e-3, −8.3e-5) |
| 4 | (−0.1400, −0.0265, 2.9e-3, −8.6e-5, 4.2e-8) |
| 5 | (−0.1446, −0.0191, 1.3e-4, 3.1e-4, −2.3e-5, 4.9e-7) |
| 6 | (−0.1409, −0.0222, 8.3e-4, 2.7e-4, −2.6e-5, 8.3e-7, −8.6e-9) |
| 7 | (−0.1412, −0.0201, 7.7e-6, 2.6e-4, 8.3e-6, −3.9e-6, 2.4e-7, −4.5e-9) |
From the table, we can find that if taking the first term as an approximation, the reaction coefficient should be β2(t) = −0.14; if taking the front two terms as an approximation, the optimal reaction coefficient should be given by β2(t) = −0.14 − 0.02t. Another thing we should mention is that for different truncated levels N = 3, 4, 5, 6 and N = 7, all of the inversion results preserve the same relative error expressed by Err = 0.0141. From this point of view, the numerical inversion is of uniqueness too. As to theoretical uniqueness of the inverse problem here, we will deal with it in our sequent papers.
Acknowledgment
This paper is supported by the National Natural Science Foundation of China (Grant nos. 10926194, and 11071148).




