Benzothiazine Dyes/2,4,6-Tris(trichloromethyl)-1,3,5-triazine as a New Visible Two-Component Photoinitiator System
Abstract
Novel photoinitiator systems working under visible radiation were studied. The photoredox pair constructed with dye derivatives of 12H-quinoxalino-[2,3-b][1,4]-benzothiazine (1–3) and 2,4,6-tris(trichloromethyl)-1,3,5-triazine (Tz) were found to be effective initiators for free radical polymerization of trimethylolpropane triacrylate (TMPTA) using VIS light. Photosensitization occurred through electron transfer, which was confirmed by the observation of a radical cation of the studied dyes. The 1•+ was also characterized in cryogenic matrices (mixture of CH2Cl2 and ionic liquid: 1-butyl-3-methylimidazolium hexafluorophosphate (BMIM+PF6 −)) and its reactivity was investigated by means of pulse radiolysis in solution at room temperature. In a halogenated solvent and in a mixture of CH2Cl2 and BMIM+PF6 −, the radical cation 1•+ underwent deprotonation to form a neutral radical 1•, which was stable in the second time scale. During photolysis of the 1/Tz photoredox pair in 1-methyl-2-pyrrolidone and monomer, the formation of a neutral radical 1• was not observed.
1. Introduction
One of the most widely used and simplest methods of polymer formation is radical chain addition polymerization. Light-induced photopolymerization has several advantages over other methods; the process occurs at low temperatures, and it can be controlled by manipulating the intensity and wavelength of irradiation. Photo-initiated free radical polymerization of multifunctional monomers produces highly crosslinked polymers with high thermal stability, mechanical strength, and resistance to solvent. These polymers have many industrial applications, specifically in coatings for flooring and furniture, dental restorative materials, optical fiber coatings, hard and soft contact lenses, and photolithography [1–3].
Compounds with an efficient absorbance at long wavelengths were the most significant photoinitiators in the past decade. Absorbance at lower energies is significant for two reasons: first, the development of efficient long-wavelength, UV and visible emitting light sources, such as lasers and LEDs for imaging, printing, and medical applications, has created an increasing demand for an initiator system that is effective in the 400 to 500 nm spectral region; secondly, in exterior durable photopolymers, the key development was photoinitiators that absorb outside of the UV absorption curve of traditional UV absorbers used to protect organic coatings from the harmful rays of the sun.
Many attempts have been made to develop efficient photoinitiating polymerization systems that can be used with visible light. Previously, two components systems that operated via photoinduced electron transfer were developed [3–20]. In such systems, dyes play an important role. Dyes are chromophores that absorb visible light and after excitation, they react with coinitiators (electron donor D or electron acceptor A) via electron transfer. In both cases, the initiating radicals are formed through the cleavage of the oxidized or reduced form of the coinitiator (Scheme 1).
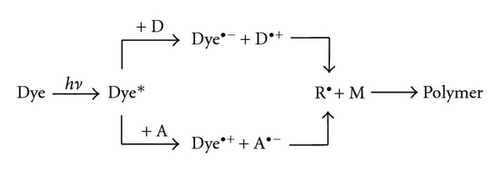
In the photoreducible sensitization, the dye was photoreduced in the presence of a suitable reductant such as phenyltioacetic acid [8–10], N-phenylglycine [9, 10], amine [11–13], and alkyltriphenylborate salt [14, 15].
In the last decade, dye/coinitiators systems working under visible irradiation via photooxidizable sensitization have been developed [3–7, 16–20]. In such photoredox pairs, N-alkoxypyridinium salts were commonly used as coinitiators. Upon electron transfer, these compounds yielded alkoxy radicals (Scheme 2) which initiated polymerization. The second important coinitiator is the derivative of 2,4,6-tris(trichloromethyl)-1,3,5-triazine. The halogenmethyl radicals formed are responsible for initiation of free radical polymerization (Scheme 3) [16–20].
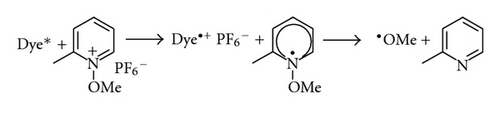
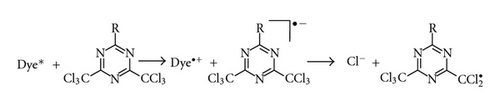
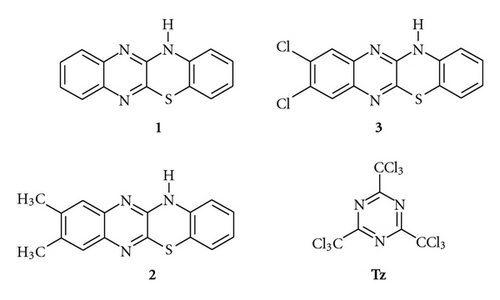
It is well known in the literature that not all dyes are able to initiate photopolymerization with the aid of 2,4,6-tris(trichloromethyl)-1,3,5-triazine (Tz) [16]. Therefore, the primary goal of the presented study is the application of dye derivatives of 12H-quinoxalino-[2,3-b][1,4]-benzothiazine (1–3) in combination with Tz as a photoinitiator for free radical polymerization of a multifunctional acrylate (TMPTA). The structure of studied dyes and Tz are presented in Scheme 4. Moreover, the photodarkening mechanism of dye 1 during photolysis was studied. The mechanism proposed is supported by spectroscopic characterization of its radical cation and neutral radical in low-temperature matrices (mixture of CH2Cl2 and ionic liquid: 1-butyl-3-methylimidazolium hexafluorophosphate). The rigid environment of these matrices at cryogenic conditions on recombination and fragmentation processes allows for the direct observation of the transient species. This effect also allows for convenient observation of the undergoing processes upon a controlled annealing and softening of the rigid matrix environment.
2. Experimental
2.1. Generals
The procedure for preparing the dyes has been described elsewhere [7]. 2,4,6-tris(trichloromethyl)-1,3,5-triazine (Tz) and 1-methyl-2-pyrrolidone (MP) were purchased from Wako-Chemicals and Sigma-Aldrich, respectively. Acetonitrile (MeCN), 2-propanol (2-PrOH), dichloromethane (CH2Cl2), and carbon tetrachloride (CCl4) were purchased from POCH (Poland). The ionic liquid, 1-butyl-3-methylimidazolium hexafluorophosphate (BMIM+), was prepared by following the method of Huddleston et al. [21].
The absorption and steady state fluorescence spectra were recorded using a Jasco V-670 spectrophotometer and Jasco FP 6300 spectrofluorimeter (Jasco, Japan), respectively. All photochemical experiments were carried out in a Rayonet Reactor RPR 200 (The Southern New England Ultraviolet Co, USA) equipped with lamps emitting light at 419 nm. A specific spectral band was isolated by the use of band-pass filters at 419 ± 10 nm. Illumination intensity was measured using uranyl oxalate actinometry [22].
2.2. Free Radical Polymerization
In the above expression, Qs is a heat flow per second during the reaction, m is the mass of the monomer in the sample, M is the molar mass of the monomer, n is the number of double bonds per monomer, and ΔHp is the theoretical enthalpy for complete polymerization of acrylate double bonds (20.6 kcal/mol) [23]. The heat flow was measured with a PT 401 temperature sensor (Elmetron, Poland), immersed in the sample. A polymerizing mixture containing the dye without Tz was used as a reference sample.
2.3. Pulse Radiolysis
Pulse radiolysis experiments were carried out with high energy (6 MeV) 17 ns electron pulses generated from an ELU-6 linear electron accelerator. All measurements were performed at room temperature. The dose absorbed per pulse was determined with N2O saturated aqueous solution of KSCN (0.01 M), assuming mol/J and M−1 cm−1 (G represents the radiation chemical yield and ε is a molar absorption coefficient at 475 nm). The dose delivered per pulse was within the range of 5 to 80 Gy. Details of the pulse radiolysis system are provided elsewhere [24, 25].
2.4. Cryogenic Measurements
Glassy samples were prepared by quench-freezing room temperature solutions in liquid nitrogen. The samples were 1.5 to 2 mm thick and were placed in a temperature-controlled liquid nitrogen-cooled cryostat (Oxford Instruments-OptiStat DN). The desired temperature (77 to 150 K) of the matrix was attained by automatically controlled heating. The samples mounted in a cryostat were irradiated with 4 μs electron pulses from the ELU-6 linear accelerator.
3. Results and Discussion
3.1. Sensitized Free Radical Photopolymerization
The efficiency of benzothiazine dye derivatives of 12H-quinoxalino-[2,3-b][1,4]-benzothiazine (1–3) in combination with Tz as photoinitiating systems for the free radical polymerization of TMPTA was evaluated. It is well known, that the most efficient dyeing photoinitiators are the photoredox pair in which electron transfer occurs with the participation of the excited triplet states [4, 6, 7, 15]. Therefore, dyes 1–3 were selected as efficient oxidizable sensitizers with a high quantum yield of triplet state generation (~0.8 [7]). The spectroscopic and electrochemical properties of these dyes are presented in Table 1. The benzothiazine dyes absorbed light at higher wavelengths than Tz (304 nm); therefore the band-pass filters were used to irradiate the dye alone in photopolymerization experiments.
| Dye | (nm) | (V) | E00 c (kJ mol−1) |
|---|---|---|---|
| 1 | 425 | 0.97 | 260 |
| 2 | 424 | 0.79 | 259 |
| 3 | 442 | 0.90 | 253 |
The efficiency of the polymerization initiated by the dye/Tz systems was measured indirectly from the reaction’s heat flow during irradiation. The overall polymerization results are summarized in Table 2.
| Dye | (μmol s−1) | Inhibition timea (s) | −ΔGet (kJ mol−1) |
|---|---|---|---|
| 1 | 42.8 | 26 | 32.0 |
| 2 | 11.6 | 41 | 48.5 |
| 3 | 9.6 | 68 | 31.8 |
- a[Dye] = 0.1 mM, [Tz] = 10 mM.
The calculated polymerization rate of TMPTA (Table 2) indicated that the efficiency of polymerization strongly depended upon the structure of the dye employed. It was apparent that dye 1 significantly accelerated triacrylate photopolymerization in comparison with dyes 2 and 3. Free radical polymerization initiated by the tested photoredox pairs showed a significant inhibition time (Table 2). This effect should be related to the molecular oxygen dissolved in the composition. The oxygen both quenched the triplet state of the dye and reacted with the radical species generated by the dye/Tz systems. After consumption of the oxygen, the initiating radicals reacted with the monomer. As seen in Table 2, the most efficient photoredox pair, 1/Tz, had the shortest inhibition time.
3.2. Photodarkening Mechanism
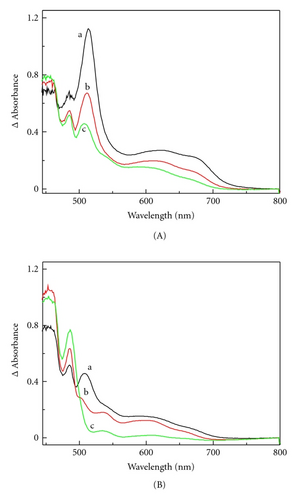
During photopolymerization of the triacrylate monomer, the studied sensitizer does not really photobleach but photodarkens. An explanation of this phenomenon demands the identification of the products formed during photolysis. Therefore, the photooxidation mechanism of compound 1 was studied in detail. Because the proton of the nitrogen heteroatom in 1 can participate in the deprotonation reaction following the oxidation of benzothiazine, the pulse radiolysis of low temperature matrices was applied to the spectroscopic characterization of its radical cation and neutral radical. The electronic absorption spectrum of the radical cation 1●+ obtained by low-temperature methodology is presented in Figure 1(A). The spectrum consisted of a strong absorption band at 514 nm and two smaller peaks at longer wavelengths of 620 nm and 675 nm. These bands decayed upon limited warming of the matrix (Figure 1(B)). The decrease of the absorption of 1●+ was accompanied by an increase of the absorption band at 485 nm. This product was stable at room temperature.
Radical cations are more acidic than their neutral precursors and some of them are even superacids [28, 29]. Therefore they readily undergo deprotonation. Under matrix conditions the radical cation deprotonation can only start upon matrix softening. Therefore, one can conclude that radical cation 1●+ undergoes deprotonation into the neutral radical 1● (Scheme 5) on annealing of the matrix. The generation of the amine radical was more apparent in pulse radiolysis in CH2Cl2 at room temperature. Transient absorption spectra recorded under oxygen conditions are presented in Figure 2.
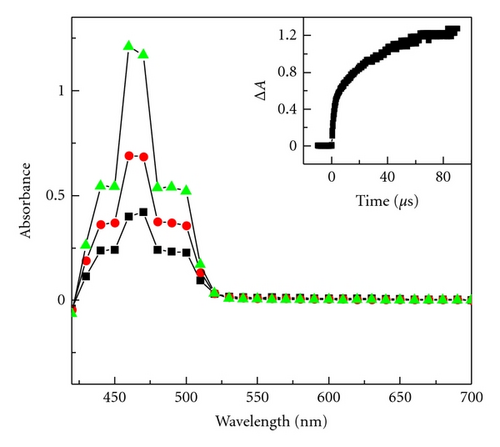
In such experimental conditions, a product with absorption bands at 440 nm, 460 nm, and 490 nm were observed. The bands were assigned to neutral radical 1●. In an oxygen-saturated solution, the ●CH2Cl radical reacted rapidly with O2 to form the peroxyl radical, which oxidized dye 1 to the radical cation 1●+ that undergoes deprotonation into the neutral radical 1●. Similar deprotonation of the radical cation of phenothiazine in n-butyl chloride was observed [30]. Therefore, the direct oxidation of compound 1 by the trichloromethylperoxyl radical to its radical cation was observed in a 2-PrOH-water solution with 0.1 M of HClO4 at room temperature. The pKa of compound 1 in the 2-PrOH-water solution was estimated to be approximately 0.4. The transient absorption spectrum recorded in the acidic condition consisted of a strong absorption band at 500 nm and two smaller peaks at longer wavelengths of 550 and 600 nm. A similar absorption was obtained in the cryogenic matrix (see Figure 1(a)). Thus, the formation of 1●+ was followed at 500 nm. The observed rate of oxidation increased linearly with dye concentration (0.56 to 0.96 mM). The plot of kobs versus concentration of the dye was used to determine the second order rate constant 2k = (2.18 ± 0.08) × 107 M−1 s−1.
After spectroscopic characterization of 1●+ and 1●, the photolysis of benzothiazine dye 1 in composition with Tz was examined in MP. The electronic spectra recorded in MP showed the characteristic broad absorption band at 625 nm (Figure 3), which was assigned to 1●+.
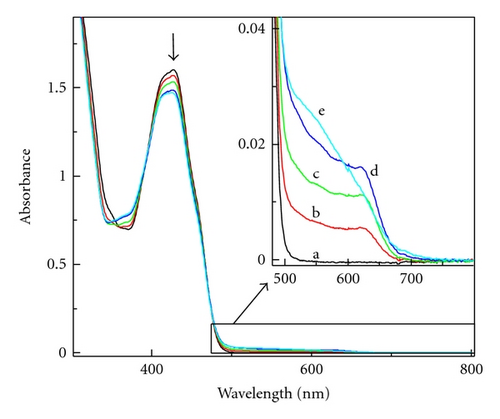
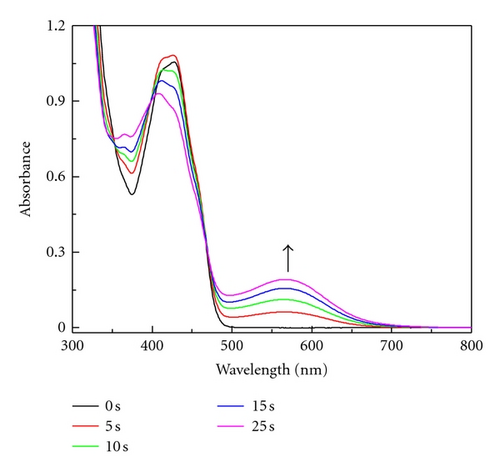
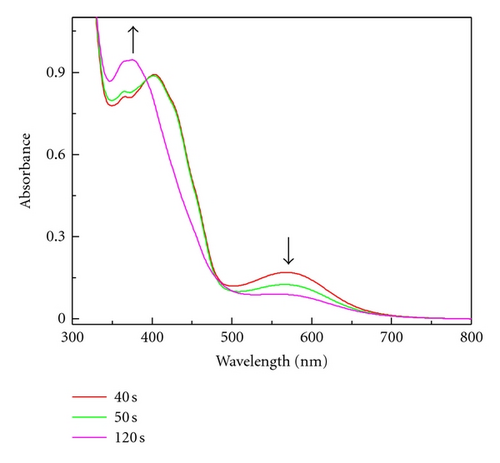
The same effect was observed during irradiation of the 1/Tz photoredox pair in MP containing 0.37 M TMPTA. Extending the irradiation time to 30 s caused an increase of the new band at 560 nm but the absorption bands of radical 1● were not detected. When the photolysis was carried out with higher irradiation intensity (6 × 1016 quant s−1) the broad absorption band at 560 nm was observed (Figure 4(a)).
For comparison the photolysis of dye 1 in CH2Cl2 was carried out. In this steady state photolysis dichloromethane was used as an electron acceptor. Electronic absorption spectrum (Figure 5) recorded after 15 minutes of irradiation showed the characteristic absorption bands at 412 nm, 435 nm, 462 nm, and 494 nm similar to the spectrum obtained during radiolysis (see Figure 2).
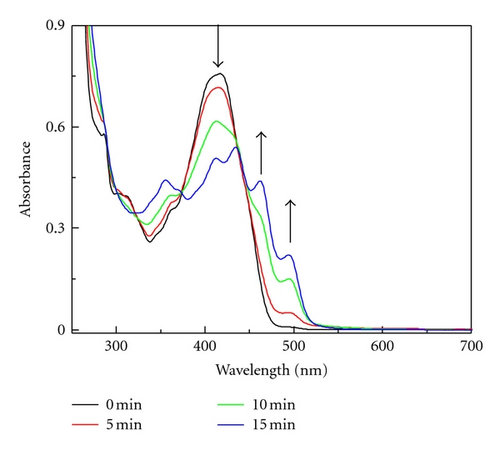
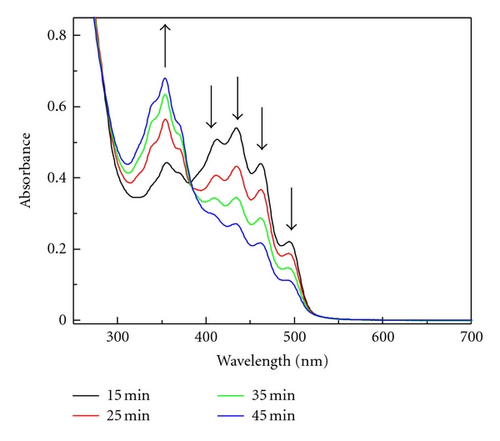
Thus, the product formed during photolysis in CH2Cl2 is neutral radical 1●. The elongation of irradiation time to 50 min caused the decrease the absorption of 1●. While the 1● disappears, the band at 353 nm increases. Therefore, we assigned this new band to 1+ cation (Scheme 5), which might be formed during oxidation of 1● by CH2Cl2 or peroxyl radical .
Based on the presented above results, one can assume that in MP, 1●+ underwent a one-electron oxidation to the dication 12+ (Scheme 5). It is concluded that the photodarkening of the polymer was caused by the radical cation or dication of the studied dyes and not the neutral radical. Such behavior is normally unwanted, particularly in composites, because it diminishes the curing of deeper layers of resin. However, in some situations, such as stereolithography, photodarkening may be an advantage, since it reduces the penetration of radiation below the top photocuring layer and allows higher pattern resolution.
4. Conclusions
Novel photoinitiator systems constructed with dye derivatives of 12H-quinoxalino-[2,3-b][1,4]-benzothiazine (1–3) and 2,4,6-tris(trichloromethyl)-1,3,5-triazine (Tz) are found to be effective initiation systems for free radical polymerization of TMPTA using VIS light. Photosensitization occurs through electron transfer, which was confirmed by the observation of a radical cation of the dyes studied during photolysis. The radical cation formed after electron transfer between benzothiazine dye 1 and Tz in MP underwent one-electron oxidation to yield the dication 12+. The formation of the neutral radical 1● was observed during radiolysis and photolysis of benzothiazine dye 1 in CH2Cl2. The cation 1+ was formed as results of two reactions: deprotonation of dication 12+ or oxidation of neutral radical 1●. The photodarkening of the polymers was caused by the radical cation or dication from the sensitizers.
Acknowledgments
This work was supported by the Polish Ministry of Science and Higher Education (Project no. N N205 1454 33).




