Photophysical Parameters, Excitation Energy Transfer, and Photoreactivity of 1,4-Bis(5-phenyl-2-oxazolyl)benzene (POPOP) Laser Dye
Abstract
The effect of solvents on the absorption and emission spectra of 1,4-bis(5-phenyl-2-oxazolyl)benzene (POPOP) laser dye has been studied in various solvents at 298 K. A bathochromic shift was observed in absorption and fluorescence spectra upon increase of solvent polarity, which indicates that this transition is π-π*. The ground and excited state dipole moments were calculated as 2.23 and 6.34 Debye, respectively. The dye solution in MeOH, n-heptane, and methyl isobutyl ketone gives laser emission in the blue region upon excitation by a 337.1 nm nitrogen pulse; the gain coefficient and emission cross section as well as normalized photostability have been determined. Excitation energy transfer from POPOP to rhodamine B and fluorescine was studied to improve the laser emission from these dyes. Such an energy transfer dye laser system (ETDL) obeys a long range columbic energy transfer mechanism with a critical transfer distance, R0, of 25 and 33 Å and kq equal to 10.4 × 1012 and 26.2 × 1012 M−1 s −1 for the POPOP/RB and POPOP/fluorescine pair, respectively. The POPOP dye is highly photostable in polar protic and polar aprotic solvents, while it displays photodecomposition in chloromethane solvent via formation of a contact ion pair. The photochemical quantum yield and rate of photodecomposition depend on the electron affinity of solvent.
1. Introduction
Fluorescent organic compounds possessing high Stokes shift values [1, 2] are prospective candidates for practical application in various fields of science and technology, where high concentrations or long optical paths are required [3, 4], for example, in scintillation techniques [5, 6], sunlight collection, and conversion of solar energy into electricity [7, 8], electroluminescent light sources (OLEDs) [9–11], and various biological applications, and so forth. Several physicochemical mechanisms can be applied to increase the fluorescence Stokes shift of organic compounds [12]; however, not all lead to emissions with high quantum yields [13]. The most popular and most studied is the excited state proton transfer reaction [14, 15]; however, it is usually connected with high radiationless excitation energy losses [16]. To our understanding, excited state conformational transformations resulting in the formation of more planar molecular structures [17–19] have several advantages in producing high Stokes shifted fluorescence emissions [20] over the alternative twisting mechanisms [21], which also induce radiationless excited state deactivation in most of the known cases [22].
Derivatives of 1,2-bis-(5-phenyloxazol-2-yl)benzene [23–25] are ortho analogs of the well-known scintillation luminophore POPOP [1,4-bis-(5-phenyloxazol-2-yl)benzene]. They belong to the class of efficient fluorescent organic compounds with abnormally high Stokes shifts [26]. In contrast to their planar paraisomers [27], molecules of the title series are characterized by essential nonplanarity caused by the steric repulsion of two bulky heterocyclic moieties introduced into the orthopositions of the central benzene ring [28]. The resulting disruption of intramolecular conjugation shifts the electronic absorption spectra of ortho-POPOPs towards the shorter-wavelength region with respect to the absorption of their planar paraisomers.
Further increase of the Stokes shift values is possible by combination of several photophysical mechanisms in one molecule: for example, excited state planarization and solvatochromic effects. Asymmetrization of the electronic density distribution by introduction of highly electron-donating and/or electron-withdrawing substituents into the ortho-POPOP molecule leads to a significant rise in its excited state dipole moment, increased sensitivity to solvent polarity, and thus enlarges the fluorescence Stokes shift in polar media [29]. In the present we report photophysical parameters, excitation energy transfer, and photoreactivity of POPOP laser dye (Scheme 1).
2. Experimental
3. Results and Discussion
3.1. Optical Absorption and Emission Spectra in Different Solvents
The steady state absorption and emission parameters of 1 × 10−5 mol dm−3 POPOP were recorded in various nonpolar, polar aprotic, and polar protic solvents and are summarized in Table 1. The absorption and emission spectrum of POPOP are shown in Figures 1 and 2, respectively. As shown in Figure 1, the solvent polarity shows a slight effect on the position of electronic absorption spectral maxima, indicating the polar character of POPOP in the ground state.
| Solvent | λabs (nm) | λf (nm) | ε×104 mol−1 cm−1 dm3 | f | kr × 108 s−1 | ϕf | τf (ns) | kisc × 108 | σa×10−16 cm2 | σe × 10−16 cm2 |
|---|---|---|---|---|---|---|---|---|---|---|
| n-Heptane | 362 | 416 | 5.65 | 1.00 | 9.79 | 0.76 | 0.78 | 3.02 | 1.34 | 2.96 |
| Carbon tetra chloride | 370 | 416 | 4.89 | 0.86 | 8.90 | 0.74 | 0.83 | 3.02 | 1.16 | 2.74 |
| Benzene | 365 | 411 | 5.73 | 1.02 | 11.48 | 0.74 | 0.65 | 3.88 | 1.36 | 2.56 |
| Toluene | 368 | 418 | 4.98 | 0.90 | 9.95 | 0.81 | 0.81 | 2.29 | 1.18 | 2.81 |
| Diethyl ether | 362 | 411 | 5.12 | 0.93 | 8.67 | 0.73 | 0.84 | 3.15 | 1.22 | 2.88 |
| Methyl isobutyl ketone | 365 | 418 | 4.81 | 0.80 | 7.93 | 0.68 | 0.86 | 3.63 | 1.14 | 2.46 |
| Amyl alcohol | 365 | 424 | 4.33 | 0.79 | 7.95 | 0.85 | 1.07 | 1.36 | 1.03 | 2.58 |
| Cyclohexane | 363 | 409 | 4.48 | 0.80 | 8.31 | 0.76 | 0.92 | 2.53 | 1.06 | 2.56 |
| Acetone | 362 | 415 | 5.17 | 0.93 | 8.78 | 0.69 | 0.78 | 3.88 | 1.23 | 2.88 |
| Ethanol | 363 | 424 | 4.92 | 0.97 | 9.15 | 0.75 | 0.82 | 2.98 | 1.17 | 3.03 |
| Methanol | 361 | 424 | 4.83 | 0.89 | 8.09 | 0.75 | 0.93 | 2.58 | 1.15 | 2.82 |
| Diethyl formamide | 368 | 425 | 5.00 | 0.95 | 9.62 | 0.77 | 0.80 | 2.83 | 1.19 | 2.89 |
| Ethylene glycol | 367 | 439 | 3.25 | 0.57 | 5.82 | 0.92 | 1.58 | 0.47 | 0.77 | 2.1 |
| Propylene carbonate | 365 | 430 | 4.87 | 0.94 | 9.58 | 0.82 | 0.85 | 2.10 | 1.16 | 3.12 |
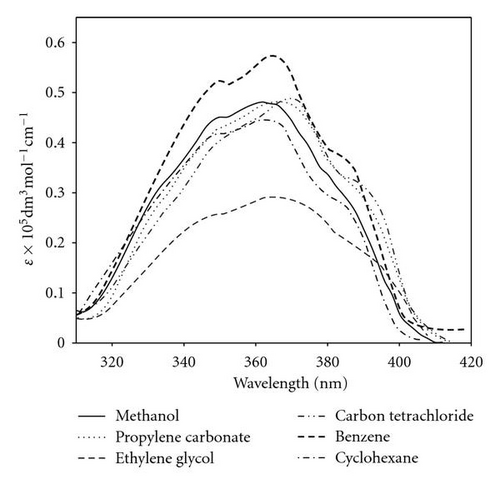
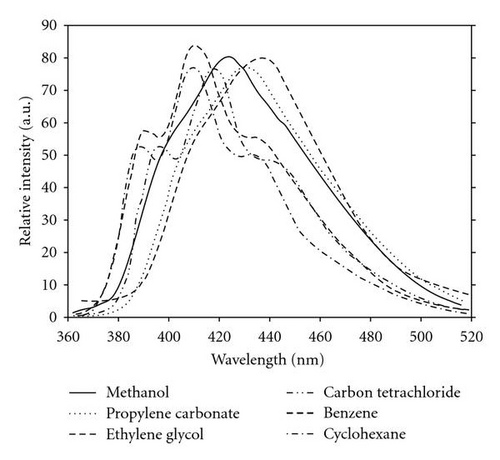
Fluorescence spectra, on the other hand, are more sensitive to solvent polarity. As solvent polarity increases, the emission spectra become red-shifted, Figure 2. This indicates that the singlet excited state of the POPOP molecule is more polar than the ground state. There is also a good mirror image relationship between absorption and fluorescence spectra. These facts, together with high molar absorptivities (ε = 32500–56500 mol dm−3 cm−1) and high oscillator strength (f = 0.57–1.02), are consistent with a strong π-π* transition with a small geometry change between electronic ground and excited states [41]. The fluorescence quantum yields ϕf are slightly affected by solvent properties; the values of ϕf in methyl isobutyl ketone (ϕf = 0.68) and acetone (ϕf = 0.69) are low due to the carbonyl group quenching the singlet excited state via enhancement of intersystem crossing (S1 → T1), while the high value of fluorescence quantum yield in ethylene glycol (ϕf = 0.92) can be explained in term of a cage effect and the role of medium viscosity which decrease the stretching and twisting molecular motion in the excited state, thereby decreasing the nonradiative process [42]. As seen in Table 1, the calculated fluorescence lifetimes obtained using the Strickler-Berg equation are very similar to the measured values. This is expected because the electronic absorption and fluorescence spectrum are nearly mirror image.
3.2. Determination of Dipole Moments
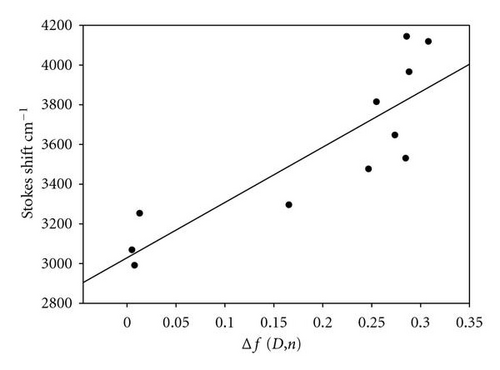
Bakhshiev’s and Kawski-Chamma-Viallet equations [47–51] have been used for the treatment of observed spectral shifts to determine the ground and excited state dipole moments of POPOP.
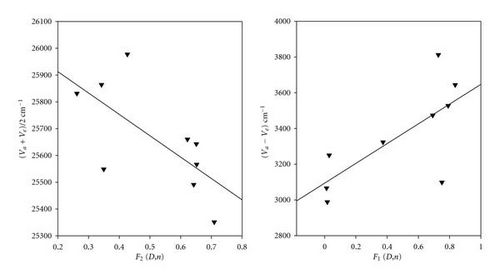
The values of S1 and S2 were found to be 553 and 1152 cm−1, respectively. Thus the calculated values of μg and μewere found to be 2.23 and 6.34, respectively.
3.3. Lasing Action and Fluorescence Quenching of POPOP
| Solvent | σe × 10−16 cm2 | Lasing range | nm | α cm−1 | Normalized photostability |
|---|---|---|---|---|---|
| Methanol | 2.82 | 408–450 | 424 | 1.25 | 20.94 MJ/mol |
| n-Heptane | 2.96 | 400–450 | 417 | 0.89 | 8.41 MJ/mol |
| MIK | 2.46 | 395–440 | 418 | 0.58 | 12.62 MJ/mol |
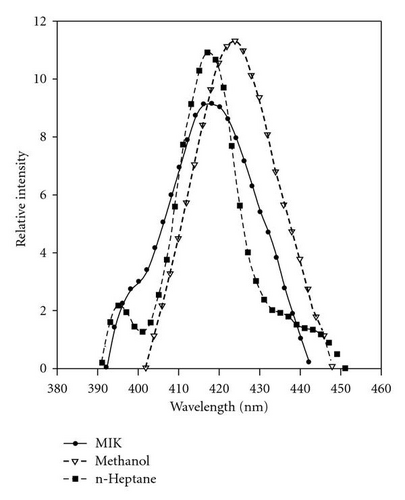
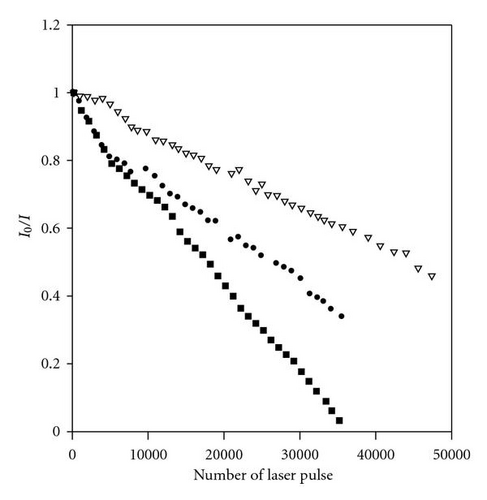
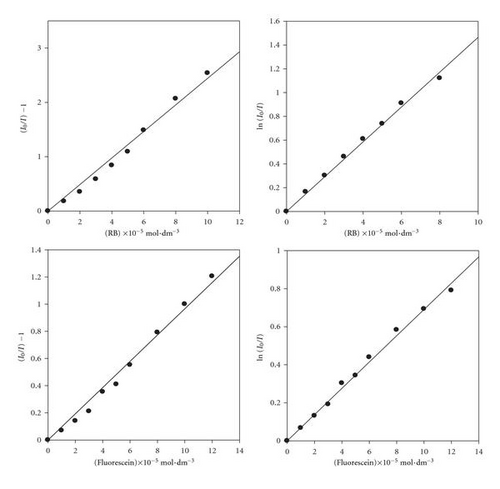
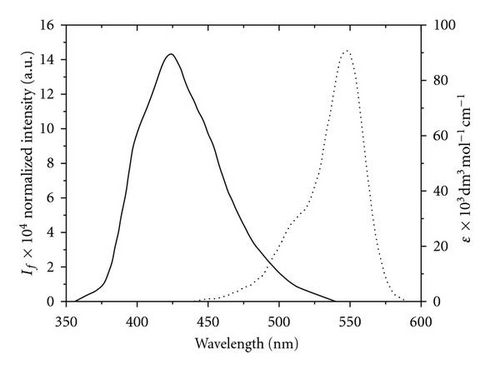
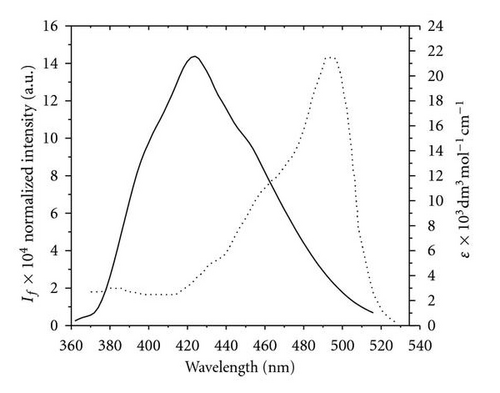
3.4. Photoreactivity of POPOP in Chloromethane Solvents
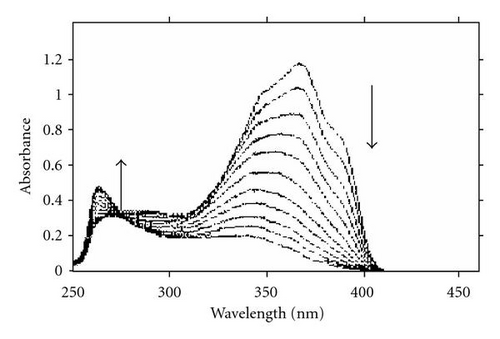
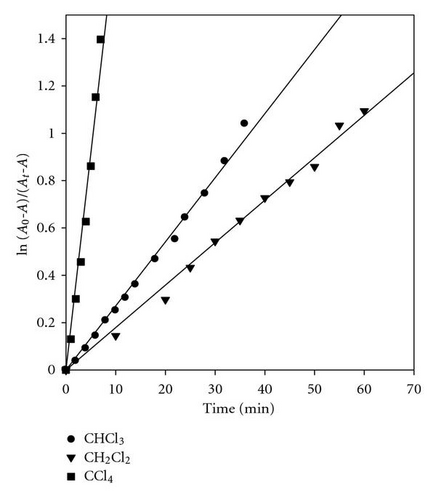
It was proposed that the electron transfer from the excited singlet POPOP to within the transient excited charge transfer complex (exciplex) is the main primary photochemical process. This leads to the POPOP radical cation, a chloride ion and a chloromethyl radical in the solvent cage Step (5).
4. Conclusion
The ground and excited state dipole moments of POPOP were determined as 2.3 and 6.34 Debye, respectively. The dye solution in methanol, n-heptane, and methyl-isobutyl ketone gives laser emission in blue region with emission maximum in the range 417–450 nm upon pumping by nitrogen laser pulse. POPOP act as a good energy donor for rhodamine B and fluorescine laser dyes, the energy transfer rate constant and critical transfer distance are determined. POPOP dye is highly photostable in polar solvents but displays photodecomposition in chloromethane solvents via electron transfer from excited dye to solvent molecules.




