CMV Serostatus of Donor-Recipient Pairs Influences the Risk of CMV Infection/Reactivation in HSCT Patients
Abstract
CMV donor/recipient serostatus was analyzed in 200 patients allografted in our institution from unrelated (122 patients) donors and 78 sibling donors in the years 2002–2011 in relation to posttransplant complications. On a group basis independently of the CMV serostatus of donor-recipient pairs sibling transplantations and those from unrelated donors that matched 10/10 at allele level had a similar rate of CMV reactivation (17/78 versus 19/71, P = ns). The rate of CMV reactivation/infection was higher in patients grafted from donors accepted at the lower level of matching than 10/10 (18/38 versus 36/149, P = 0.008). The incidence of aGvHD followed frequencies of CMV reactivation in the tested groups, being 40/156 and 25/44 in patients grafted from sibling or unrelated donors that 10/10 matched and in those grafted from donors taht HLA mismatched, respectively (P = 0.001). Regarding the rate of reactivation in both groups seropositive patients receiving a transplant from seronegative donors had more frequently CMV reactivation as compared to those with another donor-recipient matching CMV serostatus constellation (22/43 versus 32/143, P = 0 < 0.001). Multivariate analysis revealed that seropositivity of recipients with concomitant seronegativity of donors plays an independent role in the CMV reactivation/infection (OR = 2.669, P = 0.037; OR = 5.322, P = 0.078; OR = 23.034, P = 0.023 for optimally matched and mismatched patients and the whole group of patients, resp.).
1. Introduction
Donor-recipient matching for unrelated hematopoietic stem cell transplantation (HSCT) in addition to human leukocyte antigens (HLA) includes CMV serostatus of the donor and recipient to facilitate the decision [1, 2].
In the clinical practice the presence of CMV IgM antibodies is suggestive of the active infection/reactivation and the presence of IgG antibodies indicates prior infection and shows CMV immunological competence of individuals [3–5]. Unfortunately, it is very suggestive that IgG CMV antibody positive individuals harbor CMV in a latent form and their blood products are infective for CMV incompetent recipients [6]. In the present era of specific anti-CMV chemotherapy the significant impact of pretransplant donor seropositivity on the patient outcome is controversial—reviewed in the Boeckh and Nichols paper [7]. However, recipient CMV serostatus still remains an important risk factor of the patient outcome [8, 9].
HSCT involving pairs in which both donor and recipient lack CMV IgG antibodies is associated with a lower transplant mortality [10]. In the latter situation we are dealing with a donor-recipient pair in which probably neither donor nor recipient has CMV in alatent form. On the other hand, positivity of both donor and recipient should also favor the HSCT outcome—both donors and recipients likely have CMV in a latent form but the immune system of the donor should have a memory of CMV infection, which facilitates the immune response to CMV posttransplant. However, Ljungman et al. [11] in the megafile analysis showed that the latter important observation seems to be valid only for the unrelated donor transplantation setting.
To add new information to the still disputable association between the CMV donor/recipient serostatus with the outcome of transplantation the present study was undertaken.
2. Materials and Methods
Two hundred patients (F/M: 91/109; 26 and 174 patients were below and above 16 years of age, resp.) allografted from unrelated donors (122 patients), and 78 from sibling (SIB) donors in our institution in the years 2002–2011 were studied. One hundred and seventy-five suffered from hematological malignancies acute myeloid leukemia (AML; n = 67), chronic myeloid leukemia (CML; n = 24), acute lymphocytic leukemia (ALL; n = 39), other lymphoproliferative disorders (n = 23), myeloproliferative diseases (n = 10), and myelodysplastic syndromes (n = 12). The others were transplanted because of anemias (10 patients) and immunodeficiencies (14 patients) and osteopetrosis (n = 1).
One hundred and five and 95 patients received myeloablative (based on busulfan and cyclophosphamide) and reduced intensity conditioning (reduced busulfan dose or melphalan plus fludarabine and antithymocyte globulin (ATG)), respectively. Unrelated donor transplanted patients and those on reduced intensity conditioning received ATG (10 to 12.5 mg/kg b.w. cumulative dose, 125 patients) or alemtuzumab (90 mg as a dose, 38 patients) as a part of the conditioning regimen. All patients were on cyclosporin A with a dose adjusted to the blood CsA trough a level to 200 ng/L. CMV serostatus, age, gender, underlying disease, donor source, and HLA match as well as conditioning regimen (reduced or myeloablative) are given in Table 1.
| Number of patients | 200 |
| Age | |
| (median, range), yrs | 34, 1–60 |
| Adults > 16 yrs | 174 |
| Children ≤ 16 yrs | 26 |
| Recipient gender | |
| Female | 91 |
| Male | 109 |
| Donor gender | |
| Female | 83 |
| Male | 116 |
| Donor | |
| Sibling | 78 |
| Unrelated HLA matched (10/10 at the allele level), | 78 |
| Mismatched, at the allele or low resolution levels up to two mismatches | 44 |
| Transplant material | |
| Bone marrow (BM) | 28 |
| Peripheral blood progenitor cells (PBPC) | 172 |
| Diagnosis | |
| Hematological malignancies (HM) | 175 |
| Chronic myeloid leukemia (CML) | 24 |
| Chronic lymphocytic leukemia (CLL) | 5 |
| Acute myeloid leukemia (AML) | 67 |
| Acute lymphocytic leukemia (ALL) | 39 |
| Other HM | 40 |
| Anemias and immunodeficiencies | 24 |
| Osteopetrosis | 1 |
| Conditioning regimen | |
| Myeloablative | 105 |
| Reduced intensity conditioning (RIC) | 95 |
| Acute GvHD, grades | |
| 0 | 114 |
| I | 21 |
| II | 26 |
| III | 16 |
| IV | 23 |
| Chronic GvHD | |
| Extensive | 38 |
| Limited | 33 |
| EBV ≥100 DNA copies/105 cells | 45/187 |
| CMV ≥100 DNA copies/105 cells | 54/187 |
| HHV6 ≥100 DNA copies/105 cells | 34/187 |
| Polyoma (JC/BK) | 19/33 |
| CMV IgG serostatus | |
| Recipients | |
| CMV IgG negative | 32 |
| CMV IgG positive | 168 |
| Donors | |
| CMV IgG negative | 66 |
| CMV IgG positive | 132 |
| Recipient/donor CMV serostatus | |
| Recipient CMV IgG (+)/donor CMV IgG (+) | 118 |
| Recipient CMV IgG (−)/donor CMV IgG (−) | 18 |
| Recipient CMV IgG (+)/donor CMV IgG (−) | 48 |
| Recipient CMV IgG (−)/donor CMV IgG (+) | 14 |
The patients were routinely followed for clinical outcome in one-week intervals until 30 days posttransplant, then monthly until one year post-transplant and as well as when clinical symptoms were suggestive of CMV, EBV, or HHV6 reactivation or any other serious post-transplant complications including relapse or GvHD. Out of 200 patients studied viral CMV, EBV and HHV6 DNA copies in blood were determined in 187 recipients transplanted after the year 2003.
The Zeus Scientific, Inc. (NJ, USA), IgG and IgM ELISA test system was used for qualitative detection of CMV-specific antibodies in donors’ and recipients’ plasma. The ELISA kit was used according to the manufacturer’s instructions. Briefly, microtiter plates, precoated with inactivated CMV antigen, were incubated with the recipient or donor plasma. Bound IgG or IgM was detected with peroxidase labeled anti-IgG and anti-IgM antibodies by the addition of the color substrate and reading by spectrometry. Results were interpreted as seropositive or seronegative as per the manufacturer’s instructions.
DNA was extracted from peripheral blood (QiAmp Blood Kit; Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The numbers of CMV, EBV, and HHV6 DNA copies in peripheral blood cells were determined using real-time PCR and Light Cycler II (Roche, Mannheim, Germany). The sequences of the PCR primers and the probe were selected from the BALF5 region of EBV, the US17 region of CMV, and the U67 region of HHV6. PCRs were performed as described by Jaskula et al. [12].
2.1. Statistical Analysis
Statistical analysis was performed using the CSS Statistica for Windows (version 10.0) software (Stat-Soft Inc., Tulsa, OK). Univariate analyses were performed by the Fisher exact test. Logistic regression was used for the multivariate analysis, and alog-rank test to analyze the survival probability. Differences between samples were considered to be significant when P < 0.05 and those between 0.05 and 0.1 were indicative of a trend.
3. Results and Discussion
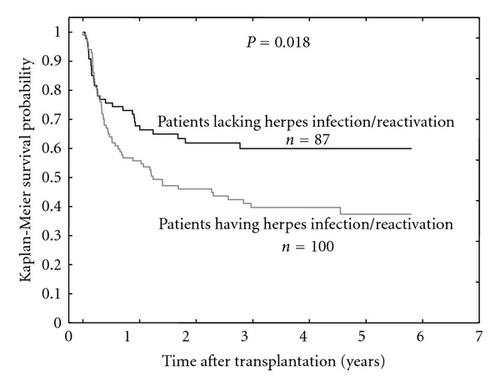
The presence of >100 CMV, EBV, and HHV6 DNA copies per 105 blood cells (clinically significant [12, 13]) was detected in 29%, 24%, and 18% of patients, respectively. Sixty out of 100 patients having during the observation period one or more reactivation events of one or more examined herpes viruses died. The mortality rate was lower in the group of patients lacking reactivations/infections (32 out of 87 patients), which resulted in a better five-year survival (59% versus 37%, P = 0.018, Figure 1).
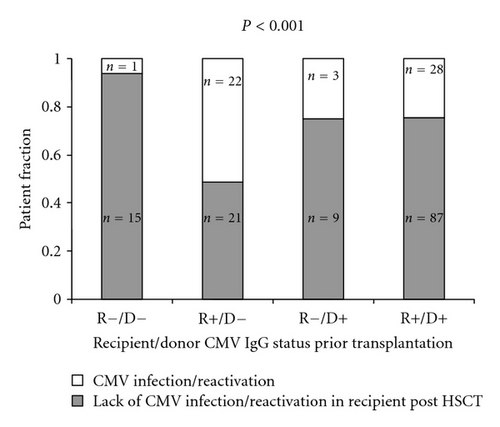
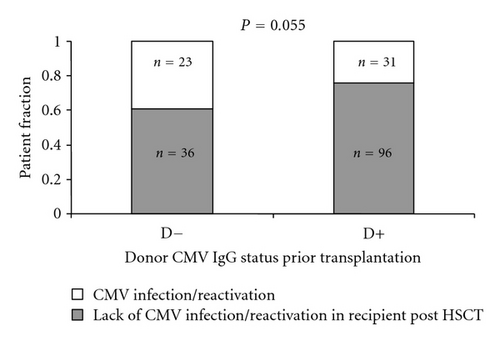
Patients receiving transplantation from the CMV IgG seronegative donors tended to suffer more frequently from CMV infection/reactivation after HSCT as compared to those grafted from CMV seropositive donors (23/59 versus 31/127, P = 0.055, Figure 2(b)). This association was valid for seropositive and seronegative recipients. However, the highest risk of CMV reactivation was when seropositive recipients were transplanted from the seronegative donors (22/43 versus 32/143, P < 0.001 Figure 2(a)). In contrast, CMV negative serostatus of both the donor and the recipient was associated with the lowest rate of the CMV reactivation (1 out of 16 patients) as compared to other recipient (R)/donor (D) CMV IgG serostatus relations, being 22/43 versus 28/115 versus 3/12, (P < 0.001) for R+/D−, R+/D+, and R−/D+, resp. (Figure 2(a)). We also found that aGvHD (grade > I) was more frequently seen in patients receiving grafts from IgG negative donors (28/66 versus 36/132, P = 0.036, Figure 2(c)). However, donor serostatus did not affect the survival of HSCT recipients (Figure 3).
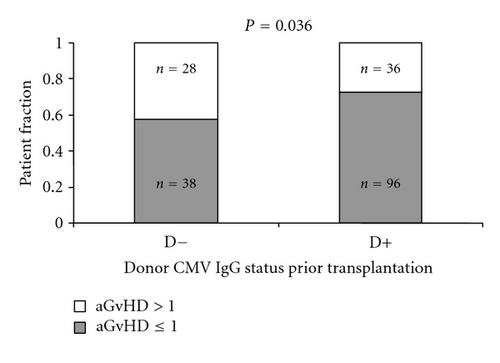
In addition to the factors associated with the serostatus of donors and recipients, a lack of optimal donor/recipient HLA matching was associated with a higher risk of grade > I aGvHD (25/44 versus 40/156, P < 0.001) and with a higher rate of CMV reactivation/infection (18/38 versus 36/149, P = 0.008). CMV reactivation was also more frequently seen in patients who were over 16 years old at the time of transplantation (52/165 versus 2/22, P = 0.042, Table 2) and in those having CMV IgG antibodies before transplantation (50/159 versus 4/28, P = 0.073, Table 2).
| Variable | aGvHD | P value | CMV absence | CMV presence | P value | |
|---|---|---|---|---|---|---|
| ≤grade I | >grade I | Infection/reactivation until 1 year post-HSCT | ||||
| Donor/recipient HLA match | ||||||
| Matched | 116 | 40 | P <0.001 | 113 | 36 | P = 0.008 |
| Mismatched at the allele or low resolution levels up to two mismatches | 19 | 25 | 20 | 18 | ||
| Source of HSCT | ||||||
| PBPC | 111 | 61 | P = 0.029 | 112 | 48 | P = 0.496 |
| BM | 24 | 4 | 21 | 6 | ||
| Type of donor | ||||||
| SIB | 65 | 13 | P <0.001 | 61 | 17 | P = 0.074 |
| MUD | 70 | 52 | 72 | 37 | ||
| Conditioning regimen | ||||||
| RIC | 67 | 28 | P = 0.450 | 60 | 30 | P = 0.202 |
| Myeloablative | 68 | 37 | 73 | 24 | ||
| Donor CMV IgG | ||||||
| CMV IgG− | 38 | 28 | P = 0.036 | 36 | 23 | P = 0.055 |
| CMV IgG+ | 96 | 36 | 96 | 31 | ||
| Recipient CMV IgG | ||||||
| CMV IgG− | 19 | 13 | P = 0.307 | 24 | 4 | P = 0.073 |
| CMV IgG+ | 116 | 52 | 109 | 50 | ||
| Donor-recipient IgG CMV serology | ||||||
| R−/D− | 10 | 8 | 15 | 1 | ||
| R+/D− | 28 | 20 | P = 0.159 | 21 | 22 | P <0.001 |
| R−/D+ | 9 | 5 | 9 | 3 | ||
| R+/D+ | 87 | 31 | 87 | 28 | ||
| R−/D−, R+/D−, R−/D+, R+/D+ | 106 | 44 | P = 0.115 | 111 | 32 | P <0.001 |
| R+/D− | 28 | 20 | 21 | 22 | ||
| Donor/recipient gender | ||||||
| Male to male, female to female, and male to female | 105 | 54 | P = 0.572 | 105 | 44 | P = 0.841 |
| Female to male | 29 | 11 | 28 | 10 | ||
| Donor gender | ||||||
| Male | 76 | 40 | P = 0.543 | 75 | 32 | P = 0.747 |
| Female | 58 | 25 | 58 | 22 | ||
| Recipient gender | ||||||
| Male | 76 | 33 | P = 0.544 | 71 | 27 | P = 0.747 |
| Female | 59 | 32 | 62 | 27 | ||
| Recipient age | ||||||
| ≤16 | 17 | 9 | P = 0.824 | 20 | 2 | P = 0.042 |
| >16 | 118 | 56 | 113 | 52 | ||
| CMV infection/reactivation event within 1 year post-HSCT | ||||||
| CMV− | 97 | 36 | P = 0.025 | |||
| CMV+ | 30 | 24 | ||||
| aGvHD | ||||||
| aGvHD ≤ grade I | 97 | 30 | P = 0.025 | |||
| aGvHD > grade I | 36 | 24 | ||||
| EBV infection/reactivation event within 1 year post HSCT | ||||||
| EBV− | 100 | 42 | P = 0.204 | 104 | 38 | P = 0.263 |
| EBV+ | 27 | 18 | 29 | 16 | ||
| HHV6 infection/reactivation event within 1 year post HSCT | ||||||
| HHV6− | 103 | 50 | P = 0.835 | 110 | 43 | P = 0.677 |
| HHV6+ | 24 | 10 | 23 | 11 | ||
- PBPC: peripheral blood progenitor cells; BM: bone marrow; R: recipient; D: donor; “−”: negative; “+”: positive; ATG: antithymocyte globulin; SIB: HLA-identical siblings; MUD: unrelated donors; RIC: reduced intensity conditioning.
Multivariate analysis devoted to the evaluation of the risk factors of aGvHD showed that unrelated donor (OR = 2.591, P = 0.036) transplantation and HLA mismatch (OR = 2.361, P = 0.042) appeared as independent and significant factors associated with aGvHD grade > I (Table 3). In spite of the univariate results multivariate analysis did not confirm the role of CMV reactivation and donor serology as independent factors associated with aGvHD (Table 3).
| Variable | Coefficient | P value | Odds ratio | 95% CI |
|---|---|---|---|---|
| CMV infection/reactivation event within 1 year post HSCT | 0.5473 | 0.1362 | 1.7286 | 0.8415 to 3.5509 |
| CMV IgG in donor serum | 0.0290 | 0.9411 | 1.0295 | 0.4762 to 2.2254 |
| Donor-recipient HLA mismatch | 0.8591 | 0.0421 | 2.3611 | 1.0310 to 5.4072 |
| Unrelated donor | 0.9520 | 0.0355 | 2.5909 | 1.0669 to 6.2921 |
| BM as a source of cells | −0.6177 | 0.3090 | 0.5392 | 0.1640 to 1.7723 |
The next statistical approach was to validate factors associated with CMV reactivation. For that also a multivariate analysis was calculated employing factors as follows: recipient IgG serology, donor-recipient HLA mismatch, transplantation recipient in CMV IgG positive/donor CMV IgG negative serology, type of donors, recipient age, and aGvHD. Among the above factors donor-recipient HLA mismatch (OR = 3.499, P = 0.016), recipient CMV IgG positive/donor CMV IgG negative serology status constellation (OR = 23.030, P = 0.023), and recipient age over 16 years (OR = 9.865, P = 0.007) were found to be significant risk factors of CMV reactivation (Table 4).
| Variable | Coefficient | P value | Odds ratio | 95% CI |
|---|---|---|---|---|
| Recipient CMV IgG seronegativity | −0.0761 | 0.9224 | 0.9267 | 0.2000 to 4.2929 |
| Donor/recipient HLA mismatch | 1.2525 | 0.0155 | 3.4992 | 1.2695 to 9.6446 |
| R CMV IgG+/D CMV IgG− | 3.1370 | 0.0227 | 23.0340 | 1.5491 to 342.4999 |
| Unrelated donor | 0.0021 | 0.9965 | 1.0021 | 0.3904 to 2.5722 |
| aGvHD > 1 | 0.5363 | 0.1755 | 1.7096 | 0.7870 to 3.7141 |
| Recipient age >16 years | 2.2890 | 0.0072 | 9.8650 | 1.8606 to 52.3036 |
To further analyze the significance of CMV serology as a risk factor of CMV reactivation similar to that above, analysis was performed for groups consisting of SIB and MUD 10/10 matched and MUD not optimally matched (Tables 5 and 6). On agroup basis independently of the CMV serostatus of donor-recipient pairs, sibling transplantations and those from unrelated donors matched 10/10 at allele level had asimilar rate of CMV reactivation (17/78 versus 19/71, P = ns). Notably, the rate of CMV reactivation was higher in patients grafted from donors accepted at the lower level of matching than 10/10 (18/38 versus 36/149, P = 0.008). Also when we considered separately the optimal match group (SIB + MUD) the highest risk of CMV reactivation was observed when donors were negative but recipients were positive (12/28 versus 24/120, P = 0.015). In MUD HLA mismatched recipients a tendency to the association seropositivity of recipients with concomitant seronegativity of donors with the CMV reactivation/infection was observed (10/15 versus 8/23, P = 0.096). Notably in the MUD HLA mismatch group recipient age >16 years was a risk factor for CMV reactivation (17/28 versus 1/10, P = 0.009, Table 5). There were no significant associations between aGvHD and variables considered in this paper in the optimally matched group (SIB + 10/10 HLA matched) and in the MUD HLA mismatched group of patients.
| Optimally matched group (SIB+ 10/10 HLA matched) of patients | MUD HLA mismatched group of patients | |||||
|---|---|---|---|---|---|---|
| Variable | CMV absence | CMV presence | P value | CMV absence | CMV presence | P value |
| Infection/reactivation until 1 year post HSCT | Infection/reactivation until 1 year post HSCT | |||||
| Source of HSCT | ||||||
| PBPC | 95 | 30 | 1.000 | 17 | 18 | 0.232 |
| BM | 18 | 6 | 3 | 0 | ||
| Conditioning regimen | ||||||
| Absence of ATG and Campath | 33 | 5 | 0.167 | 0.170 | ||
| ATG | 60 | 22 | 19 | 14 | ||
| Campath | 20 | 9 | 1 | 4 | ||
| RIC | 53 | 23 | 0.087 | 7 | 7 | 1.000 |
| Myeloablative | 60 | 13 | 13 | 11 | ||
| Donor CMV IgG | ||||||
| CMV IgG− | 26 | 12 | 0.273 | 10 | 11 | 0.532 |
| CMV IgG+ | 86 | 24 | 10 | 7 | ||
| Recipient CMV IgG | ||||||
| CMV IgG− | 18 | 2 | 0.160 | 6 | 2 | 0.238 |
| CMV IgG+ | 95 | 34 | 14 | 16 | ||
| Donor-recipient IgG CMV serology | ||||||
| R−/D− | 10 | 0 | 5 | 1 | ||
| R−/D+ | 8 | 2 | 0.032 | 1 | 1 | 0.18 |
| R+/D− | 16 | 12 | 5 | 10 | ||
| R+/D+ | 78 | 22 | 9 | 6 | ||
| R−/D−, R−/D+, R+/D+ | 96 | 24 | 0.015 | 15 | 8 | 0.096 |
| R+/D− | 16 | 12 | 5 | 10 | ||
| Donor/recipient gender | ||||||
| Male to male, female to female, and male to female | 67 | 23 | 0.698 | 13 | 14 | 0.485 |
| Female to male | 46 | 13 | 7 | 4 | ||
| Donor gender | ||||||
| Male | 67 | 23 | 0.569 | 8 | 9 | 0.746 |
| Female | 46 | 13 | 12 | 9 | ||
| Recipient gender | ||||||
| Male | 59 | 21 | 0.698 | 12 | 6 | 0.112 |
| Female | 54 | 15 | 8 | 12 | ||
| Recipient age | ||||||
| ≤16 | 11 | 1 | 0.290 | 9 | 1 | 0.009 |
| >16 | 102 | 35 | 11 | 17 | ||
| aGvHD | ||||||
| aGvHD ≤ grade I | 88 | 23 | 0.123 | 9 | 7 | 0.752 |
| aGvHD > grade I | 25 | 13 | 11 | 11 | ||
| Variable | Optimally matched group (SIB+ 10/10 HLA matched) of patients | MUD HLA mismatched group of patients | ||||||
|---|---|---|---|---|---|---|---|---|
| Coefficient | P value | Odds ratio | 95% CI | Coefficient | P value | Oddsratio | 95%CI | |
| aGvHD > 1 | 0.7265 | 0.1015 | 2.0679 | 0.8667 to 4.9336 | 0.0185 | 0.9816 | 1.0187 | 0.2113 to 4.9106 |
| Recipient CMV IgG seronegativity | 0.9058 | 0.2601 | 2.474 | 0.5113 to 11.9711 | 0.5751 | 0.6126 | 1.7773 | 0.1918 to 16.4703 |
| R CMV IgG+/D CMV IgG− | 0.9819 | 0.0374 | 2.6695 | 1.0587 to 6.7314 | 1.6719 | 0.0776 | 5.3222 | 0.8313 to 34.0733 |
| RIC conditioning regimen | −0.5655 | 0.1745 | 0.5681 | 0.2511 to 1.2849 | 0.30101 | 0.7333 | 1.3513 | 0.2391 to 7.6375 |
| Recipient age >16 years | 1.2945 | 0.2318 | 3.6492 | 0.4371 to 30.4624 | 3.1026 | 0.0148 | 22.256 | 1.8360 to 269.7835 |
Multivariate analysis results of patients optimally matched and separately those not optimally matched were similar and revealed that among factors analyzed for the risk of CMV reactivation seropositivity of recipients with concomitant seronegativity of donors plays an independent role (OR = 2.670, P = 0.037) for optimally matched and as tendency in HLA mismatched patients (OR = 5.322, P = 0.078, Table 6).
4. Conclusions
The information provided in the present paper shows that IgG negativity in donors favors the outcome of HSCT only when recipients are also CMV IgG negative. The worst is when donor IgG CMV negativity is confronting IgG CMV positivity in recipients. This confirms the importance of CMV IgG positivity likely associated with the immune competence of donors [3–5], which is of a special value in seropositive patients, very likely having CMV in alatent form [6]. Therefore, when recipients are IgG CMV positive the immune competence of donors is required to reduce the risk of CMV reactivation. This observation can be used as one of the factors that should be considered during donor selections for an optimal post-HSCT outcome.
Acknowledgment
This work was supported by Grant N R13 0082 06 from the Polish Ministry of Science and Higher Education.