Millimeter-Wave Rotational Spectra of trans-Acrolein (Propenal) (CH2CHCOH): A DC Discharge Product of Allyl Alcohol (CH2CHCH2OH) Vapor and DFT Calculation
Abstract
Millimeter-wave rotational spectrum of trans-acrolein (propenal) (CH2CHCOH) produced by applying a DC glow discharge through a low-pressure (~10–20 mTorr) flow of allyl alcohol (CH2CHCH2OH) vapor has been observed in the ground and several excited torsional states in the frequency region: 60.0–99.0 GHz. A least-square analysis of the measured and previously reported rotational transition frequencies has produced a set of rotational and centrifugal distortion constants for the ground as well as excited torsional states. Detailed DFT calculations were also carried out with various functional and basis sets to evaluate the spectroscopic constants, dipole moment, and various structural parameters of the trans conformer of propenal for the ground state and compared with their corresponding experimental values. A linear variation of the inertia defect values with torsional quantum number (v = 0, 1, 2, 3) demonstrates that the equilibrium configuration of trans-propenal is planar.
1. Introduction
The trans form of propenal (CH2CHCOH) also known as trans-acrolein has been detected largely in absorption toward the star-forming region Sagittarius B2(N) by Hollis et al. [2] through the observation of rotational transitions using 100 m Green Bank Telescope (GBT) operating in the frequency range from 18.0 GHz to 26.0 GHz. Spectroscopic measurements in the microwave [1, 3], infrared [4], and near ultraviolet region [5, 6] have confirmed that the trans-form is the most abundant and stable conformer of acrolein. The first microwave study of trans-acrolein in the J = 2←1, 3←2, and 4←3 a-type R-branch transitions was reported by Wagner et al. [3]. Later on, Cherniak and Costain [1] have measured both a- and b-type transitions for J = 2←1 and J = 3←2. First spectroscopic evidence of the existence of the less abundant cis-conformer of acrolein in the gas phase was found from studies of the near ultraviolet spectrum [7, 8]. Later on, cis-acrolein were detected in argon matrices [9, 10] and in the gas-phase Raman spectrum [11]. The first microwave detection of the cis form of acrolein in the gas phase was reported by Blom and Bauder [12]. They have reported the ground state rotational, quartic centrifugal distortion constants as well as dipole moment values. Blom et al. [13] also reported the complete substitution structures of both trans and cis conformers. The dipole moment values of the trans and cis-form of acrolein have been found to be μ = 3.117 ± 0.004 D [13] and μ = 2.552 ± 0, 003 D [12] respectively. Winnewisser [14] have extended the analysis of the ground state of the trans-form of acrolein to the millimeter-wave region up to 180.0 GHz which has yielded a set of ground state rotational and centrifugal distortion constants. Analysis of the far-infrared spectrum of trans acrolein in the ν18 fundamental and (ν17 + ν18) − ν18 hot bands were reported by McKellar et al. [15]. Very recently, 10 μm high-resolution rotational spectral analysis of the ν11, ν16, ν14 and ν16 + ν18 − ν18 bands of trans-acrolein were reported by Xu et al. [16]. In all the previous works trans-acrolein (propenal) was either procured commercially or prepared chemically.
Production, identification, and spectroscopic characterization of new stable and transient molecules by applying a DC glow discharge through a low-pressure flow of gas or a mixture of gases inside an absorption cell have become a well-established area of research in the field of molecular spectroscopy [21]. Recently, Jaman et. al have reported analysis of the millimeter-wave rotational spectra of propyne (CH3CCH) [22] and propynal (HCCCOH) [23] produced by DC glow discharge technique and carried out detailed DFT calculations for both the molecules to evaluate the spectroscopic constants and molecular parameters and compared them with their respective experimental values. In the present communication, we report the analysis of the ground state (v = 0) as well as several torsional excited states (v = 1,2, 3) rotational spectra of trans-propenal produced by a DC glow discharge through a low-pressure flow of allyl alcohol (CH2CHCH2OH) vapor in the frequency region 60.0–99.0 GHz. Asymmetric-top K−1 K+1-structures of different J+1←J transitions which falls under this frequency range have been observed and measured. The measured rotational transition frequencies along with the previously reported frequencies were fitted to standard asymmetric-top Hamiltonian to determine the rotational and centrifugal distortion (CD) constants for the ground as well as excited torsional states. A detailed quantum chemical calculation was also carried out to evaluate the spectroscopic constants, dipole moment, and the structural parameters of the trans conformer of propenal. Finally, the experimentally determined rotational and CD constants were compared with the best set of values obtained after a series of DFT calculations.
2. Experimental Details
The spectrometer used in the present work is basically a 50 kHz source-modulated system combined with a free space glass discharge cell of 1.5 m in length and 10 cm in diameter. The cell is fitted with two Teflon lenses at each end. A high voltage DC regulated power supply (6 kV, 1300 mA) procured from Glassman, Japan was used to apply a DC voltage through a flow of low pressure precursor gases. The cell is connected with a high vacuum pump at one end and to the sample holder section through a glass port on the other.
Klystrons and Gunn diodes followed by frequency doubler (Millitech model MUD-15-H23F0 and MUD-10-LF000) have been used as radiation sources. Millimeter wave radiation was fed into the cell by a waveguide horn and Teflon lens. A similar horn and lens arrangement was used to focus the millimeter-wave power onto the detector after propagating through the cell. The output frequency of the millimeter wave radiation was frequency modulated by a bidirectional square-wave of 50 kHz [24] and the signal from the detector (Millitech model DBT-15-RP000 and DXP-10-RPFW0) was amplified by a 100 kHz tuned preamplifier and detected by a phase-sensitive lock in amplifier in the 2f mode. The output of the lock in amplifier was connected to an oscilloscope or a chart recorder for signal display. The spectrometer was calibrated by measuring standard OCS signals in the entire frequency range. After calibration, the uncertainty in frequency measurement has been estimated to be ±0.10 MHz. A block diagram of the spectrometer is shown in Figure 1. Details of the spectrometer used have been described elsewhere [25, 26].
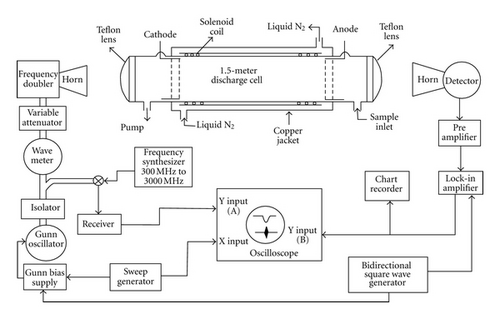
Propenal (CH2CHCOH) was produced inside the absorption cell by applying a DC glow discharge through a low pressure (~5–10 mTorr) flow of allyl alcohol (CH2CHCH2OH) vapor. The discharge current was maintained at around 5 mA with an applied voltage of 1.0 kV. A mechanical on/off type discharge was found to be suitable to observe good signals of propenal. Signals could be observed at room temperature. However, a controlled flow of liquid nitrogen vapor through the cell helps in improving the signal intensity. The observed signals of propenal appeared as sharp lines immediately after the DC discharge was applied but started losing intensity with time.
3. Computational Method
Quantum chemical computations were performed using GAUSSIAN 09W package [27]. Density functional methods with various functionals were used to calculate the structural parameters, dipole moment, total energy (sum of electronic and zero point energy) as well as the rotational and centrifugal distortion constants of trans-propenal. The geometry optimization was carried out using different functionals like Becke 3-term correlation functional(B3LYP) with basis sets 6-31+g(d, p) to 6-311++g(d, 2p), Becke three-Parameter hybrid functional and Perdew/Wang 91 nonlocal correlation functional (B3PW91) method with different basis sets from 6-31 g to 6-311++g(d, 2p), modified Perdew-Wang one-parameter hybrid model taking basis sets from 6–31 g to 6-311++g(d, 2p) and Perdew, Burke, and Ernzerhof functional (PBEPBE) with the basis sets 6-311 g to 6-311++g(d, 2p). The frequency calculation along with its anharmonicity was done on optimized geometry. The objective of this DFT calculation is to compare the structural parameters and rotational constants of trans-propenal with the experimentally observed values in its ground state. The molecular drawing is done by using GAUSSVIEW5.0 [28].
4. Rotational Spectrum and Analysis
4.1. Ground State
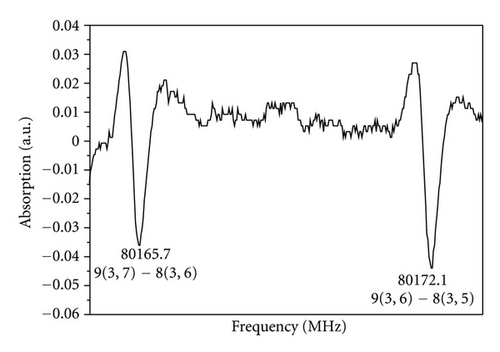
The ground state rotational spectrum of the trans conformer of propenal was predicted in the frequency range 60.0-99.0 GHz using the rotational and centrifugal distortion constants reported earlier [14]. J = 7←6 to J = 11←10 series of transitions along with their different K−1 K+1 components falls within this frequency range. Different components in each J+1←J series were measured. The observed lines were found very close to their predicted values. Finally, 224 a- and b-type R- and Q-branch transitions consisting of all previous microwave [1, 3], millimeter-wave [14], and present data were used to perform a kind of global fit to the semirigid rotor Watson’s S-reduction Hamiltonian (Ir-representation) [29] to determine a set of three rotational, five quartic, and two sextic centrifugal distortion constants. The shifts in frequency of the absorption lines from their rigid rotor positions due to centrifugal distortion effect were found to be less than that of propynal [23]. The observed and measured transition frequencies by us corresponding to J = 7←6 to 11←10 series are listed in Table 1. The ground state spectroscopic constants obtained for trans-propenal using the global fit are listed in Table 2. The small negative value of the inertia defect (Δ = − 0.018 uÅ2) demonstrates that the equilibrium configuration of trans-propenal is planar. The agreement between the derived set of spectroscopic constants and those obtained earlier [1, 3, 14] with commercial samples indicates that the newly assigned transition frequencies of Table 1 definitely belong to trans-acrolein (trans-propenal), a discharge product of allyl alcohol vapor. Figure 2 shows the observed trace of the K−1 = 3 doublet of J = 9←8 transition immediately after the DC discharge was applied. The trace remained visible for a couple of minutes on the oscilloscope screen with gradually diminishing intensity.
| Transitions | Torsional levels | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| J′ | J′′ | v = 0 | v = 1 | v = 2 | v = 3 | ||||||||
| Obs. Freq. | Obs. − cal. | Obs. Freq. | Obs. − cal. | Obs. Freq. | Obs. − cal. | Obs. Freq. | Obs. − cal. | ||||||
| 1 | 0 | 1 | 0 | 0 | 0 | 8902.19 | −.01 | ||||||
| 2 | 0 | 2 | 1 | 0 | 1 | 17801.28 | −.06 | ||||||
| 2 | 1 | 1 | 1 | 1 | 0 | 18221.12 | −.07 | 18258.18 | −.12 | 18289.86 | −.01 | ||
| 3 | 0 | 3 | 2 | 0 | 2 | 26694.35 | −.01 | 26824.88 | .01 | 26895.18 | .05 | ||
| 3 | 0 | 3 | 2 | 1 | 2 | 26765.43 | −.09 | 27487.70 | .06 | ||||
| 3 | 1 | 2 | 2 | 1 | 1 | 27329.73 | −.07 | 27385.58 | .08 | 27432.89 | .02 | 26322.50 | −.07 |
| 3 | 1 | 3 | 2 | 1 | 2 | 26079.50 | −.01 | 26165.84 | −.05 | 26237.28 | −.12 | ||
| 3 | 2 | 1 | 2 | 2 | 0 | 26718.70 | −.16 | ||||||
| 3 | 2 | 2 | 2 | 2 | 1 | 26706.76 | .04 | ||||||
| 4 | 0 | 4 | 3 | 0 | 3 | 35673.16 | −.09 | 35752.72 | .17 | 35846.64 | .15 | ||
| 4 | 1 | 4 | 3 | 1 | 3 | 34768.96 | −.11 | ||||||
| 7 | 0 | 7 | 6 | 0 | 6 | 62144.50a | .01 | 62312.22a | .01 | 62452.30a | −.11 | ||
| 7 | 1 | 7 | 6 | 1 | 6 | 60816.60a | .04 | 61018.40a | .02 | ||||
| 7 | 2 | 6 | 6 | 2 | 5 | 62290.80a | −.06 | 62456.80a | −.03 | 62597.20a | .05 | 62760.10a | −.02 |
| 7 | 2 | 5 | 6 | 2 | 4 | 62460.30a | −.05 | 62624.20a | −.08 | 62762.20a | −.07 | 62922.30a | .03 |
| 7 | 3 | 4 | 6 | 3 | 3 | 62341.20a | −.01 | 62506.50a | −.08 | 62648.90a | .00 | ||
| 7 | 3 | 5 | 6 | 3 | 4 | 62339.40a | −.07 | 62504.80a | −.04 | 62647.20a | .03 | ||
| 7 | 4 | 3 | 6 | 4 | 2 | 62331.10a | .02 | 62496.50a | −.06 | ||||
| 7 | 5 | 3 | 6 | 5 | 2 | 62493.40a | .03 | ||||||
| 8 | 0 | 8 | 7 | 0 | 7 | 70961.80a | .00 | 71154.30a | .10 | 71315.20a | .04 | 71506.60a | −.01 |
| 8 | 1 | 7 | 7 | 1 | 6 | 72820.00a | −.01 | 72969.40a | .03 | 73096.20a | .09 | 73243.30a | −.06 |
| 8 | 1 | 8 | 7 | 1 | 7 | 69489.80a | .06 | 69720.40a | −.12 | 69911.60a | .01 | 70139.80a | −.08 |
| 8 | 2 | 7 | 7 | 2 | 6 | 71179.00a | .07 | 71529.20a | .07 | ||||
| 8 | 2 | 6 | 7 | 2 | 5 | 71432.70a | .12 | 71619.40a | .08 | 71776.20a | −.03 | 71958.30a | .04 |
| 8 | 3 | 6 | 7 | 3 | 5 | 71251.50a | .06 | 71440.40a | .05 | ||||
| 8 | 3 | 5 | 7 | 3 | 4 | 71255.00a | .08 | 71443.90a | .07 | 71606.10a | −.17 | ||
| 8 | 4 | 4 | 7 | 4 | 3 | 71239.10a | .05 | 71428.20a | .09 | ||||
| 8 | 5 | 4 | 7 | 5 | 3 | 71423.10a | .12 | ||||||
| 8 | 6 | 3 | 7 | 6 | 2 | 71421.10a | −.06 | ||||||
| 9 | 0 | 9 | 8 | 0 | 8 | 80371.70a | −.01 | ||||||
| 9 | 1 | 8 | 8 | 1 | 7 | 81901.20 | .01 | 82069.50a | −.01 | 82212.30a | .06 | ||
| 9 | 2 | 8 | 8 | 2 | 7 | 80062.80a | .04 | 80276.30a | −.11 | 80457.00a | .08 | 80667.10a | .09 |
| 9 | 2 | 7 | 8 | 2 | 6 | 80424.00a | .07 | 80633.30a | .06 | 80808.70a | −.05 | 81012.40a | −.08 |
| 9 | 3 | 6 | 8 | 3 | 5 | 80172.10a | .05 | 80384.50a | .04 | ||||
| 9 | 3 | 7 | 8 | 3 | 6 | 80165.70a | .03 | 80378.20a | .12 | ||||
| 9 | 4 | 5 | 8 | 4 | 4 | 80148.40a | −.04 | 80361.10a | .04 | ||||
| 9 | 5 | 5 | 8 | 5 | 4 | 80140.70a | .08 | 80353.40a | .06 | ||||
| 9 | 6 | 4 | 8 | 6 | 3 | 80137.50a | −.01 | 80350.40a | .06 | ||||
| 10 | 0 | 10 | 9 | 0 | 9 | 88523.30a | −.01 | 88765.90a | −.05 | 89211.90a | −.12 | ||
| 10 | 1 | 9 | 9 | 1 | 8 | 90974.40a | −.05 | ||||||
| 10 | 1 | 10 | 9 | 1 | 9 | 86818.50a | −.02 | ||||||
| 10 | 2 | 8 | 9 | 2 | 7 | 89436.20a | −.01 | 89667.83a | .03 | 89861.60a | −.02 | ||
| 10 | 2 | 9 | 9 | 2 | 8 | 88941.80a | −.01 | 89179.48a | .10 | 89380.10a | .07 | 89613.80a | −.02 |
| 10 | 3 | 7 | 9 | 3 | 6 | 89093.20a | −.01 | 89329.07a | −.01 | ||||
| 10 | 3 | 8 | 9 | 3 | 7 | 89082.20a | −.08 | ||||||
| 10 | 4 | 6 | 9 | 4 | 5 | 89059.35a | −.09 | 89295.47a | −.09 | ||||
| 10 | 5 | 5 | 9 | 5 | 4 | 89048.30a | −.01 | ||||||
| 10 | 6 | 4 | 9 | 6 | 3 | 89043.55a | −.04 | ||||||
| 10 | 7 | 3 | 9 | 7 | 2 | 89041.90a | −.02 | ||||||
| 10 | 9 | 1 | 9 | 9 | 0 | 89043.17a | −.02 | ||||||
| 11 | 0 | 11 | 10 | 0 | 10 | 97263.13a | −.01 | 97531.40a | −.03 | 97756.50a | −.12 | 98025.70a | .05 |
| 11 | 1 | 11 | 10 | 1 | 10 | 95473.05a | −.03 | 95791.10a | .01 | 96054.40a | .14 | 96369.80a | .04 |
| 11 | 2 | 9 | 10 | 2 | 8 | 98470.83a | −.01 | 98724.30a | −.11 | 98936.00a | .02 | ||
| 11 | 2 | 10 | 10 | 2 | 9 | 97815.59a | .03 | 98077.10a | .00 | 98555.60a | .08 | ||
| 11 | 3 | 8 | 10 | 3 | 7 | 98019.04a | −.02 | 98278.40a | .05 | 98500.60a | .03 | 98752.70a | −.03 |
| 11 | 3 | 9 | 10 | 3 | 8 | 98001.32a | .00 | 98260.60a | .01 | 98482.90a | −.06 | 98735.30a | −.01 |
| 11 | 4 | 8 | 10 | 4 | 7 | 98231.70a | −.08 | ||||||
| 11 | 5 | 7 | 10 | 5 | 6 | 97957.00a | .00 | 98216.90a | −.05 | 98453.50a | .04 | ||
| 11 | 6 | 5 | 10 | 6 | 4 | 97950.28a | .00 | 98456.20a | −.01 | ||||
| 11 | 7 | 4 | 10 | 7 | 3 | 97947.54a | .01 | ||||||
| 11 | 8 | 3 | 10 | 8 | 2 | 97947.05a | .04 | ||||||
| 1 | 1 | 1 | 2 | 0 | 2 | 24892.58 | .01 | ||||||
| 2 | 1 | 2 | 3 | 0 | 3 | 15585.86 | .01 | ||||||
| 6 | 0 | 6 | 5 | 1 | 5 | 13444.27 | .02 | ||||||
| 7 | 0 | 7 | 6 | 1 | 6 | 23450.46 | .01 | ||||||
| 8 | 0 | 8 | 7 | 1 | 7 | 33595.68 | −.01 | ||||||
- aThis work, rest are from [1].
| Constants | Global fit using microwave and millimeter wave data | DFT calculation |
|---|---|---|
| A (MHz.) | 47353.729 ± 0.009 | 47532.149 |
| B (MHz.) | 4659.4894 ± 0.0004 | 4635.391 |
| C (MHz.) | 4242.7034 ± 0.0004 | 4223.524 |
| DJ (kHz) | 1.031 ± 0.001 | 0.983 |
| DJK (kHz) | −8.684 ± 0.006 | −9.099 |
| DK (kHz) | 361.949 ± 0.963 | 346.316 |
| d1 (kHz) | −0.1197 ± 0.0002 | −0.119 |
| d2 (kHz) | −0.0069 ± 0.0001 | −0.006 |
| HJK (Hz) | 0.014 ± 0.012 | |
| HKJ (Hz) | −0.490 ± 0.021 | |
| σb | 0.041 | |
| κc | −0.9806 | |
| Δd | −0.018 | |
| Ne | 224 |
- bStandard deviation of the overall fit.
- cAsymmetry parameter.
- dInertia defect Δ = Ic − Ib − Ia.
- eNumber of transitions used in the fit.
4.2. Excited Torsional States
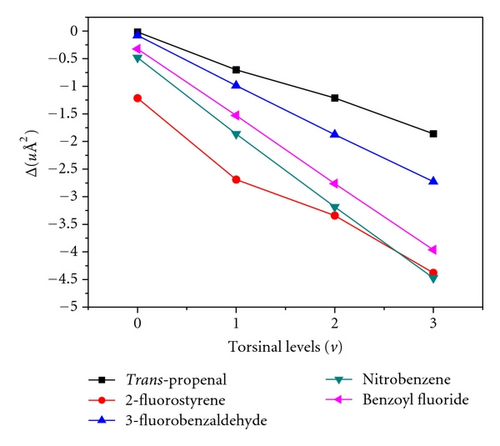
From an analysis of the ultraviolet [5] and far infrared spectrum [4] of acrolein vapor the first four excited torsional levels were found to lie around 157 cm−1 (v = 1), 312 cm−1 (v = 2), 468 cm−1 (v = 3) and 623 cm−1 (v = 4), respectively. Wagner et al. [3] have reported a few low J transitions of trans- acrolein in the 18.0–36.0 GHz for the first three (v = 1, v = 2 and v = 3) excited torsional states and determined only the rotational constants B and C for each of these excited states. In this work, we have extended the analysis of rotational transitions in each of the above three excited states up to 99.0 GHz which has resulted in the determination of three rotational and two quartic centrifugal distortion constants for all the three torsional excited states. The new assigned transitions along with those reported earlier [3] are also shown in Table 1 along with the ground state transitions. The excited state data were also used to fit to the same semirigid rotor Watson’s S-reduction Hamiltonian (Ir-representation) [29]. Three rotational and two quartic (DJ and DJK) CD constants were used to fit the data. The contribution of other CD parameters was found to be negligible while fitting the excited state data. The derived spectroscopic constants and inertia defect values for the three torsional excited states are shown in Table 3. The more negative inertia defect values for successive torsional excited states indicate that the excited state lines arise from an out-of-plane vibration, in this case, COH group torsion about C–C single bond. The observed inertia defect values for the ground and torsional excited states of trans-propenal and some other related molecules are compared in Table 4. The plots of inertia defect values with torsional quantum numbers for trans-propenal along with other molecules are shown in Figure 3 for comparison.
| Constants | v = 1 | v = 2 | v = 3 | |||
|---|---|---|---|---|---|---|
| This work | Ref. [3] | This work | Ref. [3] | This work | Ref. [3] | |
| A (MHz.) | 45782.822 ± 3.231 | 44727.881 ± 3.873 | 43420.393 ± 5.888 | |||
| B (MHz.) | 4666.210 ± 0.004 | 4666.19 ± 02 | 4672.056 ± 0.005 | 4672.10 ± 0.02 | 4678.661 ± 0.006 | 4678.69 ± 0.02 |
| C (MHz.) | 4259.668 ± 0.005 | 4259.66 ± 0.02 | 4273.558 ± 0.006 | 4273.56 ± 0.02 | 4290.297 ± 0.007 | 4290.29 ± 0.02 |
| DJ (kHz) | 1.078 ± 0.012 | 1.280 ± 0.017 | 1.168 ± 0.026 | |||
| DJK (kHz) | −8.735 ± 0.075 | −46.831 ± 0.112 | −28.714 ± 0.631 | |||
| σf | 0.077 | 0.085 | 0.075 | |||
| κg | −0.9804 | −0.9803 | −0.9802 | |||
| Δh | −0.702 | −1.212 | −1.861 | |||
| Ni | 43 | 28 | 20 | |||
- fStandard deviation of the overall fit.
- gAsymmetry parameter.
- hInertia defect Δ = Ic − Ib − Ia.
- iNumber of transitions used in the fit.
| Molecules | Inertia defect (Δ/uÅ2) values | |||
|---|---|---|---|---|
| v = 0 | v = 1 | v = 2 | v = 3 | |
| Trans-propenalj | −0.018 | −0.702 | −1.212 | −1.861 |
| (CH2CHCHO) | ||||
| o-cis 3-fluorobenzaldehydek | −0.078 | −0.988 | −1.876 | −2.726 |
| (C6H5FCOH) | ||||
| Nitrobenzenel | −0.481 | −1.863 | −3.186 | −4.470 |
| (C6H5NO2) | ||||
| Benzoyl fluoridem | −0.325 | −1.528 | −2.765 | −3.963 |
| (C6H5COF) | ||||
| 2-Fluorpstyrenen | −1.215 | −2.689 | −3.341 | −4.380 |
| (C6H4FC2H3) | ||||
4.3. Computational Results
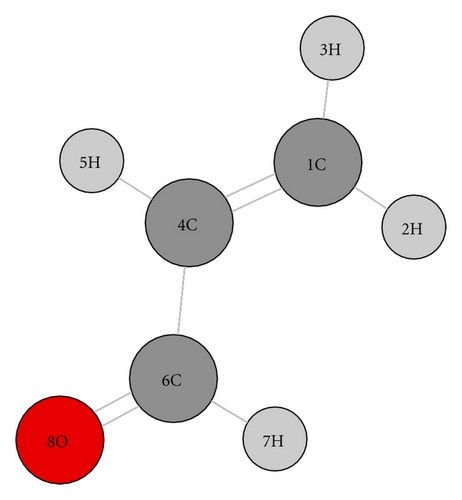
Propenal is a slightly asymmetric prolate top molecule (κ = − 0.9806). The optimization of geometry for the trans conformer of propenal was tested by employing various levels of theory and basis sets. However, the computed rotational and centrifugal distortion constants and the structural parameters obtained with model MPW1PW91 model with 6-31++g(d, 2p) basis set were found to be in good agreement with the observed values. Calculated values of ground state rotational constants of trans-propenal obtained with various models and basis sets are shown in Table 5. Results obtained with DFT MPW1PW91/6-31++g(d, 2p) have been compared with the corresponding experimental values in Table 2. For optimized geometry of trans-propenal the calculated energy is −5219.204 eV and the dipole moment is 3.481 D. The number and labeling of atoms in propenal molecule as shown in Figure 4. Bond lengths and angles have been computed using different models and basis sets and are shown in Tables 6 and 7, respectively.
| Model | Basis set | A0 (MHz) | B0 (MHz) | C0 (MHz) |
|---|---|---|---|---|
| DFT | ||||
| B3LYP | 6-31+g(d, p) | 47421.368 | 4599.276 | 4189.265 |
| B3LYP | 6-31++g(d, 2p) | 47462.833 | 4596.304 | 4190.461 |
| B3LYP | 6-311++g(d, 2p) | 47713.427 | 4621.512 | 4213.560 |
| B3PW91 | 6-31g | 47562.896 | 4564.835 | 4164.742 |
| B3PW91 | 6-31++g(d, 2p) | 47445.030 | 4617.117 | 4207.640 |
| B3PW91 | 6-311++g(d, 2p) | 47640.454 | 4642.978 | 4230.737 |
| MPW1PW91 | 6-31g | 47680.556 | 4583.545 | 4181.241 |
| MPW1PW91 | 6-31++g(d, 2p) | 47532.149 | 4635.391 | 4223.524 |
| MPW1PW91 | 6-311++g(d, 2p) | 47719.894 | 4660.612 | 4246.017 |
| PBEPBE | 6-311g | 46829.581 | 4528.525 | 4119.901 |
| PBEPBE | 6-311++g(d, 2p) | 46855.368 | 4594.974 | 4175.330 |
| PBEPBE | 6-31++g(d, 2p) | 46605.708 | 4567.810 | 4150.825 |
| Expt.o | 47353.729 | 4659.4894 | 4242.7034 |
- oThis work.
| Models | Basis sets | Bond lengths between | Dipole Moment (D) | Energy (eV) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1C–2H | 1C–3H | 1C–4C | 4C–5H | 4C–6C | 6C–7H | 6C–8O | ||||
| 6-31+g(d, p) | 1.088 | 1.085 | 1.340 | 1.087 | 1.474 | 1.112 | 1.218 | 3.515 | −5220.511 | |
| B3LYP | 6-31++g(d, 2p) | 1.087 | 1.084 | 1.340 | 1.086 | 1.474 | 1.111 | 1.218 | 3.502 | −5220.755 |
| 6-311++g(d, 2p) | 1.084 | 1.081 | 1.335 | 1.083 | 1.474 | 1.109 | 1.211 | 3.464 | −5221.979 | |
| 6-31g | 1.088 | 1.084 | 1.341 | 1.086 | 1.465 | 1.106 | 1.239 | 3.542 | −5216.647 | |
| B3PW91 | 6-31++g(d, 2p) | 1.085 | 1.082 | 1.334 | 1.084 | 1.471 | 1.111 | 1.208 | 3.491 | −5218.334 |
| 6-311+g(d, 2p) | 1.085 | 1.082 | 1.334 | 1.084 | 1.417 | 1.111 | 1.208 | 3.434 | −5219.694 | |
| 6-31g | 1.086 | 1.083 | 1.339 | 1.085 | 1.463 | 1.104 | 1.236 | 3.564 | −5217.218 | |
| MPW1PW91 | 6-31++g(d, 2p) | 1.086 | 1.083 | 1.336 | 1.085 | 1.470 | 1.110 | 1.213 | 3.481 | −5219.204 |
| 6-311++g(d, 2p) | 1.084 | 1.081 | 1.332 | 1.083 | 1.469 | 1.109 | 1.205 | 3.443 | −5220.238 | |
| 6-311g | 1.093 | 1.090 | 1.348 | 1.092 | 1.467 | 1.115 | 1.250 | 3.392 | −5213.844 | |
| PBEPBE | 6-31++g(d, 2p) | 1.096 | 1.092 | 1.349 | 1.095 | 1.475 | 1.123 | 1.228 | 3.394 | −5214.252 |
| 6-311++g(d, 2p) | 1.093 | 1.089 | 1.344 | 1.092 | 1.474 | 1.121 | 1.221 | 3.367 | −5215.531 | |
| Expt.p | 1.089 | 1.081 | 1.341 | 1.084 | 1.468 | 1.113 | 1.215 | 3.117 | ||
- pRef. [13].
| Models | Basis sets | Bond angles between | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| H2–C1–H3 | H2–C1–C4 | H3–C1–C4 | C1–C4–H5 | C1–C4–H6 | H5–C4–C6 | C4–C6–H7 | C4–C6–O8 | H7–C6–O8 | ||
| 6-31+g(d,p) | 116.799 | 121.067 | 122.133 | 122.342 | 121.089 | 116.569 | 115.154 | 124.162 | 120.684 | |
| B3LYP | 6-31++g(d,2p) | 116.834 | 121.043 | 122.123 | 122.314 | 121.153 | 116.533 | 115.123 | 124.165 | 120.712 |
| 6-311++g(d,2p) | 116.849 | 120.973 | 122.177 | 122.347 | 121.069 | 116.583 | 114.830 | 124.348 | 120.822 | |
| 6-31g | 116.488 | 121.219 | 122.22 | 122.091 | 121.314 | 116.596 | 115.381 | 123.994 | 120.626 | |
| B3PW91 | 6-31++g(d,2p) | 116.699 | 121.104 | 122.196 | 122.363 | 120.926 | 116.711 | 115.126 | 124.248 | 120.625 |
| 6-311+g(d,2p) | 116.922 | 120.852 | 122.226 | 122.414 | 120.821 | 116.766 | 114.689 | 124.414 | 120.897 | |
| 6-31g | 116.516 | 121.201 | 122.284 | 122.146 | 121.259 | 116.594 | 115.443 | 123.945 | 120.612 | |
| MPW1PW91 | 6-31++g(d,2p) | 116.938 | 120.930 | 122.131 | 122.419 | 120.864 | 116.717 | 115.023 | 124.176 | 120.800 |
| 6-311++g(d,2p) | 116.964 | 120.807 | 122.227 | 122.491 | 120.714 | 116.794 | 114.691 | 124.394 | 120.914 | |
| 6-311g | 116.573 | 121.132 | 122.295 | 122.136 | 121.724 | 116.139 | 115.404 | 123.451 | 120.604 | |
| PBEPBE | 6-31++g(d,2p) | 116.976 | 120.764 | 122.260 | 122.329 | 120.985 | 116.685 | 114.796 | 124.334 | 120.870 |
| 6-311++g(d,2p) | 116.998 | 120.664 | 122.337 | 122.394 | 120.846 | 116.762 | 114.486 | 124.549 | 120.964 | |
| Expt.q | 118.0 | 119.8 | 122.2 | 122.4 | 120.3 | 117.3 | 114.7 | 123.9 | 121.3 | |
- qRef. [13].
5. Conclusion
An efficient method of generating trans-propenal (trans-acrolein) in the gas phase by applying a DC glow discharge through a low pressure vapor of allyl alcohol inside the absorption cell has been presented. The gas phase rotational spectra of the trans conformer of propenal produced in this way has been recorded and analyzed in the frequency range 60.0–99.0 GHz for the ground as well as three torsional excited states (v = 1, 2 and 3). The asymmetric top K−1 K+1-components of different transitions having J values 6 to 10 have been measured. The observed transition frequencies along with the previously reported data [1, 3, 14] were fitted to a standard asymmetric-top Watson’s S-reduction Hamiltonian (Ir-representation) to determine ground state rotational and centrifugal distortion constants. Analysis of the rotational transitions for the three excited torsional states has been extended up to 99.0 GHz which has enabled us to determine the three rotational and two centrifugal distortion constants. The small negative value of the inertia defect (Δ = − 0.018 uÅ2) in the ground vibrational state (v = 0) and the linear variation of the inertia defect values with torsional quantum number (v = 1,2, 3) demonstrate that the equilibrium configuration of trans-propenal is planar as noticed in case of 3-fluorobenzaldehyde, benzoyl fluoride, and nitrobenzene (Figure 4). The existence of a slightly bent or twisted–COH group would have resulted in a zig-zag behavior in the variation of inertia defect values with torsional quantum number as observed in the case of 2-fluorostyrene (Figure 4). To compare the experimental results with theory, DFT calculations were performed using various models and basis sets. However, it was found that MPW1PW91 model with 6-31++g (d, 2p) basis set produced the best values of rotational and quartric centrifugal distortion constants which are close to the experimental values.
Acknowledgment
The authors would like to thank Mr. A. K. Bhattacharya for his technical assistance during the course of this work.




