Physical and Spectroscopic Properties of Yb3+-Doped Fluorophosphate Laser Glasses
Abstract
The physical properties including refractive index, Abbe number, nonlinear refractive index, microhardness and thermal expansion coefficient, and spectroscopic properties of Yb3+-doped fluorophosphate laser glasses were investigated. The results show that due to the addition of fluoride, mechanical and thermal properties are promoted, emission cross-section σemi is also greatly enhanced. The largest gain coefficient σemi · τm (0.824 pm2 · ms) can be obtained with the minimum pump intensity Imin (1.112 kw/cm2). This kind of Yb3+-doped fluorophosphate glass is an excellent candidate material for Yb3+-doped host for high-power generation.
1. Introduction
With the rapid development of laser diode (LD) recently, Yb3+ doped laser materials as the gain medium in high-energy solid-state laser systems have obtained much attention [1–3]. There are only two manifolds in the Yb3+ energy level scheme, namely, the ground state and the excited state, and the absorption band is located at about 970 nm, with a large cross-section, which enables efficient pumping by high-power III–V diode lasers that are commercially available. The Yb3+ ions are also of interest not only as high-power lasers for nuclear fusion but also as energy transfer sensitizers for infrared lasers and up conversion lasers [4, 5]. The main obstacle is mechanical and thermal problems in the development of high-average powder solid-laser materials because more than half of pumping energy precipitate in gain medium, though effective semiconductor diode is used as pumping source, which results in various problems such as thermal load, mechanical stress. Therefore, in addition to high optical and spectroscopic properties, excellent thermal and mechanical properties are necessary, to improve the repetition rate of the laser glass.
For a long term, Yb3+ doped phosphate glasses have been regarded as ideal host matrix for high-power laser due to larger absorption and emission cross-section, less nonlinear refractive index [6–8]. However, the line-like network of phosphate glass results in higher thermal expansion coefficient (TEC), inferior physical properties such as mechanical and chemical durability [9, 10]. Furthermore, the hygroscopic tendency of phosphor lowers the fluorescence lifetime of Yb3+ ions. Some early researches [11, 12] have shown that formation of P–O–B(4) bonds in borophosphate glass could greatly improve physical properties along with broad emission bandwidth. However, the photo energy of B–O bond (~1400 cm −1) is larger, so that the fluorescence lifetime of borate glass is less as compared to other glass. In order to improve the water resistance of phosphate glass, fluorophosphate glass is available because fluorophosphate glass has m advantages such as long fluorescene life time, low nonlinear refractive index [13, 14].
To obtain excellent physical and spectroscopic properties of Yb3+-doped laser glass, we investigated the relationship between composition and mechanical, thermal and spectroscopic properties of fluorophosphate glasses.
2. Experimental
Two series of fluorophosphate glasses were chosen, and the glass compositions (mol%) listed in Table 1 were prepared by melting 100 g batches using analytical grade (NH4)2HPO4, Al2O3, carbonate, Fluoride, Nb2O5, and Yb2O3 with a purity of above 99.99%. When each batch was slowly heated from room temperature up to 1000°C in a Al2O3 crucible, the crucible was covered to minimize the volatilization of phosphor, then the batch was melted at 1300~1320°C depending on the glass composition. Melts were quenched in stainless steel moulds and properly annealed. The final compositions of the glasses were checked by chemical analysis and found to be within ±1%.
| A0 | A1 | A2 | A3 | A4 | B1 | B2 | B3 | B4 | |
|---|---|---|---|---|---|---|---|---|---|
| P2O5 | 63 | 63 | 63 | 63 | 63 | 44 | 44 | 44 | 44 |
| Al2O3 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 |
| Nb2O5 | 1 | 1 | 1 | 1 | 1 | 4 | 4 | 4 | 4 |
| Li2O | 10 | 7.5 | 5 | 2.5 | — | — | — | — | — |
| BaO | 18 | 13.5 | 9 | 4.5 | — | — | — | — | — |
| LiF | — | 2.5 | 5 | 7.5 | 10 | 10 | 10 | 10 | — |
| NaF | — | — | — | — | — | 10 | 10 | — | 10 |
| MgF2 | — | — | — | — | — | 24 | — | 20 | 20 |
| CaF2 | — | 4.5 | 9 | 13.5 | 18 | — | 24 | 14 | 14 |
| Yb2O3 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
The refractive index (nD, nF, and nC) was measured on an Abbe refractometer (WZS-S) at room temperature at the wavelength of 589.3, 486.1, and 656.3 nm, respectively.
The microhardness of the investigated samples was measured using Vickers’s microhardness indentor (MET-4). The eyepiece on the microscope of the apparatus allows measurements with an estimated accuracy of ±0.5 μm for the indentation diagonal. Grinding and well polishing were necessary to obtain polished and flat parallel surfaces glass samples before indentation testing. At least five indentation readings were made and measured for each sample. Testing was conducted with a load of 30 g and loading time 15 seconds. The measurements were carried out under normal atmospheric condition.
Thermal expansion coefficient of the investigated glass was carried out on 2.0 cm long rods using AS-100 automatic recording multiplier dilatometer with heating rate of 5°C/min. The uncertainty of linear thermal expansion from room temperature to 300°C is ±5 × 10-7/°C.
The samples for measurement of spectroscopic properties were cut to a size of 20 mm × 20 mm × 2 mm with two larger sides polished. Absorption spectra were recorded with Perkin-Elmer (Lambda 900) UV/VIS/NIR spectrophotometer, at room temperature, in the range of 870–1150 nm. Emission spectra were measured with Triax 550 spectrophotometer through exciting the samples with a diode laser operating around 940 nm. The emission from the sample was focused to a monochrometer and detected by the Ge detector. The signal was intensified with a lock in amplifier and processed by a computer. Fluorescence lifetimes were measured by exciting the samples with a Xenon lamp and detected by an S-1 photomultiplier tube. The fluorescence decay curves were recorded and averaged with a computer-controlled transient digitizer.
3. Results
3.1. Physical Properties
Table 2 summarizes the data of measured refractive index, Abbe number, nonlinear refractive index, microhardness, and thermal expansion coefficient for fluorophosphate glass samples.
| A0 | A1 | A2 | A3 | A4 | B1 | B2 | B3 | B4 | |
|---|---|---|---|---|---|---|---|---|---|
| Refractive index nD | 1.556 | 1.551 | 1.548 | 1.543 | 1.540 | 1.509 | 1.510 | 1.510 | 1.510 |
| Abbe number ν | 62.23 | 66.86 | 67.90 | 67.32 | 64.32 | 63.07 | 62.72 | 64.20 | 63.94 |
| Nonlinear refractive index n2(×10−13 esu) | 1.36 | 1.20 | 1.16 | 1.15 | 1.23 | 1.15 | 1.16 | 1.12 | 1.13 |
| Microhardness (GPa) | 3.68 | 3.75 | 3.89 | 3.95 | 4.10 | 4.40 | 4.16 | 4.20 | 4.11 |
| Thermal expansion coefficient α(×10−7/K) | 105.92 | 91.41 | 82.61 | 78.86 | 75.24 | 71.98 | 89.18 | 63.71 | 88.14 |
As shown in Table 1, with increasing fluoride content in the samples of series A, the microhardness increases gradually along with a decrease of thermal expansion coefficient. In series B, B1, and B3 glasses exhibit high microhardness and low-thermal expansion coefficient when compared to B2 and B4 on the same condition.
3.2. Spectroscopic Properties
Figure 1 shows the absorption and emission spectra of the samples of series A. The line shape of absorption spectra is similar except intensity in all samples, the main absorption peak is around 975 nm (as shown in Figure 1(a)), which corresponds to the energy transition of the lowest subenergy level of and . The absorption spectra is characterized by broader line widths due to out-of-order glass structure in which Yb3+ ions are localized in different coordination site and some portion of stark splitting energy overlap. As seen in Figure 1(b), the main emission peak of all samples is around 975 nm, and subemission peak is around 1006 nm which is mostly concerned. Other spectroscopic properties have been shown in Table 3.
| A0 | A1 | A2 | A3 | A4 | B1 | B2 | B3 | B4 | |
|---|---|---|---|---|---|---|---|---|---|
| Concentration (1020 ions/cm3) | 2.753 | 2.745 | 2.740 | 2.746 | 2.744 | 3.127 | 3.120 | 3.075 | 3.027 |
| Σabs(104 pm3) | 3.93 | 3.86 | 3.54 | 3.65 | 3.73 | 4.76 | 4.84 | 5.49 | 5.51 |
| σemi(pm2) | 0.598 | 0.601 | 0.608 | 0.649 | 0.653 | 0.915 | 0.947 | 1.065 | 1.032 |
| Arad(s −1) | 982 | 1018 | 921 | 951 | 968 | 1188 | 1208 | 1371 | 1376 |
| τm(ms) | 0.84 | 0.87 | 0.98 | 1.03 | 1.08 | 0.89 | 0.87 | 0.73 | 0.75 |
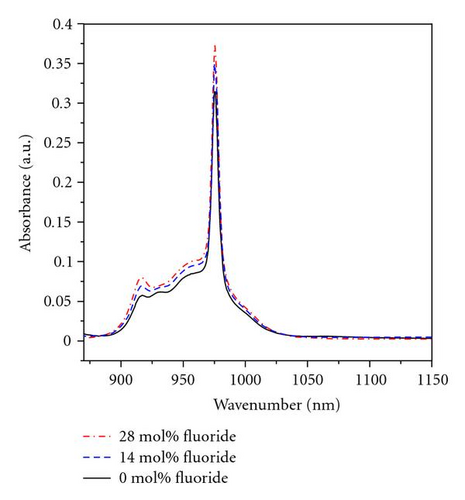
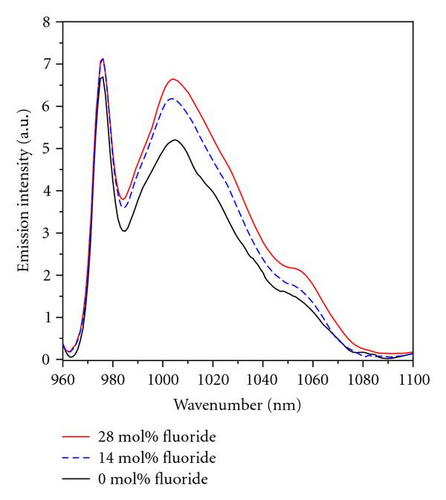
In the Table 3, emission cross-section σemi and fluorescence lifetime τm gradually increase as the fluoride content increases from 0 to 28 mol% in series A glasses. Series B glasses shows larger integral absorption cross-section ∑abs and emission cross-section σemi, but less fluorescence lifetime τm compared to series A glasses.
4. Discussion
4.1. Effect of Fluoride on Mechanical and Thermal Properties
Excellent mechanical and thermal properties are indispensable to the laser driver for inertial confinement fusion (ICF), especially low-thermal expansion coefficient can reduce the thermal load of laser glasses, enhancing thermal shock toughness. As seen in Table 2, the microhardness increases and thermal expansion coefficient decreases when fluoride content gradually increases, moreover, the mechanical and thermal properties of series B glasses with higher fluoride content are superior to those of series A glasses. This anomalous behavior should be due to the structural change caused by the fluorides. It is clear from the glass composition as shown in Table 1 that the A0 glass without fluoride content mainly consists of metaphosphate (ΣMO: P2O5 = 1) group. Addition of fluorides leads to rupturing of long metaphosphate chains, and the formation of short structural fragments of P2(O, F) 7 and P(O, F) 4. However, these smaller fragments are linked up to a greater extent by the Al(O, F) 6 polyhedra [19], which leads to strengthening of the glass network. In particular, Nb5+ with higher field strength also promotes the linkage of smaller fragments in series B glasses. In addition, the fact that B1 and B3 glasses display better mechanical and thermal properties is also explained by the high cation field strength for Mg2+ and Li+ ions.
4.2. Effect of Fluoride on Spectroscopic Properties
The emission cross-section σemi has an important effect on laser properties of Yb3+ ions because a larger σemi indicates higher laser gain [20]. From (4), it can be seen that the value of emission cross-section is only determined by the absorption cross-section, which removes the errors of reabsorption in the experiment of fluorescence spectrum. As seen in Table 3, the σemi increases with increase in fluoride content, and the increase of σemi depends on the change of structure of fluorophosphate glasses. Addition of fluoride leads to the reduction of P–O–P linkages due to a gradual transformation of to P2(O, F) 7 and P(O, F) 4, which decreases the connectivity of the glass network. This behavior is strengthened by the concentration of F − ions. Moreover, the surrounding coordination Yb3+ ions are also changed due to present mixed anions, fluorine, and oxygen. In particular, the series B glasses with higher Nb2O5 and fluoride content contain different types of structural units such as P2(O, F) 7, P(O, F) 4, Al(O, F) 6, and Nb(O, F) 6 in the framework of fluorophosphate glass, which increases asymmetry of the Yb3+ site environments and results in larger absorption and emission cross-section as shown in Table 3.
4.3. Effect of Fluoride on Laser Parameters
| A0 | A1 | A2 | A3 | A4 | B1 | B2 | B3 | B4 | |
|---|---|---|---|---|---|---|---|---|---|
| σemi · τm(pm2 · millisecond) | 0.502 | 0.523 | 0.596 | 0.668 | 0.705 | 0.814 | 0.824 | 0.777 | 0.774 |
| Imin(kw/cm2) | 1.761 | 1.68 | 1.516 | 1.615 | 1.27 | 1.073 | 1.112 | 1.176 | 1.172 |
The σemi · τm increases with increasing fluoride content in series A glasses, since addition of fluorine promotes emission cross-section σemi and fluorescence lifetime τm. In spite of shorter τm, series B glasses exhibit higher values of σemi · τm and Imin due to higher σemi and the Imin of series B glasses is superior to the known QX/Yb glass [24]. Therefore, we believe that the fluorophophate glasses are promising laser glasses for high-peak power and high-average power.
5. Conclusion
Yb3+-doped fluorophosphate laser glasses have successfully been developed. A systematic investigation of physical properties including refractive index, Abbe number, nonlinear refractive index, microhardness, and thermal expansion coefficient has been performed as a function of fluoride content. With the increase of fluoride content, the microhardness increases gradually along with the decrease of thermal expansion coefficient. The structure around Yb3+ is simultaneously changed which greatly influences the spectroscopic properties and laser parameters. The best laser performance is found in 44P2O5-7Al2O3-4Nb2O5-10LiF-20MgF2-14CaF2-1Yb2O3 glass system with the gain coefficient σemi · τm(0.824 pm2 · ms) and minimum pump intensity Imin (1.112 kw/cm2). The favorable combination of outstanding physical, spectroscopic properties and laser parameters indicates that current Yb3+-doped fluorophosphate glass is an excellent candidate material for Yb3+-doped host for high-power generation.
Acknowledgment
This work is financially supported by the Chinese National Defense New Materials Project (MKPT-05-240).




