Out of South America: phylogeny of non-biting midges in the genus Labrundinia suggests multiple dispersal events to Central and North America
Abstract
Non-biting midges of the genus Labrundinia (Chironomidae: Tanypodinae) are minute dipterans with immature stages living in a variety of unpolluted water bodies, from small streams and ponds to lakes and bays. Extensively recorded in ecological studies, the genus comprises 39 species, all except one described from areas outside the Palearctic region. Internal structure among Labrundinia species was postulated by S. S. Roback, who recognized four species groups based on morphological characters of immature stages. We examined phylogenetic relationships among known Labrundinia species using partial DNA sequences of the nuclear protein-coding gene CAD and morphological characters. Both analyses with Bayesian inference and parsimony methods recovered the monophyly of Labrundinia, strongly supported by five morphological synapomorphies. Internal relationships within the genus partly supported Roback's species groups with the addition of later described species. Biogeographical inferences were obtained by applying Bayesian binary MCMC (BBM) analysis and favoured a scenario where Labrundinia had its initial diversification in the Neotropical region and that current presence in the Nearctic region and southern South America is due to subsequent dispersal.
Introduction
The dipteran family Chironomidae probably originated in the middle Triassic approximately 248–210 million years ago (Cranston et al. 2010). It comprises at least 10 000 species in more than 400 genera (Armitage et al. 1995; Sæther et al. 2000) and roughly 6200 of these are known to science (P. Ashe, pers. comm.). Chironomidae is the most widespread of all aquatic insect families, occurring in all biogeographical regions of the world. The immature stages of most species occur in freshwater, but numerous terrestrial or marine species are known (Sæther & Ekrem 2003). Although the distribution of the species in many genera is relatively well known, detailed analyses of distribution patterns and historical biogeography are rare in chironomids, especially from the Neotropical region. Phylogenetic studies on Chironomidae have a fairly long tradition, beginning with Goetghebuer (1914) who studied the relationships between groups in the family. However, it was not until Brundin's monograph (1966) that species-level phylogenies of chironomid midges were produced (Ekrem 2003). Since then, several groups have been investigated (e.g. Sæther 1971, 1977, 1983, 1990, 2000; Brundin & Sæther 1978; Adam & Sæther 1999; Boothroyd & Cranston 1999; Ekrem 2003; Stur & Ekrem 2006; Fu et al. 2010; Carew et al. 2011; Krosch et al. 2011; Krosch & Cranston 2013). Cranston et al. (2012) reconstructed the phylogeny of Chironomidae based on DNA sequence data from multiple genes. However, within the subfamily Tanypodinae, only the genus Alotanypus has had a phylogenetic hypothesis proposed at the species level (Siri et al. 2011).
The Tanypodinae is the third most speciose subfamily in the Chironomidae, with species distributed widely across most of the globe, occupying a diverse array of habitats including small streams and ponds to lakes and bays (Silva et al. 2011). Generally regarded as predators, the larval feeding apparatus differs from other Chironomidae, with the strong development of premental structures such as ligula and paraligulae (Cranston 1995). The subfamily was erected by Thienemann & Zavřel (1916) primarily on the basis of immature stages (Cranston 1995) and their monophyly is well supported, with Podonominae as its sister group (Cranston et al. 2012). Postulated internal relationships at the generic level in Tanypodinae derive from Fittkau (1962), who erected the tribe Anatopyniini. Roback & Moss (1978) established the tribes Procladiini and Natarsiini with an unusual phenetic method to chironomid systematics (Cranston et al. 2012).
The Pentaneurini genus Labrundinia was erected by Fittkau (1962) based on Tanypus longipalpis Goetghebuer, designated as the type species. It comprises 39 species, all of them described in a separate paper (Silva et al. 2014). Except for Labrundinia longipalpis, all species have been described from areas outside the Palearctic region: five from North America, four from Central America and 29 from South America. Internal relationships among the so far known Labrundinia species were postulated by Roback (1987a), who recognized four species groups based on morphological characters of immature stages: pilosella group, maculata group, neopilosella group and virescens group. To date, no phylogeny or even hypothesis of monophyly has been proposed for Labrundinia. Fittkau (1962) suggested that the genus is closely related to the Zavrelimyia group, in particular Paramerina Fittkau, but morphological analyses of new species in Labrundinia indicate the presence of characters that contradict this argument, such as larval bifid claws, the form of the tibial spurs and phallapodemes on adults (Silva et al. 2014).
Recently, several phylogenetic analyses based on morphological characters from various groups in the family Chironomidae have been published (e.g. Sæther 2000; Cranston et al. 2002; Ekrem 2003; Donato & Siri 2010; Fu et al. 2010; Fusari et al. 2013). However, in most cases, the results have been ambiguous, and parsimony trees have been characterized by polytomies and low node support (Ekrem et al. 2010a). Molecular characters in phylogenetic analyses of chironomids have an obvious advantage, and their use has notably grown over the years (e.g. Cranston et al. 2002, 2010; Ekrem & Willassen 2004; Allegrucci et al. 2006; Stur & Ekrem 2006; Martin et al. 2007; Krosch et al. 2011). In testing the utility of three mitochondrial (COI, COII, 16S) and two nuclear (CAD and EF-1α) markers in low-level phylogenetic reconstruction in Chironomidae, Ekrem et al. (2010a) found that partial CAD (carbamoylphosphate synthase II domain) sequences performed best and had the strongest phylogenetic signal in a data set consisting of species in the genus Micropsectra (Chironomidae) and related genera.
Silva et al. (2013) analysed the applicability of cytochrome c oxidase subunit I (COI) gene sequences, so-called DNA barcodes, in species delimitation and the life stage association of Labrundinia. The molecular analysis produced monophyletic groups which were almost entirely congruent with the morphological features, except for the specimens identified morphologically as Labrundinia tenata Roback, which were divided into two separate barcode clusters. These results suggest that barcodes can be used routinely in species delimitation and identification of chironomids, but the authors acknowledge the importance of data from nuclear markers to support definitions of species boundaries. Additional studies confirm the effectiveness of DNA barcodes in species delimitation and identification in Chironomidae (Carew et al. 2003; Ekrem et al. 2010b; Stur & Ekrem 2011; Silva et al. 2012; Silva & Wiedenbrug 2014), but saturation, particularly in 3rd codon position, is so extensive that this marker is considered unsuited for phylogenetic reconstructions (Ekrem et al. 2007, 2010a). The primary purpose of this study is to explore the phylogenetic relationships of Labrundinia species using morphology as well as partial DNA sequences of the nuclear protein-coding gene CAD. The obtained phylogenetic reconstructions are used to analyse dispersal–vicariance events that best explain the biogeographical pattern seen in recent clades.
Material and methods
Taxon sampling
The taxa included in this study were selected with the aim of getting representatives from as many of the known morphotypes in Labrundinia as possible. Fieldwork was restricted to south-east Brazil, which harbours most of the described Labrundinia species so far (Silva et al. 2014). However, despite high sampling efforts at and near type localities, only one-third of the known species of Labrundinia were obtained. The larvae and pupae were collected using a hand-net in different aquatic systems. Some larvae and pupae were isolated in small vials covered with nylon screen and reared in laboratory to obtain adult life stages. Neither substratum nor food was given, except for some detritus carried over with the water. Various aquatic macrophyte species were collected and placed in a plastic tray to obtain adults emerged. Immature chironomids were preserved in 96% pure ethanol, while imagines were kept in slightly diluted ethanol (~80–85%) to avoid breakage.
Different morphotypes were recognized based on variation in all observable morphological traits such as colouration, genital structures, shapes of pupal thoracic horn and larval claws. After DNA extraction, the exoskeleton was mounted in Euparal on microscope slides with the corresponding wings, legs and antennae. Identifications were made based on taxonomic revisions, original descriptions (Fittkau 1962; Beck & Beck 1966; Roback 1971, 1987a,b; Epler 2001; Jacobsen 2008; Silva & Fonseca-Gessner 2009; Silva et al. 2011, 2014; Siri & Donato 2011) and subsequent examination of type material. All reference material for the molecular data presented in this study is deposited in the NTNU University Museum, Trondheim, Norway.
DNA extraction, PCR and sequencing
DNA was extracted from single individuals, mostly larvae but also pupae and adult males and females (for details see Appendix S1). DNA extraction and PCR were performed in the molecular laboratory at the NTNU University Museum, Trondheim, Norway. DNA extraction followed the standard tissue protocol and kit supplied with GeneMole robot (MoleGenetics, Lysaker, Norway); lysis was performed with proteinase K at 56 °C overnight.
Each PCR was made in a total volume of 25 μL and contained 2 μL DNA template (concentration not measured), 2.5 μL 10× Ex Taq Buffer, 2 μL MgCl2 in 25 μm concentration, 2 μL of dNTPs in 10 mm concentration, 1 μL of each of the CAD primers (Moulton & Wiegmann 2004) 54F (5′-GTNGTNTTYCARACNGGNATGGT-3′) and 405R (5′-GCNGTRTGYTCNGGRTGRAAYTG-3′) in 10 μm concentration, 0.2 μL of TaKaRa HS ExTaq (Takara Bio Inc., Otsu, Shiga, Japan) and 14.3 μL of ddH2O. Amplifications for the CAD region were performed in a thermocycler with an initial denaturation step of 95 °C for 15 min, followed by five cycles of 30 s annealing at 57 °C, five cycles of 30 s annealing at 52 °C and 35 cycles of 30 s annealing at 51 °C in a typical touch-down procedure. The PCR products were purified using ExoSAP-IT (USB Products; Affymetrix, Santa Clara, CA, USA) and shipped to MWG Eurofins (Ebersberg, Germany) for bidirectional sequencing using BigDye 3.1 (Applied Biosystems, Foster City, CA, USA) termination.
Sequences were assembled and edited using DNA baser Sequence Assembler 3.2.4 (Heracle Software, Pitesti, Romania), checked for stop codons and aligned as translated amino acids using default clustalw options (Thompson et al. 1994) as implemented in mega6 (Tamura et al. 2013). The alignment was trivial as no indels or introns were observed in the sequences. The nucleotide statistics were also calculated with mega6.
Phylogenetic analyses
Morphological characters showing consistent patterns of interspecific divergence, potentially providing a good phylogenetic signal for species relationships, were selected largely based on observations and experience by the first author (Appendix S2). Data from larvae, pupae and adult males were carefully considered and coded in a matrix (Appendix S3) using mesquite 2.75 (Maddison & Maddison 2004). In some instances, it was necessary to rely on published species descriptions as well-preserved relevant material was unavailable for examination. Continuous characters (e.g. ratios) were divided into character states based on intervals of variation. Although we attempted to keep the number of autapomorphies low, some single character states are listed for certain species to indicate differences to other terminal taxa. Nomenclature and abbreviations follow Sæther (1980). Despite the high number of question marks in species without CAD sequence data, morphological and molecular characters were combined into a total evidence matrix to ensure that as many of the known species as possible could be included to assess the historical biogeography of Labrundinia. Separate analyses on a reduced matrix, only including taxa with both molecular and morphological data, were also performed to evaluate whether missing data had confound influence on the major trends in the complete phylogeny.
The species used as out-groups in phylogenetic analyses were chosen a priori based on morphological similarities and existing classification (Fittkau 1962; Roback & Moss 1978). Additionally, Basic Local Alignment Search Tool (blast) was conducted using mega6, to find close matches to Labrundinia sequences. The following genera were included: Ablabesmyia Johannsen, 1905; Australopelopia Cranston, 2000; Conchapelopia Fittkau, 1962; Fittkauimyia Karunakaran, 1969; Larsia Fittkau, 1962; Monopelopia Fittkau, 1962; Pentaneura Philippi, 1865; Procladius Skuse, 1889 and Zavrelimyia Fittkau, 1962. The final data set consisted of 49 terminals: 40 in-group terminals (for details see Silva et al. 2014) and nine out-group terminals. Despite the fact that Labrundinia maculata was regarded as a junior synonym of Labrundinia longipalpis by Silva et al. (2011), the species were treated as separate to investigate the validity of the synonymy and provide higher resolution for the biogeographical analysis.
The best-fit models of nucleotide substitution were estimated using mega6 and chosen based on the Akaike information criterion. For our data set, the general time reversible model with invariable sites and gamma correction for rate heterogeneity (GTR+G+I) was the best-fit model (−lnL = 8982.168, BIC = 18461.093, AIC = 18066.650). Bayesian analyses were conducted in mrbayes 3.2.2 (Ronquist et al. 2012), using four chains for 3 million generations with a print frequency of 1000, sample frequency of 500 and a burn in of 600 sampled trees (30 000 generations). All priors used were default. The trace files were then examined with the program tracer 1.6 (Rambaut & Drummond 2008) to determine the point of Markov Chain Monte Carlo (MCMC) convergence (burn in) and to evaluate the effective sample size (ESS) of the parameter estimates.
The morphological data set was also used for parsimony analysis under implied weighting, implemented in tnt version 1.1 (Willi Hennig Society Edition) (Goloboff et al. 2008). In this method, characters are weighted during tree searches and the weights applied to each character are summed to determine the fit, such that the cladogram with maximum total character fit is chosen as the most parsimonious cladogram (MPC). Character fit is determined as a function of the values of concavity constant (k), which controls how much a character is weighted against homoplasy (Legg et al. 2013), in general, as k increases, fit decreases (Goloboff 1993). In our study, analyses with implied weighting were run with values of k ranging from 1 to 20. All characters were treated as unordered. Tree searches were conducted using ‘Traditional Search’ options: 10 000 random addition sequences, TBR branch swapping and 100 trees saved per replicate. Under ‘Settings’, the General RAM was set to 50 Mb and the maximum number of trees to be held to 10 000. Clade support was assessed using symmetrical resampling, as implemented in tnt. Both the absolute frequencies and the frequency differences (or GC values) were recorded (Goloboff et al. 2003), using the same ‘Traditional Search’ options given above with the default value of 33% change probability. Cladograms were rooted between Procladius and the remaining taxa. figtree 1.3.1 (Rambaut 2009) was used to display the resulting trees.
Biogeographical analyses
Recently introduced ancestral state inference models based on Bayesian statistics allows reconstruction of ancestral states while accounting for phylogenetic uncertainty, which according to Ronquist (2004) is essential in evolutionary inference. This feature is particularly important in topologies with polytomies and low-supported nodes and justify the choice of this model over others. To reconstruct the historical biogeography of the lineages, the Bayesian Binary Method (BBM) analysis as implemented in rasp v.2.1b (Yu et al. 2013) was used to infer the possible ancestral ranges of Labrundinia species in the phylogenetic trees resulting from analyses of the combined molecular and morphological data. In this model, ancestral distributions are reconstructed based on a full hierarchical Bayesian approach with trees from the posterior distribution of a Bayesian inference as input (Yu et al. 2013).
Biogeographical patterns of extant Labrundinia species were assigned to ecoregions as defined initially by Amorim (2012) and Morrone (2006), modified and complemented with additional areas. Herein, four simplified species distribution areas are distinguished: (A) Nearctic, (B) Central America, (C) northern and (D) southern South America. Area C is comprised of several geographical regions based on a vicariance model (Amorim 2012), namely the Atlantic Forest, the North Amazonian, south-east Amazonian and south-west Amazonian regions. We combined these regions because of observations of large distribution gaps in the ranges of Labrundinia species constrained to each of the assigned areas (Kaffenberger et al. 2012). We regard these gaps to be caused by differences in sampling effort and not true disjunct distributions. Species of Labrundinia pilosella, distributed between A and B, were coded AB, while Nearctic populations of L. longipalpis were separated as ‘Labrundinia maculata’ and given their separate distribution range in the Nearctic region. The out-group taxa were assigned in all defined areas. Ancestral area distributions were estimated for in-group nodes only.
To account for uncertainties in phylogeny inference, 12 002 trees, resulting from Bayesian inference output of the full combined molecular and morphological data set, were used in the BBM analysis. The Bayesian binary analysis was run for 3 million generations, sampling every 500th and excluding the trees of the first 10% of generations with four chains and two parallel runs of which a combined result was obtained. State frequencies were estimated applying the model F81+ C, with dirichlet distribution set to 1.0, while among-site rate variation was modelled by the gamma distribution. Ancestral distributions were mapped on each node of a majority-rule consensus tree, obtained from the stored trees with the Compute Condense option in rasp. The number of maximum areas was set to 2.
Results and discussion
Data set properties
Material suitable for genetic analyses was obtained for 13 of the 40 Labrundinia species included in the morphological character matrix. The aligned CAD sequences were 899 bp long with 524 variable sites (58.3%), of which 424 (80.9%) were potentially parsimony informative. Most variable sites occurred in the third codon position (Table 1). The sequences were somewhat AT-biased, especially in third position, which exhibited a combined average AT composition of 64.0% (Table 1). No introns were recognized in CAD sequences of Labrundinia.
| Nucleotide position | % Variable sites | % Informative sites | % Adenine | % Cytosine | % Guanine | % Thymine |
|---|---|---|---|---|---|---|
| 1st | 26.5 | 12.5 | 33.6 | 17.3 | 28.1 | 21.0 |
| 2nd | 17.9 | 0.5 | 35.2 | 19.2 | 18.1 | 27.5 |
| 3rd | 55.5 | 87.0 | 27.8 | 17.5 | 18.5 | 36.2 |
| All | 58.2 | 95.6 | 32.2 | 18.0 | 21.6 | 28.2 |
The morphological matrix included 46 characters (21 from adults, 14 pupal and 11 larval). Few species showed polymorphic character states (Appendix S3). The combined data matrix comprised 49 terminal taxa each with 944 characters; in total, 53.2% of the characters were scored as missing for the complete data matrix.
Phylogenetic patterns
The monophyly of Labrundinia is well established in our results. The group is recovered in all trees resulting from a reduced taxon–character matrix, or from a weighted morphology matrix, with five morphological traits recognized as synapomorphies: (i) apex of hind tibia without spur; (ii) tergite IX with distal margin strongly convex in the male adults; (iii) pupal tergite VIII with projections over base of the anal lobe; (iv) pupal anal lobe long and narrow; and (v) larval bifid claw on posterior parapod with inner tooth longer than outer tooth.
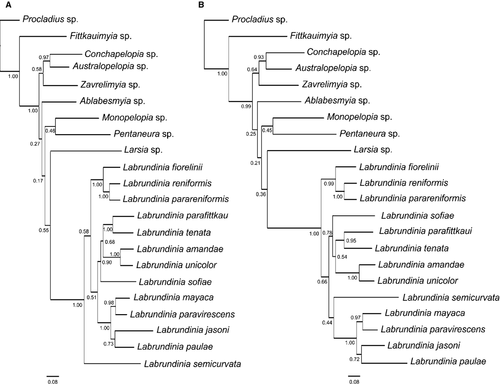
Bayesian analyses of the CAD gene sequences alone and the combined molecular and morphological data sets for 22 taxa produced almost identical trees: Labrundinia was supported as monophyletic with posterior probability support 1.0 (Fig. 1A). The Bayesian tree resulting from combined molecular and morphological data sets (Fig. 1B) differs from the molecular-only phylogeny by the placement of L. semicurvata which groups with L. mayaca + L. paravirescens + L. jasoni + L. paulae as its closest relative. However, only two clades are supported with posterior probability > 0.8. Each parsimony analysis under implied weights of the reduced taxon–character matrix alone produced only a single most parsimonious cladogram (MPC). For easier comparison with the results obtained by analyses of the full morphological character data set, we here present the MPC (fit = 5.45663, length 170, CI = 0.40, RI = 0.66) produced for k = 12 (Fig. S1). In this tree, Labrundinia is monophyletic, but only two clades are well supported by values of symmetrical resampling support above 90: L. paulae + L. jasoni + L. mayaca + L. paravirescens. Additionally, Labrundinia amandae + L. unicolor and L. reniformis + L. parareniformis were recovered as strongly supported sister species.
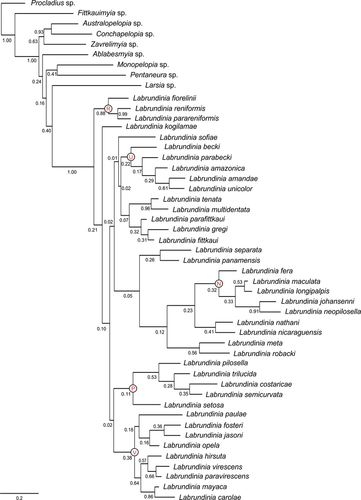
A Bayesian analysis of the combined morphology and molecular data set resulted in a fully resolved consensus tree with strong support for the monophyly of Labrundinia (Fig. 2). Despite mostly low support, the species groups concept presented by Roback (1987a) is not disproved by the internal phylogenetic relationships in our analyses and some previous observations can be recovered: Labrundinia longipalpis and L. maculata are sisters and are placed with L. fera, L. johannseni and L. neopilosella in an expanded neopilosella group (Roback 1987a) connected by node N, dissolving Roback's maculata group. The virescens group (Roback 1987a), connected by node V, is expanded with the inclusion of L. carolae, L. fosteri, L. hirsuta, L. jasoni, L. mayaca, L. opela, L. paulae and L. paravirescens. Although L. hirsuta and L. opela do not have their immature stages described, Roback (1987b) suggested placement of these species in the virescens group based on abdominal colouration patterns, concordant with our results. The postulated pilosella species group (Roback 1987a), originally included L. pilosella and L. becki, with L. parabecki likely to be placed in this group (Roback 1987b). In our study, however, L. becki are nested with L. parabecki + L. amazonica + L. amandae + L. unicolor. The members of this group, connected by node U, do share a distinctive morphological feature, the abdomen including hypopygium is completely brown. In contrast, L. pilosella is sister to L. trilucida + L. costaricae + L. semicurvata, at node P. Although we refrain from formally naming a reniformis species group, a well-supported group (0.88), comprising L. fiorelinii + L. reniformis + L. parareniformis, can be identified at node R. The reconstruction of the internal phylogenetic structure in Labrundinia is slightly unexpected given the fact the species groups suggested by Roback were based primarily on larval and pupal characters, as there were not sufficient fully reared male adults or associated life stages to clearly understand the variation across species and to delineate the suggested groups (Roback 1987a). We think that the low support values from analysis of the concatenated data set can be attributed to the high amount of missing CAD data for about two-thirds of the species. It is interesting and reassuring to observe, however, that the phylogeny based on this matrix (Fig. 2) is concordant with results from the reduced matrix (Fig. 1B).
Parsimony analyses of the full morphological character matrix under implied weights with k values ranging from 1 to 20 each produced only a single MPC. The MPCs obtained with k values of 10–11 were identical, the only difference being the values of the fits (11.03334 and 10.38862, respectively), and those for the k values ranging from 12 to 15 (fit = 9.81614, 9.30497, 8.84609 and 8.42165, respectively) differed only in the rearrangement of a small clade: (L. sofiae (L. tenata (L. multidentata (L. fiorelinii (L. reniformis, L. parareniformis))))), rather than (L. sofiae ((L. tenata, L. multidentata) (L. fiorelinii (L. reniformis, L. parareniformis)))). The MPC produced by the k values ranges of 1–9 and 16–20 presented substantial divergence in the relationships among the taxa. Therefore, the range of k = 10–15 seems to produce MPCs within which the topology is nearly unaffected by changes in k values. The MPC for k = 12 (length 289, CI = 0.24, RI = 0.63) is shown in Fig. S2.
Within the k = 12 analysis, Labrundinia appears as a monophyletic group. While the main clades found in the Bayesian analysis were also recovered in the parsimony topology, a certain degree of incongruence between the two analyses can be observed on the level of individual accessions as well as clades regarding their composition and relationships. The neopilosella group were divided into two sister clades: ((L. fera (L. longipalpapis, L. maculata)) (L. johannseni, L. neopilosella)), rather than (L. fera ((L. johannseni, L. neopilosella), (L. longipalpapis, L. maculata))). Despite the rearrangement, L. longipalpis and L. maculata remain as sister species, which is in agreement with the synonymization proposed by Silva et al. (2011). The clade comprising L. becki also grouped differently in the parsimony analysis: ((L. becki, L. amazonica) (L. parabecki (L. amandae L. unicolor))), rather than (L. becki (L. parabecki (L. amazonica (L. amandae, L. unicolor)))). Moreover, this clade presented L. pilosella + L. trilucida as its closest relative, supporting Roback's observation (1987a) that L. becki and L. pilosella might be closely related. Finally, the virescens group were also recovered with a different rearrangement in the parsimony cladogram: (L. paulae (((L. hirsuta (L. opela (L. fosteri, L. jasoni))) (L. virescens, L. paravirescens)) (L. carolae, L. mayaca)))))))), rather than ((L. paulae (L. opela (L. fosteri, L. jasoni))) ((L. hirsuta (L. virescens, L. paravirescens)) (L. carolae, L. mayaca))). Roback (1987b) described the adult male of L. fosteri, but did not place the species in the species groups previously proposed (Roback 1987a). In our study, both Bayesian and parsimony topologies suggest a relationship of L. fosteri with virescens group. Adult morphological characters that support this are reduced dorsomedian eye elongation and hypopygium colour, while the so far known immature stages share pupal thoracic horn shaped like the number ‘9’ and larval mandible with very short teeth.
Parsimony analyses under implied weights on a matrix including all morphological characters of species with described pupal and larval stages were also attempted. The single MPC found for k = 12 (fit = 8.71951, length 248, CI = 0.28, RI = 0.65) recovered Labrundinia as a monophyletic group, with members which key to neopilosella and virescens groups also forming monophyletic clusters. One notable divergence refers to the placement of Labrundinia longipalpis, which groups with L. maculata + L. johannseni + L. neopilosella as its closest relative. Excluding larval characters from the parsimony analysis, thus leaving 35 parsimony informative characters in the data matrix, resulted also in only one MPC. The topology found (fit = 6.91852, length 254, CI = 0.27, RI = 0.63) is less resolved than those in all previous analyses, which is consistent with the premise that the integration of morphological data derived from all life history stages is essential in providing accurate phylogenetic estimates (Cranston et al. 2010, 2012). As in the previous analyses, the clade comprising L. fiorelinii + L. reniformis + L. parareniformis was recovered with high support. The species within this group are morphologically similar in sharing a reniform shape of the pupal thoracic horn.
Biogeographical analyses
The reconstruction of ancestral distribution areas estimated in BBM reconstruction analysis (Fig. 3) mostly agreed with the preferred phylogenetic hypothesis for Labrundinia (Fig. 2). Posterior probability values were basically the same in both analyses. The BBM reconstruction produced a new clade comprising L. sofiae, L. kogilamae and L. setosa, however, with negligible posterior probability (2%). The divergence between the Bayesian runs 1 and 2 was 0.0004, which according to Yu et al. (2013) is adequate for indicating a proper number of generations.
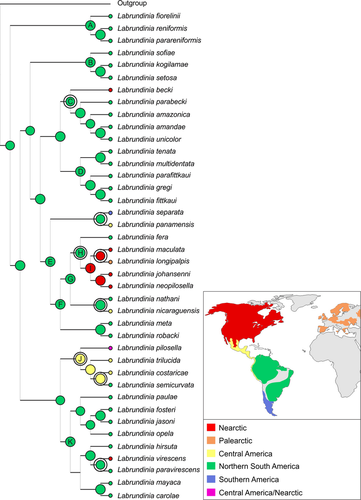
BBM results suggest that a complex biogeographical history shaped the current distribution of Labrundinia. The optimal reconstruction (Fig. 3) inferred at least eight dispersal events to explain the present distribution of Labrundinia and favoured its ancestor as having originated in Neotropical region. The major components of the distribution of Labrundinia have their origin in northern South America (Clade A–G and K), with most or all species of numerous main clades occurring in this area and highly supported (Table 2). Clade H, comprising the species L. fera, L. maculata, L. longipalpis, L. johannseni, L. neopilosella, has an initial diversification in northern South America, to subsequent diversification occurring into the Nearctic region (Clade I). Whereas, clade J comprising L. pilosella, L. trilucida, L. costaricae and L. semicurvata presents ambiguous initial diversification (Table 2), favouring Central America as its optimal ancestral area with later diversification into Nearctic and northern South America. The uncertainty on the ancestral reconstruction of this clade might be attributed to the presence of L. pilosella, which presents a range extending also into the Nearctic region.
| A | B | C | D | E | F | G | H | I | J | K | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nearctic | - | - | 0.2 | - | - | - | - | 0.2 | 83.9 | 5.6 | - |
| Central America | - | - | - | - | 0.2 | - | 0.1 | 0.1 | 0.1 | 60.9 | - |
| Northern South America | 98.4 | 99.5 | 95.9 | 99.7 | 96.5 | 98.4 | 97.5 | 96.1 | 5.9 | 5.0 | 96.6 |
| Southern South America | - | - | - | - | 0.1 | - | - | - | - | - | - |
| Palearctic | - | - | - | - | - | - | - | - | 0.3 | - | - |
| Nearctic/Central America | - | - | - | - | - | - | - | - | 0.1 | 14.3 | - |
| Nearctic/Northern South America | 0.4 | 0.1 | 3.6 | 0.1 | 0.1 | 0.1 | 0.4 | 2.7 | 9.1 | 1.2 | 0.1 |
| Nearctic/Palearctic | - | - | - | - | - | - | - | - | 0.5 | - | - |
| Central America/Northern South America | 0.9 | 0.4 | 0.2 | 0.2 | 2.4 | 1.4 | 1.9 | 0.7 | - | 12.8 | 3.3 |
| Northern SA/Southern South America | 0.2 | - | 0.1 | - | 0.6 | - | - | 0.1 | - | - | - |
| Northern/Palearctic | 0.2 | - | 0.1 | - | 0.1 | - | - | - | - | - | - |
Although all ancestral areas produced by the BBM analysis exhibit certain ambiguity, most of the putative reconstructed areas are well supported, with marginal probability values higher than 88% (Table 2), especially those occurring at an early point in the history of the group, meaning northern South America. These findings support previous ideas that the group originated in Neotropical region (Fittkau 1962; Fittkau & Roback 1983), indicating the importance of this area in the diversification of Labrundinia. So far, most Labrundinia species are distributed in only one biogeographical province. For instance, Labrundinia separata is the only species endemic to southern South America, distributed from Argentinian Pampas to Valdivian Forest, while L. longipalpis is the only one occurring in the Palearctic region. The latter species is morphologically nearly identical to L. maculata, native to North America and considered its junior synonym (Silva et al. 2011). However, the genetic similarity between Nearctic and Palearctic specimens of L. longipalpis remains unclarified (Silva et al. 2011) and requires further study of fresh material to understand the biological species boundaries in Labrundinia (Silva et al. 2013). The reconstructed Neotropical origin and later dispersal into adjacent regions is clear when analysing, for instance, clades C, E and K and their respective members. These clades diversified initially in northern South America and include terminal species occurring in the Central America, Nearctic and southern South America.
Dispersal events out of South America has also been recorded in suboscine birds and according to Ericson et al. (2002) probably occurred after the formation of the Panama isthmus (ca. 3–5 Myr ago). However, in contrast to the patterns observed for Labrundinia, suboscines birds show more geographical structuring in their phylogeny with the New World and Old World groups as monophyletic sister lineages (Sibley & Ahlquist 1990; Irestedt et al. 2001). Initial diversification in the Neotropical region with subsequent dispersal northwards is concordant with patterns observed for the Mexican and North American Tertiary floras, which relate the northern advance of the entomofauna to the expansion of the tropical forest (Lanteri 1990). During the Eocene, the Neotropical flora spread northward into western North America (Halffter 1964). As the temperature decreased in the Tertiary, the northern boundary of the flora, and possibly its related fauna, receded southwards (Lanteri 1990). The pronounced lowering of temperature during the Miocene caused a remarkable flora (Dorf 1960), in which the majority of extant phytocenosis (Howden 1969) and associated faunas were represented (Howden 1966). Initial diversification in the Neotropics with later dispersal events into the Nearctic region also has been found in diplopterous wasps (Bequaert 1940), curculionid beetles (Lanteri 1990) and toads (Pauly et al. 2004). Our results thus supports the value of studying these regions to understand species diversification processes in the New World.
Conclusion
The monophyly of Labrundinia is supported by five morphological synapomorphies. The group is recovered in all analyses using Bayesian inference and parsimony on a reduced taxon–character matrix or from a weighted morphology data set. Our findings are congruent with previous ideas regarding internal relationships within Labrundinia (Roback 1987a). Previously proposed species groups, such as the neopilosella and the virescens groups, were recovered in all analyses, although support values were low or absent for many nodes. An exception is the well-supported clade comprising L. fiorelinii, L. reniformis and L. parareniformis. The species Labrundinia maculata and L. longipalpis, currently regarded as taxonomic synonyms, are recovered as sister species in all analyses, except in the parsimony analyses on morphological characters from species with described pupal and larval stages. Bayesian binary MCMC (BBM) analysis revealed an ancestral centre of diversification in northern South America, and Labrundinia seems to have subsequently colonized the Nearctic region and southern South America in later dispersal events. This study provides an initial framework for understanding the evolutionary history of Labrundinia lineages from the New World. Although recent years have seen increased activity concerning the genus, the knowledge on phylogenetic relationships and distribution of Labrundinia as well as other Chironomidae genera, especially in Central and South America, remains fragmentary. Thus, additional inventories are required to discover and analyse possible areas of endemism and ratify the results presented in this study. Moreover, further analyses including well-supported phylogenies of clades currently containing species with missing sequences and unknown immature stages will allow verification or falsification of the biogeographical hypotheses presented here. Finally, future studies also should seek to investigate the tempo of diversification using calibrated molecular clocks on species-level phylogenies to test hypotheses on dispersal and colonization events out of South America.
Acknowledgements
The authors extend their thanks to Augusto Siri, Broughton Caldwell, Caroline S. N. Oliveira, Charles Watson, Erica McAlister, Jan Peters, Jason Weintraub, Jérôme Constant, Jon Gelhaus, John Epler, Humberto F. Mendes, Manuel Pescador, Marion Kotrba, Martin Spies, Philip Perkins, Scott E. Brooks, Sofia Wiedenbrug, Susana T. Strixino and Victor G. Smith for providing us with important material. We also thank Paula F. M. Rodrigues for comments on the phylogenetic analysis. F. L. da Silva received financial support from the National Council for Scientific and Technological Development (CNPq proc. 141092/2009-2). Financial support was also given by the Research Council of Norway (RCN) through a Personal Mobility Grant: “(proc. 211023/F11)”.




