Cold non-ischemic heart preservation with continuous perfusion prevents early graft failure in orthotopic pig-to-baboon xenotransplantation
Jan-Michael Abicht, Paolo Brenner contributed equally to this work.
Abstract
Background
Successful preclinical transplantations of porcine hearts into baboon recipients are required before commencing clinical trials. Despite years of research, over half of the orthotopic cardiac xenografts were lost during the first 48 hours after transplantation, primarily caused by perioperative cardiac xenograft dysfunction (PCXD). To decrease the rate of PCXD, we adopted a preservation technique of cold non-ischemic perfusion for our ongoing pig-to-baboon cardiac xenotransplantation project.
Methods
Fourteen orthotopic cardiac xenotransplantation experiments were carried out with genetically modified juvenile pigs (GGTA1- KO/hCD46/hTBM) as donors and captive-bred baboons as recipients. Organ preservation was compared according to the two techniques applied: cold static ischemic cardioplegia (IC; n = 5) and cold non-ischemic continuous perfusion (CP; n = 9) with an oxygenated albumin-containing hyperoncotic cardioplegic solution containing nutrients, erythrocytes and hormones. Prior to surgery, we measured serum levels of preformed anti-non-Gal-antibodies. During surgery, hemodynamic parameters were monitored with transpulmonary thermodilution. Central venous blood gas analyses were taken at regular intervals to estimate oxygen extraction, as well as lactate production. After surgery, we measured troponine T and serum parameters of the recipient’s kidney, liver and coagulation functions.
Results
In porcine grafts preserved with IC, we found significantly depressed systolic cardiac function after transplantation which did not recover despite increasing inotropic support. Postoperative oxygen extraction and lactate production were significantly increased. Troponin T, creatinine, aspartate aminotransferase levels were pathologically high, whereas prothrombin ratios were abnormally low. In three of five IC experiments, PCXD developed within 24 hours. By contrast, all nine hearts preserved with CP retained fully preserved systolic function, none showed any signs of PCXD. Oxygen extraction was within normal ranges; serum lactate as well as parameters of organ functions were only mildly elevated. Preformed anti-non-Gal-antibodies were similar in recipients receiving grafts from either IC or CP preservation.
Conclusions
While standard ischemic cardioplegia solutions have been used with great success in human allotransplantation over many years, our data indicate that they are insufficient for preservation of porcine hearts transplanted into baboons: Ischemic storage caused severe impairment of cardiac function and decreased tissue oxygen supply, leading to multi-organ failure in more than half of the xenotransplantation experiments. In contrast, cold non-ischemic heart preservation with continuous perfusion reliably prevented early graft failure. Consistent survival in the perioperative phase is a prerequisite for preclinical long-term results after cardiac xenotransplantation.
1 INTRODUCTION
Cardiac allotransplantation is the best available treatment option for patients with severe terminal heart disease. Organ demand, however, exceeds the available supply.1, 2 Xenotransplantation of genetically modified porcine organs might offer a solution to the existing organ shortage.3 Recently, we reported on non-human primates which, after having received genetically modified porcine heart replacements, survived consistently for more than 3 months,4 a period proposed as one prerequisite before initiating a clinical trial.5 In this context, a key challenge to be mastered is donor organ preservation to overcome primary graft failure.
For organ preservation, antegrade flushing of the coronary arteries with cardioplegic solution followed by ischemic storage at 4°C is the standard for clinical heart transplantation.6 In this context, ischemia lasting <4 hours is associated with higher survival rates, whereas ischemic preservation of more than 6 hours is a risk factor for increased mortality within 1 year.1 Hence, better organ protection is necessary to reduce cardiac metabolism and prevent ischemia-reperfusion injury.7 Although a variety of clinical cardioplegic solutions have been used,8 analyses of their effectiveness yielded controversial results,6 suggesting that none of the currently available compounds had a clear advantage when compared to others. At present, histidine-tryptophan-ketoglutarate (HTK), University of Wisconsin (UW), and Celsior solutions are commonly used for preservation in human allotransplantation.6, 8
With ischemic preservation, perioperative graft dysfunction occurs in 3.8%-7.4% of human allotransplantations.9, 10 After orthotopic cardiac xenotransplantation, however, early graft failure is much more frequent, posing a barrier to consistent survival since mortality has been reported to be within the range of 40%-60%.11 This phenomenon has been coined “perioperative cardiac xenograft dysfunction” (PCXD).
Recently, Steen et al12 reported successful orthotopic allotransplantion of pig hearts after 24 hours of preservation. They perfused donor hearts with an oxygenated 8°C cold hyperoncotic cardioplegic nutritive solution that contained hormones and erythrocytes. After explantation, the grafts were stored in a heart preservation system, where they were further perfused for 24 hours with the same solution before being transplanted into a recipient pig. After transplantation all recipient pigs were stable with normal blood pressures without inotropic support, normal urine production, and normal blood gases, indicating adequate organ perfusion.12 In another study using the same method of organ preservation, the porcine hearts kept normal coronary artery endothelium-dependent relaxation and myocardial contractility without edema formation.13 Very recently, a phase 2 trial demonstrated the feasibility and safety of non-ischemic heart preservation in human heart transplantation.14 To improve graft survival in xenotransplantation, we applied the Steen method to our studies by perfusing the donor hearts with the same specific solution for donor organ explantation, followed by immediate continuous perfusion; during implantation, the donor organs were perfused intermittently.
The primary findings of our recent experiments in orthotopic cardiac xenotransplantation were published elsewhere.4 Here, we will focus on the immediate outcomes of this approach and discuss the effect of non-ischemic heart preservation on PCXD.
2 METHODS
2.1 Animals
Hearts from 14 juvenile pigs (German Landrace/Large White), homozygous for alpha-1,3-galactosyltransferase knockout (GGTA1-KO), and hemizygous transgenic for human CD46 (hCD46) and human thrombomodulin (hTBM) (Revivicor, Blacksburg, USA, and Institute of Molecular Animal Breeding and Biotechnology Gene Center, LMU Munich, Germany) were transplanted into male captive-bred baboons (Papio anubis; German Primate Centre DPZ). Localization and stability of hCD46 and hTBM expression were verified post-mortem.4
2.2 Anesthesia
Transthoracic echocardiographic examinations of the donor pigs were carried out 1 week prior to surgery to exclude congenital heart defects. All animals were fasted for 12 hours prior to anesthesia. Following intramuscular premedication, general anesthesia of both baboons and pigs was induced with intravenous bolus administrations of propofol (Propofol-Lipuro 2%, B. Braun Melsungen AG) and fentanyl (Fentanyl-Janssen; Janssen-Cilag GmbH). Anesthesia was maintained with either continuous infusions of propofol (0.1-0.2 mg/kg/min) or inhalation of sevoflurane (1-2 vol% endexpiratory; sevorane, AbbVie Germany GmbH & Co. KG). Analgesia was maintained with repetitive bolus administrations of fentanyl (2.5-8 µg/kg every 30-45 minutes). After endotracheal intubation, the animals were mechanically ventilated. During anesthesia, ECG and blood pressure were monitored and ventilation adjusted to end-tidal CO2 as necessary. Post-operatively, transthoracic echocardiographic examinations of the transplanted graft were carried out at regular intervals.
2.3 Surgical procedures
After median sternotomy, the donor pigs were heparinized (500 IU/kg; heparin-natrium-25000-ratiopharm, Ratiopharm GmbH). The ascending aorta was cannulated and cross-clamped. Cardioplegia was then initiated proximally, using either standard crystalloid solution or continuous non-ischemic perfusion (see below for further information). The porcine hearts were excised and stored until needed for implantation.
Median sternotomy of the recipient baboon was performed in general anesthesia. Following heparinization (500 IU/kg), the ascending aorta and both venae cavae were cannulated and connected to the heart-lung machine. Cardiopulmonary bypass (CPB) commenced, at 34°C the ascending aorta was cross-clamped, and the donor heart transplanted using the technique of Shumway and Lower.15
2.4 Donor heart preservation
Static ischemic heart preservation (IC, ischemic cardioplegia) was initiated using 20 mL/kg 4°C unmodified crystalloid cardioplegic solutions, either Custodial HTK (Dr F. Köhler Chemie GmbH) or Belzer UW solution (Preservation Solutions Inc). Following excision, the heart was submersed in cardioplegic solution and stored on ice.
Non-ischemic continuous preservation (CP) was initiated using antegrade aortic perfusion at 20 mm Hg with 600 mL 8°C cold preservation medium (oxygenated albumin-containing hyperoncotic cardioplegic solution with hormones, erythrocytes, and additional ingredients as shown in Table 1 Ref. 12). The asystolic heart was then excised and a large cannula was inserted into the ascending aorta. Continuous perfusion with the preservation medium was then provided by an extracorporeal heart preservation system consisting of a pressure- and flow-controlled roller pump, an O2/CO2-exchanger, a leukocyte filter, an arterial filter, and a cooler/heater unit as described elsewhere in more detail.12 To prevent left ventricular dilation during perfusion, the mitral valve was made incompetent using a plastic tube. The heart was then submersed into the heart-lung-machine’s reservoir filled with approximately 3 L of preservation medium at a temperature of 8°C. During preservation, the organ was continuously perfused at a pressure of 20 mm Hg; during implantation, perfusion was changed to an intermittent mode for 2 minutes every 15 minutes.
| Na+ | 136 mmol/L |
| K+ | 23 mmol/L |
| Ca2+ | 1.3 mmol/L |
| Mg2+ | 8.0 mmol/L |
| Cl− | 142 mmol/L |
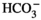
|
25 mmol/mL |
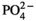
|
1.3 mmol/L |
| D-Glucose | 6.3 mmol/L |
| Albumin | 75 g/L |
| Cocaine | 6 nmol/L |
| Noradrenaline | 6 nmol/L |
| Adrenaline | 6 nmol/L |
| T3 | 3 nmol/L |
| T4 | 2 nmol/L |
| Cortisol | 420 nmol/L |
| Insulin | 8 U/L |
| Imipenem | 20 mg/L |
| Erythrocytes (Hct) | 10%-15% |
| 96% O2 + 5% CO2 | 0.2 L/min |
2.5 Immunosuppression
An immunosuppressive regimen based on Mohiuddin was used.16 In short, all animals received B cell–depleting anti-CD20 antibody (MabThera; Roche Pharma AG), ATG (Thymoglobulin; Sanofi-Aventis GmbH), and either anti-CD40 monoclonal antibody (mouse/rhesus chimeric IgG4 clone 2C10R4, NIH Non-human Primate Reagent Resource, MassBiologicalsUSA; courtesy of K. Reimann) or humanized αCD40L PAS Fab (XL-protein GmbH and Wacker-Chemie) for induction therapy. Immunosuppression was maintained with mycophenolate mofetil (CellCept, Roche), either anti-CD40 monoclonal or αCD40L PASylated Fab, and methylprednisolone (Urbason soluble, Sanofi-Aventis, Germany GmbH).
2.6 Hemodynamic monitoring of the recipient
For continuous hemodynamic measurements, a central venous catheter was placed into the internal jugular vein (Arrow International), an arterial catheter (Thermodilution Pulsiocath; Pulsion Medical Systems) into the right femoral artery. Cardiac output (CO) was assessed by transpulmonary thermodilution and indexed to body surface area using the formula 0.083 × B0.639, where B is body weight in kg17 to obtain the cardiac index (CI). Hemodynamic measurements were done in triplets at a steady state: after induction of anesthesia, one hour after weaning from CPB, after closing the chest, and before removing the arterial catheter. Data were recorded with PiCCOWin software (Pulsion Medical Systems).
2.7 Blood gas analyses and laboratory tests
Central venous oxygen saturation (scvO2 [%]) and lactate [mmol/L] were measured before surgery and at regular intervals after weaning from CPB (RAPIDlab® 1265, Siemens). Blood samples testing major organ functions were taken in the morning of the first post-operative day or before euthanasia, if the experiment had to be terminated earlier.
2.8 Anti-non-Gal antibody titers of the recipient
Preoperative plasma titers of anti-non-Gal IgM and IgG antibodies were measured by flow cytometry following the consensus protocol published previously18 and described in more detail elsewhere.4 In brief, aortic endothelial cells of genetically modified pigs (GTTA1-KO/hCD46/hTBM) were incubated with diluted baboon plasma. After washing with cold staining buffer, the cells were incubated with goat anti-human IgM-RPE (SouthernBiotech) or goat anti-human IgG-FITC (Thermo Fisher Scientific). After rewashing and resuspending, fluorescence was acquired on FACS LSRII (BD Biosciences). Data were analyzed using FlowJo software for detection of mean fluorescence intensity in the FITC or RPE channel.
2.9 Statistics
Data analysis was performed with GraphPad Prism 8.0 (GraphPad Software Inc). For analysis of two independent factors in repeatedly measured animals, a mixed model using a compound symmetry covariance matrix which is fit using restricted maximum likelihood was applied. This mixed model can manage missing values in contrast to a repeated measures (RM) two-way ANOVA. In the absence of missing values, this method gives the same p values and multiple comparisons tests as RM ANOVA. In the presence of missing values (missing completely at random), the results can be interpreted like a RM ANOVA. Statistically significant differences between two independent groups were determined using unpaired two-sided Student’s t tests. Data are presented as single measurements with group means ± standard deviations (SD). P < .05 was considered significant.
2.10 Study approval
The study was carried out according to the European Law on Protection of Animals for Scientific Purposes and was approved by the Government of Upper Bavaria, Germany. Care of the animals was in accordance with the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH publication No. 85-23, 1985) and the German Law for the Care of Experimental Animals (German Legislation for the Welfare of Laboratory Animals, article 5, §7 - §9a, revised 2006).
3 RESULTS
3.1 Initial donor graft function
Table 2 summarizes perfusion methods, perfusion times, initial graft function, and survival times of the individual experiments. For IC, HTK solution (n = 3) and UW solution (n = 2) were used as cardioplegia. Mean total ischemia time in this group was 123 ± 7 minutes. All organs preserved with HTK showed severely impaired function after CPB and the grafts failed within 24 hours; two animals could not be weaned from respirator due to hemodynamic instability. Organs preserved with UW solution showed good (ID 16755) or moderately impaired (ID 16752) function. In the case of ID 16755, cardiac function remained unchanged during the first 72 hours, whereas experiment ID 16752 had to be terminated after 72 hours due to multi-organ failure.
| ID | Preservation method | Ischemia time (min) | Perfusion time (min) | Initial graft function | Suture-extubation (min) | Post-operative survival (d) | |
|---|---|---|---|---|---|---|---|
| Continuous | Intermittent | ||||||
| 16752 | IC (UW) | 124 | n/a | n/a | Moderately impaired | 377 | 3 |
| 16754 | IC (HTK) | 128 | n/a | n/a | Severely impaired | Not extubated | 1 |
| 16755 | IC (UW) | 112 | n/a | n/a | Good | 264 | 30 |
| 16751 | IC (HTK) | 121 | n/a | n/a | Severely impaired | 785 | 1 |
| 16753 | IC (HTK) | 128 | n/a | n/a | Severely impaired | Not extubated | 1 |
| 16048 | CP | n/a | 44 | 114 | Good | 256 | 18 |
| 17138 | CP | n/a | 85 | 178 | Good | 320 | 4 |
| 17140 | CP | n/a | 94 | 105 | Good | 326 | 27 |
| 17139 | CP | n/a | 91 | 112 | Good | 296 | 40 |
| 17187 | CP | n/a | 96 | 99 | Good | 207 | 51 |
| 17290 | CP | n/a | 86 | 94 | Good | 254 | 90 |
| 17186 | CP | n/a | 124 | 120 | Good | 414 | 90 |
| 17493 | CP | n/a | 161 | 87 | Good | 390 | 195 |
| 17491 | CP | n/a | 136 | 94 | Good | 293 | 182 |
- Abbreviations: CP, continuous perfusion; HTK, histidine-tryptophan-ketoglutarate; IC, ischemic cardioplegia; UW, University of Wisconsin.
In CP-preserved organs, mean out-of-body organ perfusion times were 213 ± 35 minutes, of which continuous perfusion time inside the preservation system accounted for 102 ± 34 minutes. Organ preparation and anastomosis time was similar to that of IC-preserved hearts (111 ± 27 minutes; P = .3920). In contrast to the IC group, all CP animals were successfully extubated and suture-extubation time was significantly shorter (median 785 minutes vs 296 minutes; P = .0276, log-rank test). Organ function remained excellent throughout the first 72 hours in all experiments.
Collectively, these data suggest that CP is superior to IC in this xenotransplantation setting.
3.2 Catecholamine support in transplanted baboons
All animals needed catecholamine support for several hours after weaning from CPB (Table 3, Figure 1A). Noradrenaline, a primarily vasopressor agent administered to counteract vasodilation caused by anesthesia, extracorporeal circulation, and ischemia-reperfusion injury, was gradually reduced over time in both groups (F(3,36) = 5.679, P = .0027). However, vasopressor demand after CPB was significantly higher in animals with IC-preserved grafts, indicating that ischemia-reperfusion injury was more pronounced when compared to CP preservation (F(1,12) = 15.14, P = .0021).
| Parameter | Time point | Ischemic cardioplegia | Continuous perfusion | ||
|---|---|---|---|---|---|
| Mean ± SD | n | Mean ± SD | n | ||
| Noradrenalin [µg/kg/min] | Weaning from CPB | 0.14 ± 0.05 | 5 | 0.07 ± 0.06 | 9 |
| 1 h after CPB | 0.12 ± 0.05 | 5 | 0.02 ± 0.02 | 9 | |
| 2 h after CPB | 0.10 ± 0.06 | 5 | 0.02 ± 0.02 | 9 | |
| 3 h after CPB | 0.08 ± 0.08 | 5 | 0.02 ± 0.03 | 9 | |
| Adrenalin [µg/kg/min] | Weaning from CPB | 0.05 ± 0.04 | 5 | 0.04 ± 0.02 | 9 |
| 1 h after CPB | 0.15 ± 0.08 | 5 | 0.04 ± 0.05 | 9 | |
| 2 h after CPB | 0.18 ± 0.14 | 5 | 0.02 ± 0.03 | 9 | |
| 3 h after CPB | 0.30 ± 0.38 | 5 | 0.02 ± 0.03 | 9 | |

All animals were treated with adrenaline after weaning from CPB to improve cardiac contractility (Table 3, Figure 1B). Overall, IC-preserved hearts needed up to 15 times more inotropic support than CP-preserved hearts (F(1,12) = 7.724, P = .0167). We also observed a significant interaction between the preservation techniques and time (F(3,36) = 4.184, P = .0122): IC-preserved hearts required increasing doses of adrenaline, whereas inotropic support was reduced in recipients with CP-preserved hearts. This indicates early deterioration of systolic function in the IC group, in contrast to quick recovery from CPB in the CP group. Notably, hearts preserved with UW solution needed little inotropic support (Figure 1B, gray).
3.3 Hemodynamics of transplanted baboons
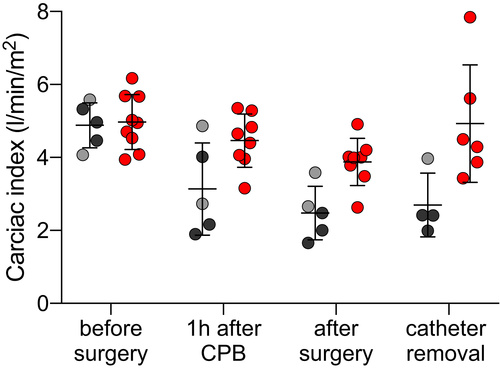
As the hemodynamic parameter of global cardiac performance, CI was measured before, during, and after surgery (Table 4 and Figure 2). In both groups, CI was similar before transplantation. After orthotopic transplantation, CI decreased in both groups (F(3,30) = 10.58, P < .0001). However, CP-preserved hearts performed significantly better in the immediate post-operative period than IC-preserved hearts F(1,12) = 12.96, P = .0036). Animals with IC-preserved hearts had a pathologically low CI after transplantation despite increasing inotropic support, indicating severe systolic heart failure, and a decreasing ability to maintain adequate organ perfusion. In these recipients, the marginal CI did not recover after surgery. By contrast, those with CP-preserved hearts had CIs that returned to normal levels within several post-operative hours (F(3,30) = 3.753, P = .0212).
| Parameter | Time point | Ischemic cardioplegia | Continuous perfusion | ||
|---|---|---|---|---|---|
| Mean ± SD | n | Mean ± SD | n | ||
| CI [L/min/m2] | Before surgery | 4.88 ± 0.62 | 5 | 4.97 ± 0.75 | 9 |
| 1 h after CPB | 3.13 ± 1.26 | 5 | 4.46 ± 0.73 | 8 | |
| After surgery | 2.48 ± 0.74 | 5 | 3.88 ± 0.65 | 8 | |
| Catheter removal | 2.70 ± 0.87 | 4 | 4.93 ± 1.61 | 6 | |
| ScvO2 [%] | Before surgery | 84.6 ± 6.5 | 5 | 84.2 ± 4.9 | 9 |
| 1 h after CPB | 41.4 ± 26.1 | 3 | 73.3 ± 8.7 | 6 | |
| 6 h after CPB | 40.4 ± 14.9 | 5 | 71.0 ± 11.7 | 9 | |
| 9 h after CPB | 44.3 ± 9.1 | 5 | 70.6 ± 3.9 | 9 | |
| 12 h after CPB | 48.5 ± 8.5 | 4 | 69.7 ± 4.2 | 9 | |
| Lactate [mmol/L] | Before surgery | 0.82 ± 0.33 | 5 | 0.89 ± 0.22 | 9 |
| 1 h after CPB | 3.89 ± 3.21 | 5 | 1.45 ± 0.54 | 9 | |
| 6 h after CPB | 8.89 ± 4.95 | 4 | 1.72 ± 0.58 | 9 | |
| 9 h after CPB | 8.69 ± 3.18 | 4 | 2.38 ± 1.79 | 9 | |
| 12 h after CPB | 9.373 ± 5.533 | 4 | 1.371 ± 0.898 | 9 | |
3.4 Blood gas analyses
Central venous oxygen saturation measurements were taken to assess the compensatory increase of oxygen extraction from the blood when tissue oxygen delivery is insufficient. Table 3 and Figure 3 show ScvO2 levels before, during, and after the transplantation procedure.
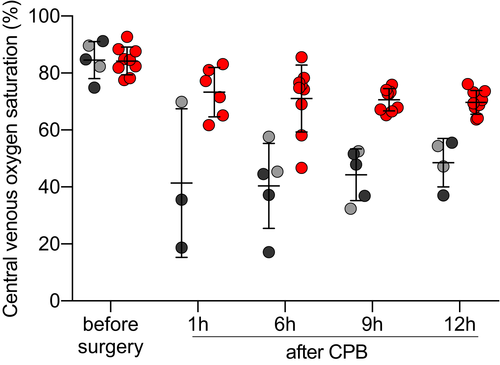
In both groups, ScvO2 was similar before transplantation. Along with the drop of CI, ScvO2 significantly decreased after transplantation in all recipients (F(4,42) = 26.21, P < .0001). In animals with CP-preserved organs, this decrease was small and ScvO2 measurements remained within acceptable ranges. In contrast, animals that received an IC-preserved graft showed highly pathological levels of ScvO2, indicating an excessive oxygen extraction from arterial blood (F(1,12) = 41.54, P < .0001). In the presence of systolic heart failure, as described by reduced CI, this indicates insufficient tissue oxygen delivery.
After surgery, ScvO2 levels minimally declined, but remained stable within normal ranges in recipients with CP-preserved hearts. In animals with IC-preserved hearts, ScvO2 levels increased slowly, but remained pathologically low for the whole immediate post-operative period (F(4,42) = 7.205, P = .0002).
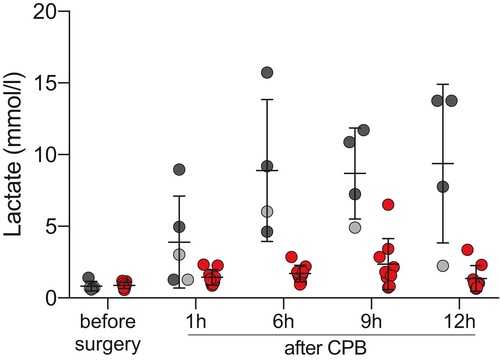
Serum lactate is considered a parameter of tissue hypoperfusion and hypoxia before, during, and after surgery (Table 4 and Figure 4). Both groups showed elevated levels of serum lactate after weaning from CPB (F(4,45) = 23.73, P < .0001). In the CP group, post-operative levels rose only moderately, whereas highly pathological levels were reached in the IC group (F(1,12) = 22.74, P = .0005), indicating severe microcirculatory hypoperfusion and hypoxia. Furthermore, serum lactate increases were transient in the animals with CP-preserved hearts and levels returned to normal within 12 hours after CPB. In contrast, there was no return to baseline in animals with IC-preserved hearts (F(4,45) = 15.86, P =< 0.0001). Notably, serum lactate levels remained also low when IC preservation was done with UW solution (Figure 4, gray).
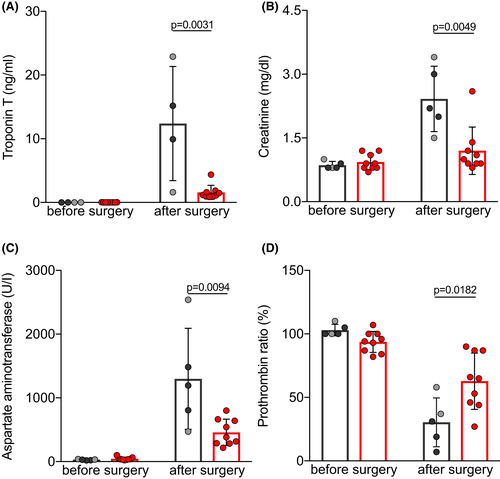
3.5 Laboratory analyses of organ functions
Serum levels of the cardiac enzyme troponin T were measured on the first day after orthotopic transplantation to assess cardiomyocyte damage (Table 5 and Figure 5A). Troponin T levels were almost eight times higher in the IC than in the CP group (P = .0031), indicating severe ischemia-reperfusion injury. Serum levels of creatinine and aspartate aminotransferase (AST), and the prothrombin ratio were measured as parameters to estimate post-operative kidney, liver, and coagulation function (Table 5). Animals with IC-preserved hearts had significantly higher serum levels of creatinine (Figure 5B; P = .0049) and AST (Figure 5C; P = .0094), and the prothrombin ratio was decreased (Figure 5D; P = .0182). When compared with the CP group, these findings indicate multi-organ dysfunction due to decreased CO of the failing graft, leading to insufficient delivery of oxygen and nutrients to the recipient’s organs.
| Parameter | Time point | Ischemic cardioplegia | Continuous perfusion | ||
|---|---|---|---|---|---|
| Mean ± SD | n | Mean ± SD | n | ||
| Troponin T [ng/mL] | Before surgery | 0.013 ± 0.000 | 4 | 0.016 ± 0.006 | 9 |
| 1 d after surgery | 12.410 ± 8.959 | 4 | 1.597 ± 1.098 | 9 | |
| Creatinine [mg/dL] | Before surgery | 0.86 ± 0.09 | 5 | 0.93 ± 0.19 | 9 |
| 1 d after surgery | 2.42 ± 0.77 | 5 | 1.20 ± 0.56 | 9 | |
| AST [U/I] | Before surgery | 29 ± 8 | 5 | 46 ± 24 | 9 |
| 1 d after surgery | 1299 ± 793 | 5 | 459 ± 207 | 9 | |
| Prothrombin ratio [%] | Before surgery | 103 ± 4 | 5 | 94 ± 8 | 9 |
| 1 d after surgery | 30 ± 19 | 5 | 63 ± 22 | 9 | |
- Donor hearts were either preserved with static ischemic cardioplegia or continuous perfusion. Data presented as mean ± SD.
- AST, aspartate aminotransferase.
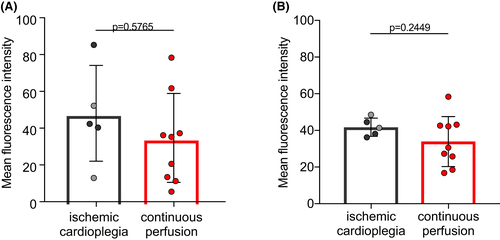
3.6 Anti-non-Gal antibody titers
To estimate preformed baboon anti-pig antibodies (Ab) as a possible cause of early xenograft failure, plasma titers of preformed anti-non-Gal Ab were measured before transplantation (Figure 6A,B). We did not observe significant differences in IgM (P = .5765) or IgG (P = .2449) Ab titers when comparing the two groups. This indicates that levels of pre-existing antibodies against non-Gal epitopes had no influence on post-operative graft dysfunction.
4 DISCUSSION
4.1 Non-ischemic preservation prevents PCXD
Perioperative graft dysfunction after orthotopic cardiac xenotransplantation was successfully prevented by non-ischemic, continuous perfusion with a hyperoncotic preservation solution containing oxygenated erythrocytes. In contrast, clinically applied crystalloid cardioplegia solutions such as HTK or UW followed by cold ischemic preservation failed to protect the xenografts from systolic pump failure, despite very short ischemia times even by clinical standards (123 ± 7 min). Under these conditions, graft failure rates of <10% would have been expected when compared to human allotransplantations.9, 10 However, three of five experiments with IC-preserved xenografts had to be terminated within 48 hours due to primary systolic failure; similar percentages have been reported by other authors.11
After IC preservation, 60% of the grafts failed to sustain an adequate circulation in spite of high inotropic and vasopressor support, and cardiogenic shock was the inevitable outcome shortly after CPB. Increased oxygen extraction could not compensate for insufficient tissue oxygen delivery, resulting in microcirculatory hypoxia and, eventually, multi-organ failure. Overall, continuous non-ischemicgraft preservation proved to be superior: cardiac function and tissue oxygenation were normal; all nine grafts showed good function, and there were no signs of organ dysfunction in the first 48 hours after transplantation.
Various experimental data suggest that pig hearts are especially susceptible to cold ischemia. Porcine grafts preserved with cold crystalloid solution and ischemic storage showed failure rates of 60%-100% within 48 hours after cardiac allotransplantations.19, 20 In a recent study, more than 40% of recipient pigs did not survive 24 hours when HTK was used as cardioplegic solution before proceeding with orthotopic allotransplantations.21 Hemodynamic measurements in the surviving pigs revealed a decrease of CO by half when compared to baseline measurements, an observation we also made in the IC group. In these allotransplantation studies, the incidence and extent of early systolic graft failure was similar to that of PCXD.
In contrast, when preserving porcine hearts with non-ischemic continuous perfusion, graft function was maintained both after allo- 12 and xenotransplantation, as our data demonstrate. We believe that a combination of several factors is responsible for these superior results: (a) By adding oxygenated erythrocytes to the perfusion solution, myocardial ischemia is absent during storage and minimized during implantation. (b) The use of hypothermia and cardioplegic solution further reduces the graft’s metabolic needs. (c) Continuous perfusion allows constant delivery of nutrients and removal of toxic metabolites. (d) High oncotic pressure in the preservation medium inhibits edema formation; strict pressure- and flow-controlled coronary perfusion helps to reduce capillary damage and aggravation of myocardial edema. (e) Lastly, physiological levels of catecholamines, cortisol, and thyroid hormones are substituted in the solution to maintain myocardial energy stores and cardiac function after cold preservation22, 23; cocaine blocks the re-uptake of catecholamines into the nerve terminals, thereby ensuring physiological concentrations around alpha- and beta-receptors in the graft.13
4.2 Hemodynamic interspecies differences
We recently showed that the systemic vascular resistance (SVR) is twice as high in anesthetized baboons as compared to size-matched, juvenile piglets.24 Elevated pulmonary vascular resistance (PVR) is an accepted risk factor for right ventricular failure after heart transplantation,25, 26 but arterial hypertension and increased SVR have not been associated with post-operative graft dysfunction in humans.10 Siepe et al reported that SVR and PVR increased 2-fold 24 hours after porcine allotransplantation.21 This group concluded that in the absence of diminished contractility, vascular resistances may be the main cause of myocardial dysfunction.
To our understanding, the function of our IC-preserved porcine grafts was severely damaged by prolonged ischemia-reperfusion injury, resulting in myocardial stunning with reduced contractility (“stone heart”). Although hearts have the potential to recover after ischemia-reperfusion injury, such dysfunctional grafts will initially have difficulties providing a sufficient CO. When confronted with a species-specific afterload mismatch, the donor hearts might not be able to cope with the increased workload, eventually leading to early systolic graft failure. Histology of the failed IC grafts is consistent with changes caused by ischemia-reperfusion damage, inadequate cardioplegia or inotropic agents (Figure 7): multiple small foci of myocyte injury with homogenization and hypereosinophilia of the sarcoplasm, but no signs of acute cellular or hyperacute rejection nor inflammatory infiltrate.
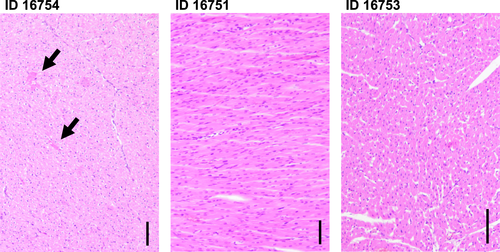
With CP preservation, ischemia-reperfusion injury is nearly absent, allowing for optimal graft function and coping with the baboon’s high-pressure cardiovascular system.
4.3 Anti-non-Gal antibodies and thromboregulation incompatibilities
Genetically modified pigs with GGTA1-KO are not prone to hyperacute rejections caused by preformed antibodies against galactose-α-1,3-galactose. However, residual preformed anti-non-Gal antibodies bind to GGTA1-KO pig cells and are widely present in humans and non-human primates such as baboons.27 Anti-non-Gal antibodies have been reported to contribute to early and late graft rejections and are likely the primary cause of delayed xenograft rejection.28-31 In our study, we did not observe any differences in preoperative plasma levels of anti-non-Gal IgM or IgG antibodies between the two groups. Thus, the complete absence of any case of PCXD in the CP group suggests that anti-non-Gal antibodies did not cause PCXD in the IC group.
Ischemia-reperfusion injury is believed to be initiated by recognition of neo-epitopes exposed on injured cells, leading to activation of the complement system and acute inflammation, ultimately resulting in tissue injury.32 This mechanism might be aggravated by incompatibilities between the porcine and primate coagulation and complement systems. In our study, donor genetic modifications were identical in both groups; all donor animals were hemizygous transgenic for hCD46 and hTBM, which have been shown to reduce any type of rejection reaction as well as thrombotic microangiopathy.33, 34 As we did not observe any case of early graft failure in the CP group, it is unlikely that complement or coagulation incompatibilities may cause PCXD in absence of ischemia-reperfusion injury.
4.4 Differences in cardioplegic solutions for ischemic preservation
We observed differences in immediate outcome depending on the cardioplegia solution applied. However, as only a small number of experiments has been done with either solution, these observations may be arbitrary. Hearts preserved with HTK solution showed poor overall results; hearts preserved with UW solution seemed to perform better, as indicated by lower catecholamine support, post-operative oxygen extraction, and serum lactate levels. One baboon (ID 16755) was the longest survivor in this group, whereas the other one (ID 16752) worsened when conferred to his cage, requiring high doses of catecholamines over several hours. In the following 48 hours, graft function fully recovered. At this point, multi-organ dysfunction had already developed, and the experiment was terminated. In a recent meta-analysis in human allotransplantation, UW was associated with better survival as compared to HTK.35 Steen et al12 stressed the importance of a hyperoncotic preservation solution for organ storage to prevent myocardial edema during perfusion of cardioplegic hearts. Containing large amounts of the colloid hydroxyethyl starch, UW might be the better IC solution for ischemia-sensitive pig organs. However, no amount of colloid active substances can prevent ischemia, only oxygen can.
4.5 Limitations
Tissue sampling was not possible immediately at the time points of hemodynamic data collection. Myocardial biopsies would have given detailed insights into histological and molecular changes but would have endangered the outcome of the experiment (bleeding, perforation).
Blood gas analyses and hemodynamic measurements were not performed in all experiments at all times due to premature termination or technical reasons. We tried to compensate for missing values by applying a mixed model for data analysis.
During preservation, the CP-preserved hearts received several substances which IC-preserved hearts did not, such as thyroid hormones and catecholamines (Table 1). We did not try to improve standard cardioplegic solutions (HTK, UW) for xenotransplantation purposes; with such optimized cardioplegic solutions, more consistent survival after orthotopic cardiac xenotransplantation might also be feasible. At the moment, CP preservation is about 100-fold more expensive than IC preservation; in our opinion, however, IC preservation of porcine hearts will always carry a higher risk of systolic pump failure, thus jeopardizing both the outcome of animal experiments and the future success of clinical cardiac xenotransplantation.
5 CONCLUSION
Cold non-ischemic heart preservation with continuous perfusion guarantees optimal xenograft function in the early post-operative period after orthotopic cardiac pig-to-baboon xenotransplantation and thus prevents from PCXD. Hence, consistent long-term survival as a prerequisite for clinical application of xenotransplantation is possible.
ACKNOWLEDGMENTS
The authors thank the German Primate Center and the Walter Brendel Centre of Experimental Medicine for support and provision of facilities, especially D. Merkus, U. Pohl, M. Shakarami, and all animal caretakers. Financial support was provided by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG) TRR 127. Open access funding enabled and organized by Projekt DEAL.
CONFLICT OF INTEREST
David Ayares is chief executive officer and chief scientific officer of Revivicor Inc. The other authors have no conflicts of interest to disclose.