Target-site resistance to ALS-inhibiting herbicides in Stellaria media, Papaver rhoeas, Glebionis segetum and Veronica persica from Ireland
Subject Editor: Lena Ulber, Julius Kühn-Institut, Braunschweig, Germany
Abstract
The increasing reliance on acetolactate synthase (ALS)-inhibiting herbicides alone has resulted in the evolution of resistance in key broad-leaf species in cereal farms in Ireland. Our objective was to confirm and characterise the resistance levels and mechanisms in resistance-suspect populations (R) of Stellaria media (STEME-R1, STEME-R2 and STEME-R3), Papaver rhoeas (PAPRH-R), Glebionis segetum (GLESE-R) and Veronica persica (VERPE-R) to two different ALS-inhibiting herbicide chemistries, sulfonylurea (SU) and triazolopyrimidine (TP). Following single-dose testing that confirmed inadequate control of all six R populations, dose–response experiments revealed varying resistance levels to SU and/or TP, which were associated with target-site resistance mutations. Weed species populations, STEME-R1 and STEME-R3 (both Trp-574-Leu), and STEME-R2 (combined Trp-574-Leu and Pro-197-Ser; first report for this species), and PAPRH-R (Trp-574-Leu, which was detected for the first time in all population plants, combined with Pro-197-His or Pro-197-Leu) and VERPE-R (Trp-574-Leu; first worldwide case) showed high resistance or cross-resistance to SU and TP. All herbicide-surviving plants tested were heterozygous for single or combined mutations. The G. segetum population, GLESE-R, had multiple substitutions and zygosity (Pro/Leu, Leu, Leu/Gln or Thr/Leu) at Pro-197, which was identified for the first time for this species conferred SU resistance only. Cytochrome P450 inhibitor studies on selected species did not detect metabolism-mediated resistance to SU or TP. Alternative herbicide modes of action alone or in co-formulation with ALS-TP should be used to manage these resistant populations. This study has added new information to the current knowledge of the resistance spectrum to ALS inhibitors in several broad-leaved weeds.
1 INTRODUCTION
In both winter and spring cereal crops, post-emergence acetolactate synthase (ALS) (HRAC Group 2) sulfonylurea (SU) or triazolopyrimidine (TP) inhibitors, and auxin-mimics (HRAC Group 4) form the basis of most broad-leaved weed control programmes (Hada et al., 2021). Auxin-mimics are active on broad-leaved weeds, but often with a limited weed spectrum and application timing window, and are often partnered, either tank-mix or pre-mix, with ALS inhibitors (Palma-Bautista, Portugal, et al., 2022). The ALS-SU and TP inhibitors can be either broad-leaf-active or broad-spectrum with grass and broad-leaf activity (Alwarnaidu Vijayarajan et al., 2023; Palma-Bautista, Portugal, et al., 2022). Some SU (e.g., metsulfuron, thifensulfuron, tribenuron or amidosulfuron) and some TP (e.g., florasulam) herbicides offer control of a wide range of broad-leaf species in wheat, barley or oat crops (Hada et al., 2021). Other SU (e.g., mesosulfuron or iodosulfuron) and TP (e.g., pyroxsulam) herbicides are intended mainly for grass-weed control while also targeting certain broad-leaf species, but can only be used in winter wheat (Vijayarajan et al., 2022). In addition, residual herbicides (e.g., broad-leaf-active diflufenican or broad-spectrum pendimethalin, prosulfocarb and flufenacet) applied pre-emergence or early post-emergence, also offer effective control of grass and broad-leaved weeds, especially in winter cereal crops (Vijayarajan et al., 2022). To date, the availability of different modes of action for broad-leaved weed control has made weed control practice relatively simple on most cereal-producing farms.
In Ireland, cereal crops dominate with spring barley (Hordeum vulgare L.) (120 000 ha), winter wheat (Triticum aestivum L.) (60 000 ha) and winter barley (60 000 ha), along with increasing levels of oats (Avena sativa L.) (25 000 ha) (Central Statistics Office [CSO], 2022). Spring-applied ALS inhibitors are only available in two- or three-way formulations, resulting in a large range of SU herbicide-formulated mixtures (Alwarnaidu Vijayarajan et al., 2023). Wetter autumns (average rainfall ranging from 78.6 to 126.2 mm) and fewer pre-emergence spray windows, along with the withdrawal of the broad-spectrum photosynthesis inhibitor isoproturon in 2017, have resulted in a greater reliance on ALS inhibitors (Met Eireann, 2024; Vijayarajan et al., 2022). Consequently, the risk of herbicide resistance developing in key broad-leaf species is increasing where broad-leaf-active ALS-SU is used without an auxin-mimic partner, or with sub-optimal rates of an auxin-mimic partner, or with a partner that has a too-narrow weed spectrum. Equally, herbicide resistance risk may be increased by grass- and broad-leaf-active ALS being used at rates lower than the recommended either alone or in sequence with broad-leaf-active ALS-SU (winter wheat only).
Stellaria media L. (common chickweed), Papaver rhoeas L. (common poppy), Glebionis segetum L. (corn marigold) and Veronica persica L. (common field-speedwell), among other broad-leaved weeds, are key species and will affect crop yield and quality in Ireland if not controlled (Alwarnaidu Vijayarajan, unpublished). Their seed production and longevity result in large soil weed seedbanks, requiring continuous control strategies such as herbicide use to manage them and to protect crop yields (Marshall et al., 2010; Palma-Bautista, Portugal, et al., 2022; Papapanagiotou et al., 2023). The most widely used formulated mixtures for these weeds are: metsulfuron + tribenuron or thifensulfuron + tribenuron (two ALS-SU inhibitors), mesosulfuron + iodosulfuron + amidosulfuron (three ALS-SU inhibitors), and florasulam + pyroxsulam (two ALS-TP inhibitors) (Department of Agriculture, Food and the Marine [DAFM], 2016).
Globally, 175 weed species (107 broad-leaf and 68 grass) have been identified as resistant to ALS inhibitors, mainly caused by target-site resistance (TSR), where amino acid substitutions at one of the nine positions on the ALS protein decrease herbicide target-binding (Heap, 2024; Murphy & Tranel, 2019). Non-target-site resistance (NTSR) is less common in broad-leaved weeds (Hada et al., 2021). To date, there are globally 21 confirmed reports of ALS resistance in S. media, 16 in P. rhoeas, one report in G. segetum, and none in V. persica (Heap, 2024). The ALS mutations at positions Pro-197 and Trp-574 were the most frequently detected, and, depending on the amino acid change, varying resistance levels and/or cross-resistance patterns have been recorded in these species (Tranel et al., 2024). For instance, Pro-197-Gln and/or Pro-197-Ser in S. media confer high resistance to SU but not to TP (Laforest & Soufiane, 2018; Marshall et al., 2010). Similarly, Pro-197-His or Pro-197-Leu in P. rhoeas confer high SU but no TP resistance, while Pro-197-Thr, Pro-197-Ser, Pro-197-Ala or Pro-197-Arg confer high SU but only intermediate or no TP resistance (Chtourou, Osuna, Mora Marín, et al., 2024; Délye et al., 2011; Kaloumenos et al., 2011; Scarabel et al., 2015). On the other hand, Trp-574-Leu in S. media is associated with both SU and TP resistance, but this mutant position is less frequent in P. rhoeas (Délye et al., 2011; Kati et al., 2019; Koreki et al., 2024). In the only known resistance report in G. segetum, Pro-197-Thr and/or Trp-574-Leu conferred high SU and TP resistance (Papapanagiotou et al., 2023). NTSR has been found in ALS-resistant P. rhoeas due to enhanced metabolism via cytochrome P450 monooxygenase (P450) (Chtourou, Osuna, Mora Marín, et al., 2024; Rey-Caballero et al., 2017). Recently, multiple resistance mechanisms, involving TSR and different forms of NTSR, have been found within a single field population of P. rhoeas conferring resistance to ALS inhibitor and auxin-mimic herbicides (Chtourou, Osuna, Mora Marín, et al., 2024; Kati et al., 2019; Palma-Bautista, Portugal, et al., 2022), making future control of this species even more challenging.
In Ireland, the first resistant broad-leaved weed species, a S. media population not controlled by ALS-SU-metsulfuron, was formally identified in 1996, using the testing service of ADAS, UK, but the mechanisms remain unknown (Heap, 2024). Resistance monitoring has only recently commenced in Ireland (Vijayarajan et al., 2022). This programme has dramatically increased resistance awareness and mitigation strategies (Alwarnaidu Vijayarajan et al., 2023; Vijayarajan et al., 2022). Given that little was known about broad-leaf herbicide resistance in this region, six broad-leaved weed populations suspected of being resistant to ALS-SU inhibitors were received from fields with a long history of ALS-SU use in 2023. The objectives of this study were to: (1) confirm the resistance levels and cross-resistance patterns in the resistance-suspect (R) population of three S. media (STEME-R1, STEME-R2 and STEME-R3) and one each of P. rhoeas (PAPRH-R), G. segetum (GLESE-R) and V. persica (VERPE-R) to ALS-SU (metsulfuron + tribenuron, thifensulfuron + tribenuron or mesosulfuron + iodosulfuron + amidosulfuron) and TP (florasulam + pyroxsulam) inhibitors, (2) explore the molecular mechanisms involved in ALS resistance; and (3) determine the efficacy of alternative modes of action in controlling ALS-resistant populations.
2 MATERIALS AND METHODS
2.1 Plant materials
Mature seeds and seed capsules of R populations of S. media, P. rhoeas, G. segetum and V. persica were received for testing in August 2023 (Table 1). The STEME-R2 and STEME-R3 were both from County Wicklow but from separate fields located approximately 23 km apart. The seed sampling by growers resulted in a limited seed quantity for the assays, especially for GLESE-R. All populations survived the recommended label rate of 4.1 + 60.1 g active ingredient (a.i.) ha−1 of ALS-SU-metsulfuron + thifensulfuron (Harmony Max SX, 29 + 429 g a.i. kg−1, Water soluble granule (WSG), FMC Agro Ltd), or 6 + 6 g a.i. ha−1 of ALS-SU-metsulfuron + tribenuron (Ally Max SX, 143 + 143 g a.i. kg−1, WSG, FMC Agro Ltd), applied alone (STEME-R) or tank-mixed with narrow-spectrum auxin-mimics. Herbicide-sensitive populations of S. media and P. rhoeas (from WeberSeeds Botany & Ethnobotany, Vaals, the Netherlands) and G. segetum and V. persica (from Yellow Flag Wildflowers, Gloucester, UK) were obtained from commercial seed suppliers for use as sensitive (S) standards.
| Species | Population code | Field position | County | Harvested crop |
|---|---|---|---|---|
| Stellaria media | STEME-R1 | 51°89′ N–8°69′ W | Cork | Spring barley |
| STEME-R2 | 52°82′ N–6°16′ W | Wicklow | Spring barley | |
| STEME-R3 | 52°98′ N–6°08′ W | Wicklow | Spring barley | |
| Papaver rhoeas | PAPRH-R | 53°46′ N–6°93′ W | Meath | Winter barley |
| Glebionis segetum | GLESE-R | 52°39′ N–6°72′ W | Wexford | Spring barley |
| Veronica persica | VERPE-R | 51°75′ N–8°74′ W | Cork | Spring barley |
2.2 Growing conditions and herbicide application
Seeds of the R and S populations were planted in 70 × 70 × 80 mm (nominally 240 mL) plastic pots filled with a standard soil mix containing 70% loam, 20% horticultural grit, 10% peat (medium) and 2 g L−1 Osmocote Mini™ (National Agrochemical Distributors Ltd., County Dublin, Ireland). Each replicate consisted of a plastic tray (230 × 176 × 55 mm) holding six individual (240 mL) pots, each containing one plant. For GLESE-R only, three pots were used per replicate because of insufficient seed availability. Herbicide treatments were applied using a Generation III Research Track Sprayer (DeVries Manufacturing, Hollandale, MN, USA) with a Teejet 8002-EVS flat fan nozzle, which delivers a total volume of 200 L ha−1 at a speed of 1.2 ms−1 at 250 kPa spraying pressure. The treated and untreated control plants were placed in a glasshouse maintained at a temperature of 18°C/12°C (day/night) with a 16 h photoperiod where sunlight was supplemented with artificial lighting to maintain a minimum light intensity of 250 μmol quanta m−2 s−1.
2.3 Preliminary screening with herbicide single dose
Single-dose herbicide resistance testing was conducted on R and S populations grown to the four-to-six true leaf stage (BBCH 14-16) using the recommended label rate of four ALS-inhibiting herbicides (Table 2). To find effective alternative herbicides, R and S populations of S. media, P. rhoeas and V. persica were also tested with the recommended label rate of auxin-mimic herbicide halauxifen + fluroxypyr (Pixarro, 12 + 403.5 g a.i. L−1, EC, Corteva Agriscience) at 6.3 + 140 g a.i. ha−1 and co-formulated auxin-mimic and ALS-TP herbicide halauxifen + florasulam (Zypar, 6.3 + 5.0 g a.i. L−1, OD, Corteva Agriscience) at 4.8 + 3.8 g a.i. ha−1 and G. segetum with auxin-mimic herbicide clopyralid (Dow Shield, 100 g a.i. L−1, SC, Corteva Agriscience) at 200 g a.i. ha−1. These auxin-mimic herbicides had the target weeds listed as susceptible on their labels. At least three replicates of each population were included for each herbicide treatment, and the entire experiment was repeated. Based on a visual check, treated plants were rated as alive (new active growth) or dead (no new active growth, severe stunting, chlorosis or no green material present) compared to untreated control plants at 30 days after treatment (DAT).
| ALS chemical family | Active ingredient (a.i.) | Dose range used in dose–response assays (g a.i. ha−1) | Recommended label rate used for preliminary screening (g a.i. ha−1) |
|---|---|---|---|
| Sulfonlyurea (SU) | Metsulfuron + tribenurona | 0.4 + 0.4, 0.8 + 0.8, 1.5 + 1.5, 3 + 3, 6 + 6, 9 + 9, 12 + 12, 24 + 24, 48.1 + 48.1 and 0 | 6 + 6 |
| Thifensulfuron + tribenuronb | - | 15 + 15 | |
| Mesosulfuron + iodosulfuron + amidosulfuronc | 0.9 + 0.3 + 1.6, 1.9 + 0.6 + 3.1, 3.8 + 1.3 + 6.3, 7.5 + 2.5 + 12.5, 15 + 5 + 25, 22.5 + 7.5 + 37.5, 30 + 10 + 50, 60 + 20 + 100, 120 + 40 + 200 and 0 | 15 + 5 + 25 | |
| Triazolopyrimidine (TP) | Florasulam + pyroxsulamd | 0.3 + 1.2, 0.5 + 2.4, 0.94 + 4.7, 1.9 + 9.4, 3.8 + 18.8, 5.6 + 28.1, 7.5 + 37.5, 15 + 75, 30.1 + 150.1 and 0 | 3.8 + 18.8 |
- a Ally Max SX, 143 + 143 g a.i. kg−1, Water soluble granule (WSG), FMC Agro Ltd.
- b Cameo Max SX, 250 + 250 g a.i. kg−1, WSG, FMC Agro Ltd. Treatment used only in preliminary screening.
- c Pacifica Plus, 30 + 10 + 50 g a.i. kg−1, Water dispersible granule (WDG), Bayer CropScience. Treatments were applied with 1% v/v biopower (alkylethersulfate sodium salt) adjuvant.
- d Broadway Star, 1.42% + 7.08% w/w, WDG, Corteva Agriscience. Treatments were applied with 1% v/v Kantor (alkoxylated triglycerides) adjuvant.
2.4 Dose–response to ALS-inhibiting herbicides
At the four-to-six true leaf stage (BBCH 14–16), dose–response assays with three ALS inhibitors were carried out to determine the levels of resistance in R populations compared to S populations (Table 2). As the response of the R and S populations to the recommended label rate of metsulfuron + tribenuron and thifensulfuron + tribenuron was similar, only metsulfuron + tribenuron was used in the dose–response assays. The selected dose rates for R populations ranged from 0.25 to 8 times the recommended label rate, and for S populations ranged from 0.0625 to 2 times the recommended label rate. The experiment was randomised with four replicates per application dose rate for each herbicide treatment, determined by the availability of seed samples from the field populations. At 30 DAT, above-ground shoot biomass was harvested from each replicate and weighed. The shoot biomass (fresh weight) for each replicate was expressed as the percentage of the mean shoot biomass of the corresponding untreated controls.
2.5 TSR analysis
Leaf samples were sampled from eight individual R plants that survived the recommended label rate (6 + 6 g a.i. ha−1) of ALS-SU-metsulfuron + tribenuron or from untreated control for S plants (n = 8) 30 DAT from the preliminary screening.
IDENTXX GmbH company (Stuttgart, Germany), performed the DNA isolation, amplification and sequencing. Air-dried leaf samples (∼0.5 cm2) were placed in tubes containing two steel beads of 4.5-mm diameter and homogenised in a shaker mill (TissueLyser II, Qiagen, Hilden, Germany), and DNA was extracted from a total of 80 leaf samples using a customised kit (Perkin Elmer, Rodgau; Chemagic Plant400 Kit) using KingFisher™ Flex Magnetic Particle Processor (Thermo Fisher Scientific, Schwerte, Germany). Polymerase chain reaction (PCR) was performed on the genomic DNA (5–10 ng μL−1) using specific primer combinations for amplification of gene fragments covering known mutated positions Pro-197 and Trp-574 of the ALS gene (Murphy & Tranel, 2019). The primer sets were designed by retrieving the partial ALS gene sequences of reference S. media (HE998774.1), P. rhoeas (AJ577316.1) and Glebionis coronaria L. (crown daisy) (MK125324.1) from the GenBank database, as well as V. persica from IDENTXX own database (not publicly available). Target DNA was amplified in a thermal cycler (T100 PCR thermal cycler, Bio-Rad Laboratories GmbH, Feldkirchen, Germany) using initial denaturation for 3 min at 95°C, followed by 40 cycles consisting of 95°C for 10 s, 60°C for 35 s, 72°C for 30 s and 72°C for 5 min for final extension. Successful amplification was checked using agarose gel electrophoresis. The PCR products were analysed for single nucleotide polymorphism (SNP) using pyrosequencing on a PyroMark Q24 (Qiagen, Hilden, Germany) using specific sequencing primers (Table 3). During the sequencing reaction, all incorporated nucleotides of a short region covering the position of interest were detected and reported by creating a pyrogram in a pyrorun file. Subsequently, the file was read by the PyroMark Q24 software (2.0.8) and visually evaluated for mutations.
| Species | Primer name | Primer sequence (5′ → 3′) | Product size (bp) | Targeted mutation site |
|---|---|---|---|---|
| S. media | Ste197-for | CTGACGCGCTGCTTGACTC | 103 | ALS Pro-197 |
| Ste197-rev | CACCTCGACAATTGGCGTTTC | |||
| Ste197-seq | GTCCCGATCATCCGT | |||
| Ste574-for | TATCGATGGTGACGGGAGTTTC | 185 | ALS Trp-574 | |
| Ste574-rev | CTGAATCATCGGAAGGATCCC | |||
| Ste574-seq | GCTTTGTAGAATCGATCTTC | |||
| P. rhoeas | Pap197-for | TAATCTTGTTAGCGGTCTTGCC | 111 | ALS Pro-197 |
| Pap197-rev | GGAGTTTCCTGAAATGCATCAGTA | |||
| Pap197-seq | TGCTGTAACTGGTCAAGT | |||
| Pap574-for | AGGGTGGAGAATTTACCAGTCA | 112 | ALS Trp-574 | |
| Pap574-rev | CAAGATATGTGTGAGCCCTGTTT | |||
| Pap574-seq | TGGGTATGGTTGTTCA | |||
| G. segetum | Chy197-for | GGTGCTACTAACCTTGTTTCTGG | 194 | ALS Pro-197 |
| Chy197-rev | ACAACACGAGGAATATCGTCAAC | |||
| Chy197-seq | CAGTACCAATCATCCTACG | |||
| Chy574-for | AGCATTTGGGTATGGTGGTACA | 58 | ALS Trp-574 | |
| Chy574-rev | TGCGCCCTATTTGCCTTA | |||
| Chy574-seq | TGCCTTATAAAACCTATCCT | |||
| V. persica | Ver197-for | ATAGTGTGCCCATGATTGCG | 73 | ALS Pro-197 |
| Ver197-rev | GTCTCTTGAAAAGCATCAGTACCA | |||
| Ver197-seq | CGATTACGGGTCAGG | |||
| Ver574-for | AGGGTGGAAAACCTTCCTGTTA | 100 | ALS Trp-574 | |
| Ver574-rev | GAGCCCGATTTGCCTTATAGA | |||
| Ver574-seq | GCCTTATAGAAACGGTCT |
- Abbreviation: ALS, acetolactate synthase.
2.6 Effect of malathion or piperonyl butoxide on ALS-SU and TP resistance
Two cytochrome P450 inhibitors malathion and piperonyl butoxide (PBO) were used to evaluate the possible involvement of metabolic NTSR to ALS inhibitors in R populations. Dose–response assays were conducted on S. media, P. rhoeas and V. persica only, using herbicide dose rates (0.125×, 0.25×, 1×, 2× and 4×) of SU-mesosulfuron + iodosulfuron + amidosulfuron (x = 15 + 5 + 25 g a.i. ha−1 respectively) or TP-florasulam + pyroxsulam (x = 3.8 + 18.8 g a.i. ha−1 respectively). At the four-to-six true leaf stage (BBCH 14-16), the R and S populations were treated with all combinations of herbicides and P450 inhibitors: malathion, PBO, SU or TP herbicides alone, malathion + SU and malathion + TP, and PBO + SU and PBO + TP, along with untreated control plants. Malathion was applied at a rate of 1000 g ha−1 and PBO at a rate of 2222.2 g ha−1 (along with 0.1% v/v Tween-80; Merck Life Science Limited, County Wicklow, Ireland), both administered approximately 3 h before herbicide application. The R populations received herbicide dose rates of 1×, 2× and 4× and S populations received 0.125×, 0.25× and 1×. There were four replicates of each treatment and dose. At 30 DAT, above-ground plant material was harvested from each replicate, and shoot fresh weight was recorded.
2.7 Statistical analysis
Data analyses were performed in R (v.3.6.3) (R Core Team, 2020). Analysis of variance (ANOVA) revealed no significant variation for treatment × experiment interactions (p > 0.05); therefore, data from single-dose testing were averaged over the six replicates. Dose–response models were fitted to the shoot fresh weight data using the drc package through the lack-of-fit F-tests (p > 0.05) (Ritz et al., 2015). For S. media, G. segetum or V. persica, a three-parameter log-logistic model was used to model the data of SU and TP. For P. rhoeas, a three-parameter log-logistic model was used to model the data of metsulfuron + tribenuron and a four-parameter log-logistic model to model the data of mesosulfuron + iodosulfuron + amidosulfuron and florasulam + pyroxsulam. Fitted models estimated the growth rate GR50 (i.e., the effective dose rate required to obtain a growth reduction of 50% relative to untreated plants) for each a.i. in the SU and TP formulated mixtures. The resistance index (RI) was calculated as the GR50 of the R population divided by the GR50 of sensitive standard.
3 RESULTS
3.1 Preliminary screening with herbicide single dose
Compared to the S populations, control of the R populations with the recommended label rate of ALS-SU (metsulfuron + tribenuron, thifensulfuron + tribenuron or mesosulfuron + iodosulfuron + amidosulfuron) and/or TP (florasulam + pyroxsulam) inhibitors was inadequate. All plants (100%) from STEME-R1, STEME-R2 and STEME-R3 survived SU and TP herbicide application. The PAPRH-R also had 100% surviving plants to SU but 58.3% (±3.73) to TP. The GLESE-R had varying plant survival to SU formulations, with 100% surviving two-way and 77.8% (±7.03) surviving three-way, while they were sensitive (i.e., all treated plants died) to TP. The VERPE-R had 100% surviving plants across all four ALS inhibitors. These results confirm that STEME-R, PAPRH-R and VERPE-R populations exhibited SU and TP cross-resistance, while the GLESE-R population was only SU-resistant. However, where alternative herbicides (HRAC Group 4) were evaluated, the R populations behaved similarly to S populations, showing sensitivity to alternative herbicides (i.e., S. media, P. rhoeas and V. persica sensitive to halauxifen + fluroxypyr or halauxifen + florasulam and G. segetum sensitive to clopyralid) tested at the recommended label rate. These results confirm that auxin-mimic herbicides applied alone or in co-formulation with ALS-TP remain effective in controlling these ALS-resistant populations.
3.2 Dose–response to ALS-inhibiting herbicides
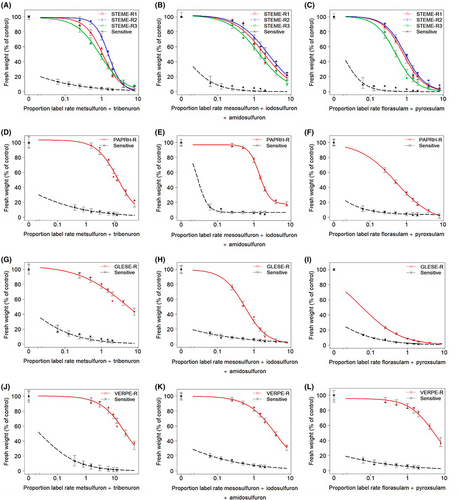
Dose–response experiments showed variable resistance levels as indicated by resistance indices to ALS-SU (metsulfuron + tribenuron and mesosulfuron + iodosulfuron + amidosulfuron) or TP (florasulam + pyroxsulam) chemistry (Figure 1; Table 4) in populations of STEME-R and PARPH-R. In contrast, the resistance indices in GLESE-R varied with each herbicide tested. The resistance indices in VERPE-R were consistent.
| Metsulfuron + tribenuron | Mesosulfuron + iodosulfuron + amidosulfuron | Florasulam + pyroxsulam | |||||
|---|---|---|---|---|---|---|---|
| Recommended label rate | 6 + 6 g a.i. ha−1 | 15 + 5 + 25 g a.i. ha−1 | 3.8 + 18.8 g a.i. ha−1 | ||||
| Species | Population | GR50 (g a.i. ha−1) | RI | GR50 (g a.i. ha−1) | RI | GR50 (g a.i. ha−1) | RI |
| S. media | STEME-R1 | 6.5 (0.31) + 6.5 (0.31) | >16.3 | 27.5 (2.19) + 9.2 (0.94) + 45.9 (3.64) | >30.6 | 3.1 (0.20) + 15.1 (0.97) | >10 |
| STEME-R2 | 9.3 (0.33) + 9.3 (0.33) | >23.3 | 35.0 (2.91) + 11.7 (0.97) + 58.3 (4.85) | >38.9 | 3.4 (0.22) + 17.0 (1.10) | >11.3 | |
| STEME-R3 | 5.4 (0.36) + 5.4 (0.36) | >13.5 | 20.1 (1.82) + 6.7 (0.61) + 33.4 (3.04) | >22.3 | 1.9 (0.13) + 9.5 (0.66) | >6.3 | |
| Sensitive | <0.4 + <0.4 | - | <0.9 + <0.3 + <1.6 | - | <0.3 + <1.2 | - | |
| P. rhoeas | PAPRH-R | 12.5 (1.19) + 12.5 (1.19) | >31.3 | 21.2 (0.67) + 7.1 (0.22) + 35.3 (1.12) | >23.6 | 1.6 (0.16) + 7.7 (0.81) | >5.3 |
| Sensitive | <0.4 + <0.4 | - | <0.9 + <0.3 + <1.6 | - | <0.3 + <1.2 | - | |
| G. segetum | GLESE-R | 28.1 (4.52) + 28.1 (4.52) | >70.3 | 8.5 (0.71) + 2.8 (0.24) + 14.1 (1.18) | >9.4 | 0.2 (0.02) + 1.1 (0.12) | >0.7 |
| Sensitive | <0.4 + <0.4 | - | <0.9 + <0.3 + <1.6 | - | <0.3 + <1.2 | - | |
| V. persica | VERPE-R | 24.6 (3.33) + 24.6 (3.33) | >61.5 | 58.8 (4.53) + 19.6 (1.51) + 98.0 (7.55) | >65.3 | 21.5 (2.43) + 107.6 (12.13) | >71.7 |
| Sensitive | <0.4 + <0.4 | - | <0.9 + <0.3 + <1.6 | - | <0.3 + <1.2 | - | |
- Abbreviation: GR50, the effective dose rate required to obtain a growth reduction of 50% relative to untreated plants.
The S populations of S. media, P. rhoeas, G. segetum and V. persica showed total mortality or high efficacy at the lowest doses of SU and TP herbicides with a biomass reduction of >80% (Figure 1A–L); the GR50 values were estimated to be lower than the lowest dose used (Table 4).
Among the three STEME-R populations, STEME-R1 and STEME-R2 showed a common response to both SU and TP, having high resistance indices (RI >10) (Figure 1A–C; Table 4). In contrast, STEME-R3 had high resistance (RI >10) to SU, but intermediate resistance (RI <10) to TP.
The PAPRH-R population had high SU resistance (RI >10), while intermediate TP resistance (RI <10) with GR50 values of 1.6 + 7.7 g a.i. ha−1 (Figure 1D–F; Table 4).
The GLESE-R population showed variable responses to the SU herbicide application (Figure 1G,H); high resistance (RI >10) to metsulfuron + tribenuron, but intermediate resistance (RI <10) to mesosulfuron + iodosulfuron + amidosulfuron (Table 4). While the TP dose–response assays confirmed that GLESE-R was highly sensitive (Figure 1I), with very low RI (<1) (Table 4).
The VERPE-R population had high SU and TP resistance (RI >10) (Figure 1J–L; Table 4).
3.3 TSR analysis
Compared to the S populations, the R populations possessed mutations at Pro-197 and/or Trp-574 of the ALS genes (Table 5), confirming that TSR is the dominant resistance mechanism in STEME-R, PAPRH-R, GLESE-R and VERPE-R to ALS-SU and/or TP inhibitors.
| Species | Population | Pro (CCN)-197 | Trp (TGG)-574 | Pro (CCN)-197 + Trp (TGG)-574 | |||
|---|---|---|---|---|---|---|---|
| Codon | Aa | Codon | Aa | Codon | aa | ||
| S. media | STEME-R1 | CCG (8) | Pro | T-G/T-G (8) | Trp/Leu | ||
| STEME-R2 | CCG (6) | Pro | T-G/T-G (6) | Trp/Leu | C/T-CG + T-G/T-G (2) | Pro/Ser + Trp/Leu | |
| STEME-R3 | CCG (8) | Pro | T-G/T-G (8) | Trp/Leu | |||
| Sensitive | CCG (8) | Pro | TGG (8) | Trp | |||
| P. rhoeas | PAPRH-R | CCT (2) | Pro | T-G/T-G (2) | Trp/Leu | C-C/A-T + T-G/T-G (4) C-C/T–T + T-G/T-G (2) |
Pro/His + Trp/Leu Pro/Leu + Trp/Leu |
| Sensitive | CCT (8) | Pro | TGG (8) | Trp | |||
| G. segetum | GLESE-R | CTA (2), C-C/T-A (1), C-T/A-A (3), C/A-C/T-A (2) | Leu, Pro/Leu, Leu/Gln, Thr/Leu | TGG (8) | Trp | ||
| Sensitive | CCA (8) | Pro | TGG (8) | Trp | |||
| V. persica | VERPE-R | CCA (8) | Pro | T-G/T-G (8) | Trp/Leu | ||
| Sensitive | CCA (8) | Pro | TGG (8) | Trp | |||
With STEME-R populations, all eight plants tested in STEME-R1 and STEME-R3 had heterozygous Trp-574-Leu substitutions (Table 5). In STEME-R2, six plants had heterozygous Trp-574-Leu and two plants had heterozygous Trp-574-Leu and Pro-197-Ser together. Such a Trp-574-Leu substitution conferred high SU (RI >10) and intermediate (RI <10; STEME-R3 only) or high TP resistance (Table 4).
With PAPRH-R, of the eight plants that were heterozygous, two had Trp-574-Leu, four had Trp-574-Leu plus Pro-197-His and two had Trp-574-Leu plus Pro-197-Leu (Table 5). Such a combination of mutation (Trp-574 and Pro-197) conferred high SU (RI >10) and intermediate TP (RI <10) resistance (Table 4).
With GLESE, all eight plants had Pro-197 mutations (Table 5). Three plants had Pro-197-Leu (two homozygous and one heterozygous), and five plants had trans-heterozygous (Pro-197-Leu/Gln or Pro-197-Thr/Leu). Different amino acid substitutions at Pro-197 conferred different patterns of cross-resistance, with RI and GR50 values being higher with SU-metsulfuron + tribenuron than with SU-mesosulfuron + iodosulfuron + amidosulfuron, but no TP resistance (Table 4).
With VERPE-R, all eight plants had heterozygous Trp-574-Leu that conferred high SU and TP resistance (RI >10) (Tables 4 and 5).
3.4 Effect of malathion or PBO on ALS-SU and TP resistance
The R populations experienced no significant change in biomass when treated with different herbicide dose rates of SU or TP in combination with P450 inhibitors malathion or PBO (data not shown). These results suggest that P450-mediated ALS NTSR likely did not occur in STEME-R1, STEME-R2, STEME-R3, PAPRH-R and VERPE-R populations. This assay for GLESE-R was not possible because the seed sample was limited; thus the possibility of P450-mediated ALS NTSR cannot be entirely ruled out.
4 DISCUSSION
Over-reliance on ALS-SU inhibitors to control a broad spectrum of weeds greatly increases resistance risks (Alwarnaidu Vijayarajan et al., 2023). The suspected resistant populations of S. media, P. rhoeas, G. segetum and V. persica allowed us to confirm and characterise resistance to two different herbicide chemistries of ALS formulations, SU-metsulfuron + tribenuron, mesosulfuron + iodosulfuron + amidosulfuron, and TP-florasulam + pyroxsulam. This is the first study in Ireland examining the molecular basis of ALS resistance in broad-leaved weeds, while this is the second resistant report for G. segetum and the first for V. persica documented globally. Previous studies have confirmed TSR and/or NTSR to ALS-inhibiting herbicides in two- or three-way herbicide formulations, especially in grass weeds (e.g., Alwarnaidu Vijayarajan et al., 2023; Davies et al., 2020).
Preliminary screening confirmed that STEME-R, PAPRH-R and VERPE-R had developed SU and TP cross-resistance, while GLESE-R had developed SU-resistance only. Dose–response experiments demonstrated that STEME-R1, STEME-R2 and STEME-R3 had developed high SU (RI >10) and intermediate (RI <10) (STEME-R3 only) or high (RI >10) TP resistance. Gene sequencing revealed amino acid substitutions, Trp-574-Leu in STEME-R1 and STEME-R3, and a combination of Trp-574-Leu and Pro-197-Ser in STEME-R2. In PAPRH-R, the Trp-574-Leu in combination with Pro-197-His or Pro-197-Leu conferred high SU (RI >10) and intermediate TP (RI <10) resistance. In GLESE-R, the substitutions of Pro-197 by Pro/Leu, Leu, Leu/Gln or Thr/Leu conferred varying resistance levels; high resistance to metsulfuron + tribenuron (RI >10), intermediate resistance to mesosulfuron + iodosulfuron + amidosulfuron (RI <10) and no TP (RI <1) resistance. In VERPE-R, the Trp-574-Leu conferred high SU and TP (RI >10) resistance.
The same target-site (Trp-574-Leu) conferred SU and TP cross-resistance in STEME-R, PAPRH-R and VERPE-R. This corresponds with most existing literature on grass and broad-leaf resistance, where Trp-574-Leu usually causes high resistance or cross-resistance across all herbicide chemistries of ALS inhibitors (Tranel et al., 2024). The combined Trp-574-Leu and Pro-197-Ser identified in STEME-R2 were confirmed for the first time in S. media. Co-existing Pro-197-Ser and Trp-574-Leu usually confers high SU and TP resistance as previously noted in Sinapis alba L. (white mustard) and Capsella bursa-pastoris L. (shepherd's-purse) (Chtourou, Osuna, Vázquez-García, et al., 2024; Lu et al., 2023). Previous to this study, Pro-197 substitutions were most frequent in P. rhoeas (Kaloumenos et al., 2011; Marshall et al., 2010; Palma-Bautista, Portugal, et al., 2022; Scarabel et al., 2015; Stankiewicz-Kosyl et al., 2023). Although Trp-574-Leu has been identified in P. rhoeas on three previous reports (Délye et al., 2011; Kati et al., 2019; Koreki et al., 2024); this is the first case where this substitution has been consistently detected in all PAPRH-R-sequenced plants (100%) that survived herbicide application in a population.
Different substitutions (Pro/Leu, Leu, Leu/Gln or Thr/Leu) at Pro-197 target-site in GLESE-R were identified for the first time in G. segetum. Previous studies in Glebionis spp. have found that the substitution of Pro-197 by Thr confers SU and TP cross-resistance (Papapanagiotou et al., 2023; Tal & Rubin, 2004). Typically, the patterns of ALS cross-resistance caused by Pro-197-Leu or Pro-197-Gln depend mostly on the individual weed species (Tranel et al., 2024). Nevertheless, Pro-197 is often considered SU-specific (Murphy & Tranel, 2019), which explains the TP-sensitivity recorded in GLESE-R in this study. Interestingly, the magnitude of resistance in GLESE-R based on RI and GR50 values varied within the SU-formulated mixtures, which suggests that differences in resistance indices can occur within the same chemistry. For instance, in SU-resistant populations of Raphanus sativus L. (feral radish) (Pondolfo et al., 2016), Descurainia sophia L. (flixweed) (Deng et al., 2014), Amaranthus retroflexus L. (redroot pigweed) (Sibony et al., 2001) and P. rhoeas (Stankiewicz-Kosyl et al., 2023), resistance to one SU herbicide does not necessarily result in the same resistance indices to every SU herbicide.
Though NTSR due to enhanced metabolism mediated by cytochrome P450 monooxygenases (P450) to ALS inhibitors is relatively uncommon in broad-leaved weeds (Hada et al., 2021), previous studies have found P450-mediated NTSR in P. rhoeas (Chtourou, Osuna, Mora Marín, et al., 2024; Rey-Caballero et al., 2017), G. coronaria (Hada et al., 2021), Sinapis spp. (Chtourou, Osuna, Vázquez-García, et al., 2024; Palma-Bautista, Vázquez-Garćıa, et al., 2022; Veldhuis et al., 2000), D. sophia (Yang et al., 2016); A. tuberculatus L. (waterhemp) (Guo et al., 2015) and Rapistrum rugosum L. (turnipweed) (Hatami et al., 2016). This study suggests that P450-mediated NTSR is less likely to be the cause of ALS-SU or TP resistance in STEME-R, PAPRH-R and VERPE-R populations from Ireland.
The wide crop-selectivity, broad-spectrum weed control and excellent efficacy of ALS inhibitors have resulted in widespread and intensive use, often in products containing multiple SU a.i. (i.e., two- or three SU formulated mixtures), to selectively control broad-leaved weeds in cereal fields in Ireland (Alwarnaidu Vijayarajan et al., 2023; DAFM, 2016). The excellent efficacy of alternative auxin-mimic herbicides remains a reliable tool for protecting the efficacy of ALS-SU inhibitors and slowing down the evolution and spread of ALS-resistant broad-leaf populations. As a result, herbicide control failures with broad-leaved weeds in this region are not as serious as those with grass weeds (Vijayarajan et al., 2022), but appropriate use and retained availability of auxin-mimic herbicides on the market are essential for future weed control.
In conclusion, this study has confirmed and characterised ALS inhibitor resistance in key broad-leaf species for the first time in Irish populations. Where cross-resistance was identified, growers will be unable to control these populations with an ALS herbicide. The alternative auxin-mimic herbicides, either alone or in co-formulation with ALS-TP can be used to control or eliminate resistant populations of STEME-R, PAPRH-R and VERPE-R in cereal crops. While the control of SU-resistant GLESE-R can be achieved initially by using ALS-TP in winter wheat only or auxin-mimic clopyralid in broad-leaved crops (e.g., oilseed rape). The results will help the industry and growers to develop agronomic practices based on effective herbicides to control these ALS-resistant populations. As cultural control of broad-leaved weeds is difficult, the primary control measure will likely be appropriate use of non-ALS chemistry such as auxin-mimic herbicides. Growers must note that resistance to auxin-mimics (e.g., S. media) and to both ALS inhibitors and auxin-mimics (e.g., P. rhoeas) has already been reported in Europe (Heap, 2024). A similar situation will threaten future weed control in Ireland, making active monitoring essential. Effective stewardship practices like stacking pre-emergence herbicides (in winter cereals) and using the correct rate and right herbicide combinations are also critical to reduce the weed seedbank and to delay/minimise the risk of further resistance problems.
ACKNOWLEDGEMENTS
This research was supported by funding from the project EVOLVE (Evolving grass-weed challenges and their impact on the adoption of carbon smart-tillage systems, Grant No: 2021R528), which is funded by the Department of Agriculture, Food, and the Marine (DAFM) under the Research Stimulus Fund (RSF) Programme. This work is a part of the Teagasc Climate Centre, which co-ordinates agricultural climate and biodiversity research and innovation in Ireland.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data supporting the findings of this study are available from the corresponding author upon request.


