CEREBRAL GLUCOSE UTILIZATION MEASURED WITH HIGH RESOLUTION POSITRON EMISSION TOMOGRAPHY IN EPILEPTIC FINNISH SPITZ DOGS AND HEALTHY DOGS
This work was supported by the Archimedes Foundation (Estonia), and the Finnish Kennel Club (Finland).
Portions of this paper have been presented as an oral presentation at 12th Annual Symposium of the EAVDI, Naples, Italy 2005, and at 19th Annual Symposium of the ESVN, Barcelona, Spain 2006.
Abstract
In human epileptic patients, changes in cerebral glucose utilization can be detected 2-deoxy-2-[18F] fluoro-d-glucose positron emission tomography (FDG-PET). The purpose of this prospective study was to determine whether epileptic dogs might show similar findings. Eleven Finnish Spitz dogs with focal idiopathic epilepsy and six healthy dogs were included. Dogs were examined using electroencephalography (EEG) and FDG-PET, with epileptic dogs being evaluated during the interictal period. Visual and semi-quantitative assessment methods of FDG-PET were compared and contrasted with EEG findings. Three independent observers, unaware of dog clinical status, detected FDG-PET uptake abnormalities in 9/11 epileptic (82%), and 4/8 healthy dogs (50%). Occipital cortex findings were significantly associated with epileptic status (P = 0.013). Epileptic dogs had significantly lower standardized uptake values (SUVs) in numerous cortical regions, the cerebellum, and the hippocampus compared to the control dogs. The lowest SUVs were found in the occipital lobe. White matter normalized and left-right asymmetry index values for all pairs of homologous regions did not differ between groups. Visual evaluation of the EEGs was less sensitive (36%) than FDG-PET. Both diagnostic tests were consensual and specific (100%) for occipital findings, but EEG had a lower sensitivity for detecting lateralized foci than FDG-PET. Findings supported the use of FDG-PET as a diagnostic test for dogs with suspected idiopathic epilepsy. Visual and semiquantitative analyses of FDG-PET scans provided complementary information. Findings also supported the theory that epileptogenesis may occur in multiple brain regions in Finnish Spitz dogs with idiopathic epilepsy.
Introduction
Idiopathic epilepsy is a common canine neurological problem, with an estimated prevalence of 0.6% in the general dog population.1 Published prevalence figures for different dog breeds and populations vary widely (1 to 33%).2, 3 Idiopathic epilepsy is defined as recurrent, unprovoked seizures for which no underlying brain abnormalities can be identified and a familial or genetic predisposition may be suspected.2 In humans, magnetic resonance imaging (MRI) has been reported to identify the epileptogenic lesion in 76% of patients with temporal lobe epilepsy and 46% of patients with extratemporal epilepsy.4 Improved sensitivity has been described using a higher magnetic field (3–7 T) and advanced MRI techniques.5 In dogs with idiopathic epilepsy, structural imaging with MRI or computed tomography (CT) is within normal limits.6, 7 Electroencephalography (EEG) is the standard functional method used in human medicine to characterize epileptic abnormalities originating from the cerebral cortex. Electroencephalography has limitations for veterinary use because the reliability of EEG data can be low in interictal recordings of sedated patients. One previous study reported that epileptic activity detection was as high as 86% in these types of recordings.8 We found paroxysmal epileptogenic activity in only 20% of dogs in a group of epileptic Finnish Spitz dogs in our previous study.9
Positron emission tomography (PET) has been used extensively during the last couple of decades to investigate changes in cerebral blood flow, oxygen consumption, glucose metabolism, and receptor binding in epileptic human patients using various radioligands.4, 10, 11 In human clinical epileptology, PET has mainly been used for presurgical evaluation of patients with medically refractory seizures and normal or nonspecific brain MRI findings.12 In dogs, an improved method for detecting epileptogenic foci could be helpful for localizing affected neuroanatomical structures, improving our understanding of the pathophysiology of different seizures, better characterizing epilepsy syndromes, and developing novel antiepileptic therapy options. The tracer 2-deoxy-2-[18F]fluoro-d-glucose (FDG) is the most widely used in PET studies and reflects cerebral glucose utilization.10 Recently published studies have described cerebral glucose metabolism measured by FDG-PET in healthy control dogs.13-16 In addition, FDG-PET has been used to detect focal cerebral glucose hyper- or hypometabolism in dogs with seizures due to different types of encephalitis.17, 18 The purpose of the current study was to determine whether FDG-PET with high-resolution research tomography could detect changes in cerebral glucose utilization in dogs with focal idiopathic epilepsy. We hypothesized that epileptic dogs would have areas of decreased cerebral glucose metabolism related to epileptic foci interictally, and that these would appear similar to those described in FDG-PET studies of epileptic human patients.
Materials and Methods
This prospective study was performed at the Small Animal Clinic of the University of Helsinki and Turku PET Centre in collaboration with the Finnish Spitz Breeding Club. The study protocol was approved by the Ethics Committee on Animal Trials. Eleven epileptic (eight males, three females) and eight healthy control Finnish Spitz dogs (three males, five females), were included in this study. Inclusion criteria for epileptic dogs were the clinical diagnosis of suspected idiopathic (focal) epilepsy and a complete seizure history demonstrating presence of at least two focal seizure episodes. Inclusion criteria for both epileptic and control dogs were absence of abnormalities in general physical and neurological examinations, blood examinations, and urinalysis.
The FDG tracer was synthesized at the Turku PET Centre Radiopharmaceutical Chemistry Laboratory from mannosyl triflate using a nucleophilic method. The radiochemical purity exceeded 95% in every production batch. Animal preparation and scanning procedures were performed in a quiet and darkened room. Before the PET examination, dogs were fasted for a minimum of 8 h with freely available water and then sedated with medetomidine hydrochloride (Domitor 1 mg/ml, Orion Pharma, Espoo, Finland; 30 μg/kg) and butorphanol (Torbugesic Vet 10 mg/ml, Fort Dodge Veterinaria S.A., Girona, Spain; 0.2 mg/kg intramuscular injection). After 10–15 min, an IV catheter was inserted to a cephalic vein. Dogs received oxygen through a facemask at the rate of 2 L/min throughout the period of sedation. Pulse rate and oxygen saturation were monitored during the study with a pulse-oxymeter. The FDG tracer (5.2 ± 1.1 MBq/kg) was administered intravenously at 15–25 min after the sedation. Forty minutes after tracer injection, an additional dose of intramuscular medetomidine (15 μg/kg) and midazolam (Midazolam Hameln 5 mg/ml, Hameln Pharmaceuticals, Hameln, Germany, 0.2 mg/kg) was given to ensure satisfactory depth of sedation during the scanning period.
The PET scans were performed using a brain dedicated, high resolution PET scanner (ECAT HRRT, Siemens Medical Solutions, Knoxville, TN).19 Dogs were positioned in sternal recumbency with the head inserted in the scanner. The brain area was centered in the middle of the scanning area and fixed with foam wedges specially designed for this study. The body temperature was maintained by covering the dogs with thick terry towels. Scanning was started 55 min after the injection of FDG and emission data were collected for 40 min. After the emission scan, a 7 min transmission scan for attenuation correction was performed. This protocol was possible because a point source material for transmission in the HRRT scanner is 137Cs (662keV of gamma energy). In these conditions, postinjection transmission data produce virtually identical emission results when compared to cold transmission.20
True events (i.e., prompt-random events), attenuation and scatter correction,21 and normalization factors were processed in 3D sinogram mode with an axial compression of span 9 and maximum ring difference of 67, resulting in 2209 sinograms in 16 segments. Normalization and all corrections were applied during the iterative ordered subsets expectation maximization 3D reconstruction.22 Images were reconstructed into a volume of 256 × 256 × 207 cubic voxels of size 1.81 mm3. For all dogs a 20 min long frame of the scanning-period (following 55–75 min of tracer injection) was reconstructed.
Visual analysis of PET images was performed independently by three authors (L.M., M.H-S., and M.S.) using noncommercial image analysis software (Vinci 2.56, Max-Planck-Institute for Neurological Research, Cologne, Germany). The scans of healthy and epileptic dogs were reviewed in a randomized blinded manner. Coregistration with MRI multiplanar reconstruction 3D images was used for anatomical localization of the findings. Examiners were asked to record any localized or lateralized visual asymmetries of FDG uptake that were visible through at least three slices and to classify the findings as obvious or mild. For subsequent analyses, presence or absence of FDG uptake changes required an agreement of at least two of the reviewers.
To perform the semiquantitative analysis, volumes of interests (VOIs) were manually drawn (R.V.) over dorsal (coronal) slices using image analysis software (Imadeus Academic 1.0, Forima Inc, Turku, Finland). Multiplanar reconstructions of 3D MRI images were used as anatomical references. Bilateral VOIs covered frontal, temporal, parietal, and occipital cerebral cortices; and the hippocampus, caudate nucleus, caudal colliculus, thalamus, and cerebellum. Maximal symmetry of bilateral VOIs and homology between individuals was sought. Additionally, unpaired VOIs were drawn to include cerebral white matter, the cingulate gyrus, and cerebellar vermis. For further analysis, VOIs were normalized against the white matter value of the same dog. Standardized uptake values (SUVs) were also calculated using normalization with injected FDG dose (MBq) / body weight (kg). The left-right asymmetry index (AI) for all pairs of homologous VOIs was used to estimate lateralization of changes. In the formula AI = (left – right) × 100 / ([left + right] / 2)% positive AI indicated reduced glucose metabolism in the right and negative in the left side VOI. For statistical analysis all AIs were changed to the positive sign.
Interictal EEG recordings were performed under medetomidine sedation as described elsewhere.7, 9 All EEG records were examined via blinded visual analysis (blinded for clinical status of the dogs).
Statistical tests were performed by one of the authors (T.O.) using commercially available statistical software (Stata 11.0, Stata Corporation, TX, USA). Ages and weights for dogs in epileptic and control groups were compared using a Wilcoxon rank sum test, and the Fisher exact test was used to compare sex distribution of the two groups. The Fisher's exact test was used to compare associations between epileptic status and visual findings of asymmetry in different brain regions. A nonparametric Wilcoxon rank sum test was used to compare differences between epileptic and control dogs in AI values, SUVs and white matter normalized values by brain region. A P-value less than 0.05 was considered to be statistically significant for all tests. For the visual findings of EEG and FDG-PET examinations sensitivity, as a measure of the proportion of epileptic dogs with findings, and specificity, as a proportion of control dogs without findings were calculated.
Results
Epileptic dogs had a median (range) age of 56 (41–120) months and control dogs had a median (range) age of 56 (18–114) months at the time of FDG-PET examination. Median (range) body weights for epileptic and control dogs were 14 (10–19) kg and 12 (10–15) kg, respectively. Genders, ages, and weights for dogs in the epileptic and control groups did not differ. All epileptic dogs had focal onset seizures with secondary generalization as the main type of seizures (10 dogs had seizures with complex focal and one with simple focal onset). Two dogs had additional focal seizures, and two dogs had generalized seizures. Median (range) age for the seizure onset was 22 (7–72) months. The median (range) seizure duration and seizure frequency values were 10 (0.5–15) min and 3 (0.5–12) per year, respectively. One epileptic dog had a seizure episode of 14 h and one 24 h before FDG-PET examination. Four epileptic dogs were on treatment with anti-epileptics (three dogs were on phenobarbital monotherapy and one dog was on both phenobarbital and potassium bromide treatment). Magnetic resonance imaging was performed for 18 dogs (11 epileptic and 7 healthy) and EEG was performed for 17 dogs (11 epileptic and six healthy). Results of MRI for eight epileptic and two healthy dogs included in this study have been published previously.7 The transmission FDG-PET scan was not used for the three first epileptic dogs (transmission was not possible to perform due to technical reasons) and for the sixth epileptic dog (dog moved before the end of scanning). For these four dogs, as dogs were of the same breed and the same size, an attenuation correction model was created on the basis of the transmission scanning (mu-map image) data set collected from one of the healthy dogs.
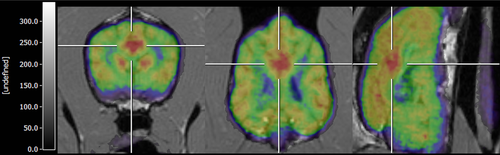
Visual evaluation of FDG-PET scans revealed the presence of 17 changes in 13 dogs (five of which were agreed upon by three examiners and 12 by two examiners). Six dogs had no changes (agreed upon in three dogs by three examiners and in three dogs by two examiners). Changes were classified as mild in five epileptic and three control dogs, and obvious in six epileptic and three control dogs. Changes in FDG uptake were present (abnormalities/asymmetries detected by at least two reviewers) in nine epileptic (sensitivity 82%), and four control dogs (50%). Some changes involved multiple brain regions. Dogs had changes in the occipital cortex (seven epileptic, zero control; sensitivity 64% and specificity 100%; Fig. 1), lateral temporal cortex (three epileptic, three control), frontal cortex (three epileptic, one control), parietal cortex (two epileptic, three control), caudal colliculus (two epileptic, two control), cingulate gyrus (one epileptic, zero control; Fig. 2), and caudate nucleus (one epileptic, zero control). No changes were present in the thalamus or cerebellum. Reviewers most often recorded the changes as hypometabolic foci (although it is impossible to evaluate absolute hypometabolism/hypermetabolism based on visual analysis alone). An exception was an area of hypermetabolism in the cingulate gyrus (gyrus cinguli) that was recorded for one dog that had experienced a seizure episode 24 h before FDG-PET examination. Four of eight healthy, and two of 11 epileptic, dogs had no changes (specificity 50%). The presence of visual asymmetry in the occipital cortex was significantly associated with epileptic status (P = 0.013; Fig. 1).
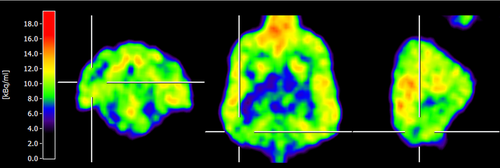
There were no significant differences in regional AI values between control and epileptic dogs (Fig. 3). For control dogs with absence of visual FDG-PET changes, AI values ranged from 0.01 to 2.7 in the cortical regions, 0.07 to 1.8 in the caudate nucleus (nucleus caudatus), 0.19 to 1.11 in the thalamus, and 0.15 to 1.65 in the cerebellum. The highest asymmetry indices were detected in the caudal colliculus (2.42) of control dogs. For hypometabolic cortical regions in epileptic dogs, the highest AI values ranged from 0.5 (direction not in agreement with visual changes) to 0.71 (direction in agreement with visual findings). In 22 of the 28 areas with visual changes (14 of 19 in epileptic dogs), the direction of asymmetry indices agreed with visual findings. All four dogs (two epileptic and two control dogs) with visual asymmetry changes in the caudal colliculus also had the highest AI in that region (from 2.7 to 4.34).

White matter normalized values and SUVs from different brain regions in epileptic and control dogs are provided in Table 1. The highest relative uptake of FDG was in the tectum (caudal colliculus) and nearly as high uptake was found in all cortical areas and in the caudate nucleus (nucleus caudatus). The lowest uptake was in the cerebellar hemispheres and paraventricular white matter, with the exception of the cerebellar vermis that had comparable uptake to the hippocampus. The epileptic dogs had significantly lower SUVs in numerous cortical regions (frontal, parietal, temporal, occipital lobes), cerebellum, and hippocampus compared to control dogs (Table 1). Treatment with anti-epileptics in four epileptic dogs caused no statistically detected effect on SUVs when compared to the epileptic dogs without treatment. White matter normalized values did not differ between the epileptic and control groups.
| WMV | SUV | |||
|---|---|---|---|---|
| Control dogs | Epileptic dogs | Control dogs | Epileptic dogs | |
| VOI | (n = 8) | (n = 11) | (n = 8) | (n = 11) |
| Region | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) |
| Fcx sn | 6.93 (1.62) | 6.45 (1.33) | 5.73 (1.11) | 4.49 (1.28)* |
| Fcx dx | 6.76 (1.45) | 6.40 (1.30) | 5.63 (1.20) | 4.46 (1.29)* |
| Fcx all | 6.85 (1.53) | 6.42 (1.31) | 5.68 (1.15) | 4.47 (1.28)* |
| Pcx sn | 7.21 (1.80) | 6.58 (1.60) | 5.94 (1.14) | 4.55 (1.41)* |
| Pcx dx | 6.88 (1.56) | 6.57 (1.49) | 5.70 (1.11) | 4.56 (1.37) |
| Pcx all | 7.05 (1.68) | 6.56 (1.54) | 5.82 (1.12) | 4.56 (1.39) |
| Tcx sn | 7.06 (1.51) | 6.63 (1.60) | 5.89 (1.32) | 4.62 (1.47)* |
| Tcx dx | 6.94 (1.28) | 6.57 (1.54) | 5.83 (1.31) | 4.59 (1.45) |
| Tcx all | 7.00 (1.39) | 6.61 (1.57) | 5.86 (1.31) | 4.61 (1.46) |
| Hip sn | 5.32 (1.20) | 4.72 (1.02) | 4.41 (0.92) | 3.29 (0.98)* |
| Hip dx | 5.31 (1.12) | 4.88 (1.17) | 4.44 (1.03) | 3.39 (1.04) |
| Hip all | 5.32 (1.15) | 4.80 (1.10) | 4.43 (0.98) | 3.34 (1.01)* |
| Ocx sn | 6.77 (1.56) | 6.20 (1.30) | 5.62 (1.24) | 4.32 (1.28) |
| Ocx dx | 6.59 (1.44) | 6.18 (1.29) | 5.46 (1.09) | 4.31 (1.28) |
| Ocx all | 6.68 (1.49) | 6.19 (1.29) | 5.54 (1.16) | 4.31 (1.28)* |
| Cd sn | 7.60 (1.88) | 7.20 (1.54) | 6.32 (1.55) | 5.04 (1.52) |
| Cd dx | 7.63 (1.81) | 7.16 (1.84) | 6.35 (1.60) | 5.00 (1.62) |
| Cd all | 7.61 (1.84) | 7.18 (1.70) | 6.33 (1.57) | 5.02 (1.57) |
| Coll sn | 9.42 (2.40) | 8.71 (2.87) | 7.85 (2.15) | 6.01 (2.07) |
| Coll dx | 8.89 (2.11) | 9.08 (2.83) | 7.40 (1.93) | 6.31 (2.18) |
| Coll all | 9.17 (2.25) | 8.90 (2.83) | 7.64 (2.03) | 6.16 (2.11) |
| Th sn | 6.41 (1.28) | 6.11 (1.47) | 5.38 (1.30) | 4.23 81.24) |
| Th dx | 6.34 (1.35) | 6.3 (1.61) | 5.28 (1.19) | 4.38 (1.34) |
| Th all | 6.38 (1.31) | 6.21 (1.54) | 5.33 (1.25) | 4.31 (1.29) |
| Cer sn | 4.74 (1.16) | 4.48 (0.70) | 3.91 (0.74) | 3.12 (0.85)* |
| Cer dx | 4.79 (1.20) | 4.51 (0.66) | 3.93 (0.75) | 3.16 (0.90) |
| Cer all | 4.77 (1.18) | 4.49 (0.67) | 3.92 (0.74) | 3.14 (0.87) |
| Ver | 5.67 (1.41) | 5.29 (0.79) | 4.65 (0.82) | 3.69 (1.01)* |
| Gc | 7.19 (1.63) | 6.90 (1.71) | 5.99 (1.43) | 4.83 (1.68) |
| Wm | 3.89 (0.73) | 2.95 (0.78)* | ||
- WMV, white matter normalized value; SUV, standardized uptake value; Volumes of interest (VOI) are covering frontal (Fcx), parietal (Pcx), temporal (Tcx), and occipital cortices (Ocx), hippocampus (Hip), nucleus caudatus (Cd), caudal colliculus (Coll), thalamus (Th), cerebellum (Cer), vermis cerebelli (Ver), gyrus cinguli (Gc), and white matter (Wm), presenting left (sn), right (dex), or consolidated (all) values.
- *Significant difference from control dogs (P ≤ 0.05)
Visual evaluation of electroencephalograms revealed changes in three epileptic (sensitivity 36%) and four control dogs. Changes were classified as paroxysmal epileptiform activity in four dogs (three epileptics and one control dog) and epileptiform with uncertain clinical relevance in three control dogs (midline spikes). For dogs that had both FDG-PET and EEG examinations, EEG localized changes were seen in two epileptic dogs (occipital region) and FDG-PET also supplied lateralization information (locations agreed upon by three reviewers, Fig. 1). Electroencephalography localized and lateralized changes (right temporo-occipital derivation) were present in one epileptic dog where FDG-PET also localized hypermetabolic changes in the cingulate gyrus (gyrus cinguli, Fig. 2). None of the control dogs had EEG and PET changes at the same locations.
Discussion
Visual focal abnormality/asymmetry FDG-PET changes demonstrated high sensitivity (82%) but low specificity (50%) for epilepsy. Several healthy dogs also had focal findings in FDG-PET. The most consistent focal abnormalities on the epileptic dogs were seen in the occipital regions (with a sensitivity of 64% and specificity of 100%). Electroencephalography was less sensitive but as specific, as all the paroxysmal posterior discharges in EEG were seen in the epileptic dogs and in none of the control dogs. In individual dogs, occipital changes agreed between FDG-PET and EEG. The lowest SUVs for the epileptic dogs occurred in the occipital region. Based on the FDG-PET and EEG findings from the current study, authors hypothesize that the occipital cortex is the epileptic focus for genetic epilepsy of Finnish Spitz dogs.
In human patients with focal seizures, epileptic foci can be localized based on the presence of hypometabolic areas in interictal FDG-PET scans [with the highest sensitivity occurring in patients with temporal lobe epilepsy (up to 90%)].4, 10 During the ictal period, cerebral metabolism and blood flow are markedly increased in the epileptic focus, but interictally (when the majority of functional examinations are performed) cerebral metabolic activity declines below normal levels.11 Suggested mechanisms for interictal cerebral glucose hypometabolism include neuronal loss, reduction of synaptic density in pathways involved in seizure onset and spread, and interictal inhibitory processes.4 In patients with idiopathic epilepsy, hypometabolism is most likely an indication of interictal inhibitory processes (however, pathological substrates for idiopathic epilepsies are not fully understood for dogs). To the authors’ knowledge, no reference standard is currently available to confirm the validity of functional cerebral examinations in dogs with idiopathic epilepsy. After completion of the current study, conventional brain histopathology examinations were performed for one control and three Finnish Spitz dogs with epilepsy and did not demonstrate abnormalities.
All paroxysmal discharges in EEG originated from the posterior derivations involving occipital location in epileptic dogs and in none of the control dogs. Although we were not able to correlate other cortical regions with epilepsy in our study group, we hypothesize that Finnish Spitz dogs suffer genetic epilepsy and that epileptogenesis occurs in multiple cortical regions, particularly in the occipital region. This hypothesis is supported by our clinical experience with this breed. Despite the focal onset seizures in the majority of Finnish Spitz dogs, initial ictal signs may vary from behavioral to autonomic or motor signs.23 Initial ictal signs related to vision may be difficult to distinguish, but vomiting, frequent in epileptic Finnish Spitz dogs and quite unusual for epileptic dogs generally, is reported in humans with posterior epilepsy.24, 25
We aimed to critically compare visual and semiquantitative assessment methods of FDG-PET in epileptic and control Finnish Spitz dogs. Visual evaluation of FDG images is the basic assessment method in clinical conditions in human epileptology. However, to minimize the bias it is advised to make the initial interpretation without clinical information or structural imaging information.12 We used a blinded visual evaluation method where changes were only recorded as present when they were identified by at least two of three examiners. Examiners included a veterinary radiology specialist (M.S.), a human children epileptologist with FDG-PET experience (L.M.), and a scientist working with small animal PET imaging (M.H-S.). There was full agreement on five findings and partial (2/3) agreement on 12 findings. In addition, 22 findings were identified by only one of the examiners.
Detection of cortical hypometabolic areas in some of the control dogs was unexpected. Finnish Spitz dogs often live outdoors and those with seizures generally have them infrequently (mean 2 per year). Therefore we cannot exclude the possibility that some control dogs for the current study had idiopathic epilepsy but owners did not witness the seizures. However, follow-up interviews of owners 5 years after the FDG-PET scans were done for the current study did not reveal evidence of seizures. Control dogs were not specially selected from bloodlines without epilepsy and had epileptic relatives in 1–3 generations. The high frequency of cerebral hypometabolic changes in control dogs could possibly be explained by polygenic inheritance, which we have found in some of our initial genetic investigations. It is possible that some of the control dogs may have genes influencing their cerebral activity, but are missing the critical genes needed for final seizure development. It is also possible that minor variations in cortical FDG uptake are normal variants in this breed.
The range of the AIs was wide in the control dogs and the AIs on the visually identified hypo/hypermetabolic regions did not exceed the normal range. In epileptic human patients, asymmetry between 10–15% indicates possible areas of reduced glucose utilization and over 15% indicates clear regions of hypometabolism.26 In our study, AIs of control and epileptic dogs had no significant variance in any regions and the highest value was 4.3. Highest AI was detected in the caudal colliculus, a minor structure where it also had a perfect match with asymmetry findings detected in the visual evaluation (Fig. 3). We suspect that the lack of asymmetry findings could have been related to the VOI drawing method used in our study. Partial hypometabolic findings can be masked with higher uptake of neighboring cortical areas when the VOI covers the whole lobe. Therefore further studies are needed to validate asymmetry characteristics for cerebral glucose metabolism in epileptic dogs. New studies examining dogs with homologous clinical epilepsy manifestation using well-defined regions of interests (ROIs) that are guided by the results of visual analysis and drawn on the high resolution magnetic resonance images (MRIs) would be optimal. Unfortunately, for technical reasons, we were not able to apply fusion of 1.5 T MRI and PET images for all of the dogs and therefore were not able to take all benefit from the superior detection of anatomical structures as we were drawing VOIs on the FDG-PET images. We instead aimed to draw VOIs for individual dogs with maximal similarity and bilateral VOIs symmetrically and not to follow possible visual findings of FDG-PET. Volumes of interest covered the maximal volumes of the selected brain structures. In a previous study, the authors examined cerebral blood flow with technetium-99m-ethyl cysteinate dimer SPECT using a template which was generated from the normal dogs for defining ROIs.27 Later a similar template was used as a control to examine the brain perfusion of epileptic dogs during the interictal state.28 A template of ROIs blinds the examiners to individual changes; however, we have seldom well characterized epilepsy syndromes with suspected homologous epileptic foci in dogs. Therefore escaping visual analysis and concentrating on quantitative measuring defined by a template is likely to mask possible findings on an individual basis. A group from Belgium found significant hypoperfusion in the subcortical/thalamic area in epileptic dogs using ROI analysis.28 They defined epileptic dogs as suffering from the generalized seizures, but ignored the fact that 10 dogs had signs of focal seizure onset. Therefore, their findings possibly reflect seizure propagation and not the epileptogenic focus. Decreased cerebral metabolism in the thalamus has also been reported in human patients with temporal and frontal epilepsy studied by FDG-PET.29 Those changes were associated with long duration of epilepsy and to secondary generalization of seizures.
Standardized uptake value is the most often used semiquantitative measure in human and veterinary FDG-PET literature.13, 14, 17, 30 When interpreting SUVs, large variations related to scanners, image acquisition, reconstruction, and ROIs methodology should be taken into account.31, 32 Authors of the current study believe that the importance of these factors is not fully appreciated by veterinary researchers and therefore the SUVs characterizing the cerebral glucose uptake published to date are not comparable. To improve this comparison, the relative SUV ratio where the regional SUV is normalized with SUV of the whole brain could be used.16 Normalization of ROI values with white matter, cerebellar, or brainstem regional activity could be helpful in some situations.33, 34 Normalization would minimize interobserver variability and therefore make comparison of data easier. In the current study, we normalized VOI values with the regional activity of the white matter (minimal uptake) in order to also reveal global or extensive differences in glucose uptake in the epileptic dogs. Indeed, epileptic dogs in our study had lower cortical glucose uptake than control dogs (Table 1).
Recently some studies have focused on the description of normal cerebral glucose utilization measured by FDG-PET in control dogs.13-16 Published mean brain SUVs for dogs ranges from 3.4 to 7.4 and the relative SUV has been 1.0. Mean SUV for whole brain for our control dogs was consistent with these results. We found the highest regional uptake of FDG in the tectum (caudal colliculus). This has also been described in earlier reports and may be explained by the auditory reflex, active also in sedated dogs, that is more prominent in some dog breeds.13, 16 We found the highest cortical SUVs in the temporal and parietal lobes and the lowest in frontal and occipital lobes. This finding disagreed with previous publications where the highest uptake in dogs was detected in nearly the opposite order.14-16 These findings did not change even when excluding the epileptic dogs, however, there was no significant variance between FDG uptake of these cortical structures. In addition, dogs studied by SPECT have shown a perfusion index highest in the occipital lobe, with significant rostrocaudal perfusion gradient, and epileptic dogs were not significantly different from the healthy controls.28, 35 Therefore, it is possible that such relative reduction of FDG uptake in the occipital lobe in the Finnish Spitz dogs could be a unique finding and may indirectly reflect the lowered seizure threshold in the occipital lobe possibly characteristic for the breed or group of dog breeds. The lowest FDG uptake in dogs is reported to be in the brainstem, but this area was not measured in our current study.14 In our study, FDG uptake in the cerebellar hemispheres was lower than in the vermis. Therefore the uptake variations within the substructures of larger anatomical structures should be taken into consideration when interpreting quantitative data.16
FDG-PET reflects glucose metabolism from the time of the tracer injection until the uptake reaches a steady state (at least 20 min). Therefore making the patient feel comfortable, placing them in a quiet, dimly lit room 10 min prior to the examination, and avoiding physical exercise and heavy meals are emphasized as important preparatory factors for human patients undergoing FDG-PET studies.12, 32 Given the breed selected for the current study, injecting the FDG without sedation was not an option. We considered the possible effect introduced by sedation would be better than having inhomogeneous and hardly interpretable study data. Sedation is considered to be an important factor influencing brain glucose metabolism and blood perfusion. Unfortunately no consistency exists between anesthesia protocols and sedatives used for veterinary PET/SPECT studies.13-15, 30, 35-37 Limited published studies have suggested that medetomidine, compared with other sedation protocols, may increase cerebral glucose metabolism and brain perfusion in dogs and cats when given prior to tracer injection and, in the case of FDG-PET even when administrated after tracer.30, 36, 37 This effect is in accordance with the knowledge that medetomidine causes no dose-dependent insulin suppression and therefore increases glucose levels in dogs.38 Moreover, results from some human studies have suggested that the relationship between cerebral glucose metabolism and blood flow can be altered by some anesthetics.39-42 We have not found any reports examining medetomidine or butorphanol influence on cerebral glucose metabolism and blood flow coupling. Combined medetomidine, butorphanol, and midazolam sedation was used on our patients when initial sedation was given prior to tracer injection. There is a lack of data for the use of such a combined sedation protocol for functional imaging studies. A study evaluating the influence of different sedative protocols on quantitative EEG in dogs found no significant differences between low-dose medetomidine and combined medetomidine-butorphanol-midazolam sedations.43 Therefore, we suspect that the influence of sedation on global cerebral glucose utilization was mainly determined by medetomidine, but was similar for all the dogs sampled in our study. Alternating effects of different anesthesia protocols on FDG uptake in various canine cortical regions have been previously published.30 A Belgium group has also found some regional left-right imbalance in cortical perfusion, likely related to medetomidine when examined by SPECT.36, 37 To have a baseline for functional brain imaging in animals would be optimal, but it is rarely feasible to collect information about FDG-PET in awake animals.44 Therefore, when interpreting the results of functional imaging in animals, the influence of anesthesia should always be considered. Different antiepileptic drugs have also been shown to affect cerebral metabolism. Phenobarbital has been reported to be the most widely used depressant (up to 37%) in humans.4 Four epileptic dogs in our study were on treatment, but this caused no statistical influence on SUVs when compared to the epileptic dogs without treatment.
Although EEG and FDG-PET results were largely in concordance, our findings are based on a small number of dogs. Therefore, future studies in a larger number of dogs will be needed to confirm these findings. The association of changes in regional cerebral metabolism with clinical signs of epilepsy and evolution of the cerebral metabolic pattern over time in dogs would be of great scientific interest.
In conclusion, findings from functional examinations (FDG-PET and EEG) in the current study most consistently detected involvement of the occipital region in Finnish Spitz dogs with idiopathic epileptic. Our results support the use of FDG-PET as a functional modality for detecting and quantifying changes originating from the occipital region in epileptic dogs. Authors recommend using both visual changes and quantitative measurements for evaluating epileptic dogs with FDG-PET, as they provided complementary information.
ACKNOWLEDGMENTS
The authors acknowledge K. Sainio (Department of Clinical Neurophysiology, Helsinki University) for performing blinded review of electroencephalograms and the Finnish Spitz Breeder Club for their active collaboration.




