COMPUTED TOMOGRAPHIC FEATURES OF CANINE NONPARENCHYMAL HEMANGIOSARCOMA
Abstract
The purpose of this retrospective study was to describe pre- and postcontrast computed tomographic (CT) characteristics of confirmed nonparenchymal hemangiosarcoma in a group of dogs. Medical records were searched during the period of July 2003 and October 2011 and dogs with histologically confirmed nonparenchymal hemangiosarcoma and pre- and postcontrast CT images were recruited. Two observers recorded a consensus opinion for the following CT characteristics for each dog: largest transverse tumor diameter, number of masses, general tumor shape, character of the tumor margin, precontrast appearance, presence of dystrophic calcification, presence of postcontrast enhancement, pattern of postcontrast enhancement, presence of regional lymphadenopathy, and presence of associated cavitary fluid. A total of 17 dogs met inclusion criteria. Tumors were located in the nasal cavity, muscle, mandible, mesentery, subcutaneous tissue, and retroperitoneal space. Computed tomographic features of nonparenchymal hemangiosarcoma were similar to those of other soft tissue sarcomas, with most tumors being heterogeneous in precontrast images, invasive into adjacent tissue, and heterogeneously contrast enhancing. One unexpected finding was the presence of intense foci of contrast enhancement in 13 of the 17 tumors (76%). This appearance, which is not typical of other soft tissue sarcomas, was consistent with contrast medium residing in vascular channels. Findings indicated that there were no unique distinguishing CT characteristics for nonparenchymal hemangiosarcoma in dogs; however, the presence of highly attenuating foci of contrast enhancement may warrant further investigation in prospective diagnostic sensitivity and treatment outcome studies.
Introduction
Spontaneous hemangiosarcoma represents approximately 5–7% of all canine malignant tumors.1, 2 Histologically, hemangiosarcoma consists of neoplastic spindle-shaped endothelial cells and unusual irregular vascular channels.3 Since hemangiosarcoma arises from vessels, it can occur in any tissue, but most commonly arises in the highly vascular spleen and liver.2, 4 Splenic and hepatic hemangiosarcomas commonly have a poor prognosis despite intense therapy due to their aggressive biologic behavior, propensity for distant metastasis, and fragility, which can cause serious hemorrhage.2, 5 The prognosis following treatment has been related to the size of the mass and the existence of metastasis at diagnosis.4
Contrast-enhanced computed tomography (CT) is commonly used for tumor staging; however, few previous references have described the CT characteristics of hemangiosarcoma in dogs. Identification of morphologic features that could allow distinction between nonparenchymal hemangiosarcomas and other soft tissue tumors, or that could be tested as predictors of biologic behavior in prospective trials, would be helpful. Additionally, because there is a risk of hemorrhage associated with biopsy of hemangiosarcoma, identification of highly vascular areas in CT images could assist direction of a CT-guided biopsy to a region with less vasculature. One report on CT findings of splenic hemangiosarcoma described these tumors as appearing hypoattenuating compared to benign splenic masses (such as hyperplasia and hematomas) in pre- or postcontrast CT images.6 Individual case reports have described CT characteristics of nonparenchymal hemangiosarcomas involving pleural, uterine, retroperitoneal, lateral ventricle, orbital, pelvic, and popliteal artery tissues.7-12 These tumors were characterized as having heterogeneous precontrast attenuation and local invasion of surrounding tissues. If contrast medium was given, poor contrast enhancement was described, with mainly a peripheral distribution. However these findings were nonspecific, and could also be seen in other kinds of malignant tumors.13 The objective of the current study was to describe the contrast-enhanced CT characteristics of nonparenchymal hemangiosarcomas in a group of dogs and determine whether any distinguishing characteristics could be identified.
Material and Methods
Medical records from the Veterinary Teaching Hospital of North Carolina State University (NCSU) and the Japan Small Animal Medical Center (JSAMC) formulated between July 2003 and October 2011 were searched for a diagnosis of hemangiosarcoma of nonparenchymal origin. Gender, breed, and age data were recorded. Confirmation of the diagnosis as hemangiosarcoma was based on a review of the histology report from the medical record. Original tissue specimens were not reviewed. Only dogs with pre- and postcontrast CT images of the nonparenchymal hemangiosarcoma, and no coexisting involvement of spleen or liver were selected. Splenic and liver involvement was determined based on reviews of the CT images, any available ultrasound images, and medical record entries. Two authors, one a veterinarian with multiple years of experience in treating oncology patients and in interpreting CT studies (S.F.) and the other a board certified veterinary radiologist (D.E.T.), reached a consensus opinion on tumor CT characteristics for each dog. Readers were allowed to vary window widths and levels for pre- and postcontrast image evaluations. The following parameters were recorded from CT images: (i) largest diameter of mass in the transverse plane, (ii) number of masses; if more than one mass was present the diameter of the largest was measured, (iii) general shape of the mass (round, lobular, or contained by bone, such as in the nasal cavity), (iv) whether infiltration of adjacent tissue was likely, as judged subjectively, based on lack of distinct tumor margins in postcontrast images, (v) whether the precontrast appearance was visually homogeneous or heterogeneous, (vi) presence of dystrophic calcification, based on intense hyperattenuation in precontrast images that was not associated with bone involvement, (vii) presence of contrast enhancement based on visual increase in attenuation of the tumor in postcontrast images, (viii) pattern of contrast enhancement (heterogeneous, ring, intense focal), (ix) presence of regional lymphomegaly, assessed subjectively, and (x) presence of associated pleural or peritoneal fluid if the tumor involved the thorax or abdomen, respectively. If more than one mass was present, the evaluations reflected assessment of all abnormal tissue in the dog and not individual masses. Due to the relatively small number of patients, statistical analysis was not performed.
Results
A total of 17 dogs met inclusion criteria. The mean age of the dogs was 9 years (median 9; range 3–14). There were 11 neutered males (65%), 3 intact males (18%), 2 neutered females (12%), and 1 intact female (6%). The patients were primarily large dogs. Breeds were Golden Retriever (2/17), German Shepherd (2/17), Labrador Retriever (2/17), and one each Shar-Pei, Pekingese, German Shorthaired Pointer, Siberian Husky, Poodle, Boxer, American Cocker Spaniel, Bichon Frise, Burnese Mountain Dog, Flatcoated Retriever, and Mixed breed. Tumor locations are summarized in Table 1. The tissue from which the diagnosis was made was obtained by biopsy (n = 9), or tissue retrieved at surgery (n = 8). Spleen and liver were included in the CT study in seven dogs and in the other 10 either only liver was included (n = 2) or neither was included (n = 8). Ultrasound images of spleen and liver were available in eight of the 10 dogs that did not have liver or spleen included in the CT study. Two dogs had hypoechoic nodules in spleen, and one dog had hepatic lymphomegaly that was assumed to be nonspecific by the ultrasonographer.
| Tumor Location | Number (n = 17) | Percent |
|---|---|---|
| Trunk | 8 | 47 |
| Mesentery | 3 | 18 |
| Retroperitoneal | 2 | 12 |
| Nasal cavity | 1 | 6 |
| Neck | 1 | 6 |
| Limb | 1 | 6 |
| Mandible | 1 | 6 |
All dogs had been imaged using multidetector CT; five with a 4-slice scanner (Toshiba Asteion Super4 Edition, Toshiba Medical Systems Corporation, Otawara, Tochigi, Japan), 11 with a 16-slice scanner (Siemens Somatom, Siemens Medical Solutions, Malvern, PA), and one with a 64-slice scanner (Siemens Sensation, Siemens Medical Solutions, Malvern, PA). Nine dogs were in dorsal, five were in ventral and three in lateral recumbency for imaging; with positioning varying based on the location of the mass. The field-of-view was selected to include all known gross tumor, based on physical examination and/or radiographs, and the thorax was not included routinely in all dogs. All dogs were under general anesthesia and a variety of anesthesia protocols were used depending on the preference of the attending veterinarian. The kVp and mA were not standardized. Slice thickness ranged from 1 to 3 mm. Scans were acquired before and after IV administration of iohexol (Omnipaque 300, 880 mgI/kg, GE Healthcare, Waukesha, WI) at NCSU and iopamidol (Oiparomin 300, 600 mgI/kg Konika Minolta Medical & Graphic, Inc., Hino, Tokyo, Japan) at JSAMC via peripheral venous catheter. Postcontrast images were acquired approximately 1 min after contrast-medium administration in five dogs. The specific delay time was unknown in 12 dogs, but was in the venous phase in 16 and in the arterial phase in one based on the appearance of the vasculature in the images.
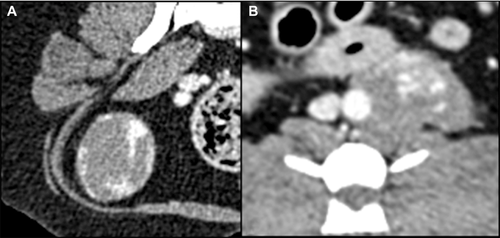
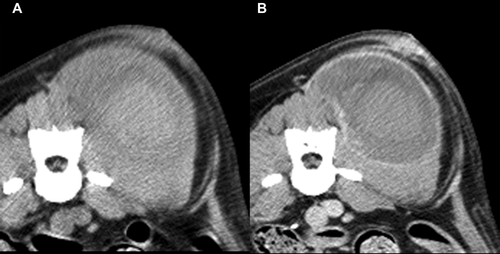
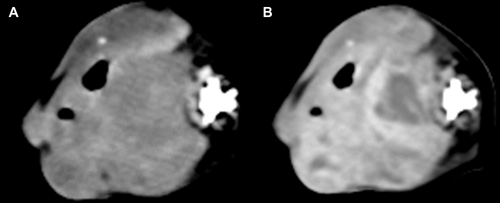
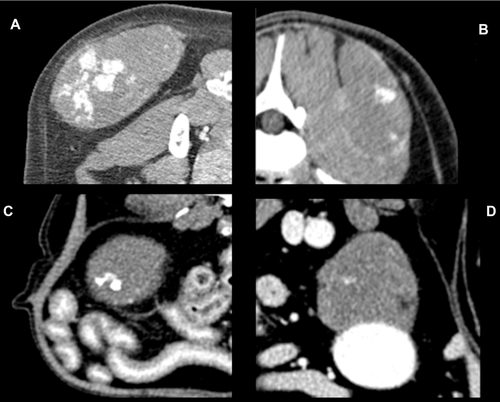
The mean maximal transverse tumor diameter was 6.2 cm (median 6.5; range 2.7–10.3 cm). Fifteen dogs (88%) had a solitary mass and two (12%) had multiple masses, two in one dog and four in the other. Not all masses in dogs with multiple masses were confirmed histologically as hemangiosarcoma, but were assumed so for this report. This was based on the similar imaging features of masses in dogs with multiple masses. Ten of 17 (59%) dogs had a generally round mass, five of 17 (29%) had a mass with some lobular features, and in two (12%) the mass was contained by the surrounding bone, for example, nasal tumor. Thirteen of 17 masses (76%) were considered subjectively to be invasive and four (24%) were considered to be well defined (Fig. 1). Well-defined masses were located in the mesentery (n = 2), retroperitoneal space (n = 1), and subcutaneously in the body wall (n = 1). Overt vascular invasion, based on intraluminal filling defects, was not identified in any dog even though one retroperitoneal mass encircled the aorta (Fig. 1B) and another retroperitoneal mass compressed the right and left iliac vein and left external iliac artery ventrally. In 12/17 dogs (71%), the tumor had a heterogeneous texture in precontrast images. In seven dogs, this was due to regions of hypoattenuation compared to muscle (Fig. 2), and in two dogs this was due to areas of hyperattenuation resulting from bone involvement with hyperostosis. Hypoattenuating regions were less attenuating than muscle. Precontrast hyperattenuation consistent with dystrophic calcification (separate from any bone production due to invasion), was identified in only one tumor, and this was minimal and faint. Sixteen tumors (94%) were characterized by contrast enhancement and in one (6%) there was no visible enhancement (Table 2). In the tumors with contrast enhancement, characteristics included generalized heterogeneous enhancement (Fig. 3), and ring enhancement (Fig. 2). In 13 of 17 dogs (76%), the pattern of contrast enhancement included regions of intense focal enhancement (Figs. 1B and 4). Lymphadenopathy was found in eight dogs (47%). Tumors with associated lymphadenopathy were of retroperitoneal (n = 1), mandibular (n = 1), intramuscular (n = 3), and subcutaneous (n = 3) origin. One out of six masses involving the abdominal cavity had associated peritoneal fluid that was confirmed as hemoabdomen.
| Pattern of Contrast Enhancement | Number (n = 17) |
|---|---|
| Intense focal | 5 |
| Intense focal with ring | 4 |
| Intense focal with heterogeneous | 4 |
| Heterogeneous | 2 |
| Ring | 1 |
| None | 1 |
Discussion
Hemangiosarcoma of nonparenchymal origin was not confined to a particular site in the current study. Tumors were typically heterogeneous in precontrast images, although high-contrast window/level settings were necessary to detect this in some dogs. Hemangiosarcoma tends to contain a large proportion of hemorrhage and necrosis, which may contribute to this heterogeneous appearance. This appearance was not qualitatively different from that seen with many other soft tissue tumors in our experience. We judged involvement of the spleen based on the CT imaging characteristics and other available results, such as ultrasound or necropsy. Therefore, we are not certain that the spleen was normal in all of these dogs. However, we found no evidence to the contrary.
Tumors generally had an aggressive appearance due to apparent invasion of adjacent tissues, based on an indistinct transition zone in postcontrast images. In our experience, this is also consistent with other soft tissue sarcomas. Masses that appeared to be noninvasive were not intramuscular, being located subcutaneously, in the mesentery and in the retroperitoneal space. While it is possible for less aggressive appearing masses to have microscopic extension, hemangiosarcoma forming in less-contained spaces than muscle could have a higher chance of being resected completely if detected sufficiently early. There may also be a site-specific biologic behaviors, as others have suggested that subcutaneous hemangiosarcoma is less aggressive compared to hemangiosarcoma at other sites.4, 14 We had inadequate sample size to prove this theory, but it may warrant further investigation in future studies. No mass infiltrated a major vessel.
Dystrophic calcification was detected in only one tumor in this study, and this was very faint. Dystrophic calcification is commonly seen in a wide range of epithelial tumors such as prostatic adenocarcinoma, thyroid carcinoma, primary lung tumors, and hepatocellular carcinoma,15-19 There are two reports of dystrophic calcification in hemangiosarcoma,7, 11 though it is rare in soft tissue sarcomas in our experience.
Sixteen of the 17 tumors were characterized by nonhomogeneous contrast enhancement, which is a common feature of most of malignant tumors15-18, 20 and previous reports on hemangiosarcoma. However, 13 of 17 (76%) tumors had highly attenuated postcontrast foci of enhancement, which are generally not found in other soft tissue sarcomas, or in epithelial tumors. As far as we know, this unusual contrast enhancement pattern has been reported in vascular origin tumors in people20-24, but not previously in animals. In reviewing the available postcontrast CT images of the previously published individual case reports of nonparenchymal hemangiosarcoma in dogs, we did not find this characteristic described. There are two possibilities for this difference between the prior results and ours. First, we did not find this enhancement pattern in every tumor thus it may not have been present in the tumors included in the previous reports. Second, perhaps the window/level settings at which the tumors were examined were not the same as those used by the observers in this study for detecting these foci. Faint foci may require high-contrast viewing. It is also possible that differences in scanner resolution and postcontrast scan times for the current study patients vs. previously reported patients could have had an effect. We always used a wide range of window/level settings in the examination of the tumors in this study. Nevertheless, this enhancement pattern appeared to be a feature of a majority of the nonparenchymal canine hemangiosarcomas in the current study. Usually the attenuation of a mass is lower than arterial attenuation, even in bone sarcoma, which has higher perfusion than soft tissue sarcomas,25 but that was not the typical finding in nonparenchymal hemangiosarcomas of the current study. The attenuation characteristics of the enhancing foci were similar to that of contrast media in vessels, and these foci were not present in precontrast images leading to the conclusion that these foci could represent vascular channels. This theory would require validation in a prospective study. We do not know why highly attenuated foci were not seen in all dogs. The extent of differentiation of the tumor could be a factor. The formation of enhancing foci might also be related to biologic behavior, similar to the difference in aggressiveness of intramuscular hemangiosarcoma (aggressive) vs. subcutaneous hemangiosarcoma (less aggressive).4, 14 However, there was not sufficient evidence to evaluate the correlation between the highly attenuated foci and the vasculature in this study.
Findings from the current study identified only one CT characteristic that could be eligible as a possible distinctive characteristic of nonparenchymal hemangiosarcoma in dogs: the presence of highly attenuating postcontrast foci (consistent with vascular channels). This CT characteristic was not described in previously published case reports. Future prospective studies are needed to determine the diagnostic sensitivity for this CT sign as a predictor of nonparenchymal hemangiosarcoma in dogs. Otherwise, the features of nonparenchymal hemangiosarcoma were similar to those seen in other soft tissue sarcomas, such as a heterogeneous appearance in precontrast images (consistent with hemorrhage and necrosis), and an indistinct transition zone in postcontrast images (consistent with regional tissue infiltration).




