Should adding pain, oxygen saturation and physical assessment to vital signs become the new standard of care for detecting blood transfusion reactions?
Funding information: The authors received no specific funding for this work.
Abstract
Background and Objectives
Clinicians sought to ascertain what frequency of vital signs best detects blood transfusion reactions. This review discusses early and delayed blood product transfusion reaction detection through the lens of scientific literature.
Methods
A comprehensive appraisal of published literature was conducted using Integrative Research Review methodology through June 2022 not limited to English or research in Cumulative Index to Nursing and Allied Health Literature, Cochrane Library of Systematic Reviews, Medline and PubMed.
Results
Full-text articles in the final sample included four articles discussing vital signs detecting blood transfusion reactions and four articles reporting the importance of adding physical assessments for early reaction detection. None of the studies provided evidence regarding how often vital signs should be monitored to detect transfusion reactions. No studies included identical screening components for detecting blood product transfusion reactions. Main themes emerged including variations in what was included in vital signs, importance of respiratory assessment, inclusion of physical assessment, nurse documentation and reporting compliance, and patient and family inclusion in transfusion reaction recognition.
Conclusion
Vital sign components varied across reviewed studies. Respiratory rate and pain were not always included in ‘vital signs’ to identify transfusion reactions. Only low-level data and no clinical trials loosely informing frequency of vital sign monitoring to transfusion reaction detection were found. Respiratory (to include oxygen saturation, lung sounds and respiratory rate) and pain assessment emerged as crucial to acute and delayed transfusion reaction recognition. The disconnect between ‘vital signs’ and the varied vital sign components reported to detect transfusion reactions in scientific literature requires further exploration.
Highlights
- The findings of this review suggest that respiratory rate, pulse oximetry and pain are crucial to detecting clinical deterioration and should be officially incorporated into vital sign assessment to detect both early and late transfusion reactions.
- No standard contextual vital sign derangement guidelines were identified to inform clinicians as to what should be viewed as ‘abnormal’ or to direct them towards transfusion reaction consideration.
- This review communicates the need for further research to define: the frequency of the vital sign measurement needed to detect blood component transfusion events; what assessments should be included when using the term ‘vital signs’ and what deviation from baseline represents an ‘abnormal’ finding.
INTRODUCTION
Much like other medical emergencies, blood transfusion reaction detection may improve patient outcomes at the earliest possible time. The U.S. transfusion adverse event rate associated with 20,933,000 blood products transfused during 2011 was 0.24%; thus, demonstrating a time burden on nurses administering blood products, monitoring for transfusion reactions and managing reaction events while already overstretched in complex health systems [1, 2]. Being able to recognize and intervene rapidly in medical emergencies remains imperative for frontline nurses despite many tasks needing to be completed. Competing interests for a nurse's time make early, accurate and evidence-based blood transfusion reaction detection methods necessary.
With reported blood transfusion reaction rates ranging from 0.14% to 2.1% in reviewed literature [1-4], nurses require clear guidance on vital sign measurement frequency, vital sign composition and abnormal findings to recognize a patient reacting to a transfusion promptly [5, 6]. Incomplete inclusion of vital signs (especially respiratory rate, pain and pulse oximetry), lack of guidelines to interpret the data [5, 7], absence of physical assessment (especially lung sounds) and not including family members and patients as partners in detection contribute to adverse events. This review examined best practices for reaction detection published in peer-reviewed literature.
Background
Screening criteria for transfusion reactions must be developed based on sound evidence to improve the quality of patient care and decrease costs to treat. Using translational research methodologies, healthcare agencies are committed to implementing evidence-based process solutions for accurate and timely recognition and management of adverse transfusion reaction events. Our hospital in the Southwestern United States recorded 51/12,077 (0.422%) transfusion-related adverse reactions in 2017, highlighting the importance of discovering evidence to support ways to detect transfusion reactions. Blood transfusion reaction detection exhibits clinical implications for practitioners and patients. Our institutional transfusion reaction rate was in the lower quartile of rates found in reviewed literature; however, even one transfusion reaction event can represent a life-changing incident for a patient.
Significance
Early reaction detection during blood product administration may reduce or even mitigate adverse transfusion-related events. Although the U.S. Federal Drug Administration reported one fatality per 697,767 (0.00013%) blood component transfusions [8], one study reported a 3% mortality rate [3]. Despite mortality rate variance in reviewed literature, the fact remains blood transfusions cause severe complications and fatalities [3, 9]. Adverse reactions may occur immediately or within several hours post-transfusion, depending on the type of reaction [3, 4, 9-11]. Consequently, clinical observations, patient verbalized complaints and visitor recognized changes are pivotal to patient survival.
Aims
This integrative research literature review aimed to examine the frequency of vital sign measurement needed to identify blood transfusion reactions. The review and evaluation of literature were conducted to examine how often vital sign assessment and what physiologic and physical parameters ought to be included to identify blood transfusion reactions in patients. The secondary purpose of this project was to ensure best transfusion reaction detection practices are employed by nurses administering blood products by partnering frontline acute care nurses with doctorally prepared nurses to conduct the structured literature review.
METHODS
Design
A comprehensive review of peer-reviewed published literature was used as the study design to guide this project. This literature review was conducted using Integrative Research Review (IRR) methodology adapted from Whittemore and Knafle [12] and Brown [13]. This methodology permitted inclusion of studies reporting both qualitative and quantitative findings and a narrative approach for synthesizing results from a wide range of study designs. Additionally, sources using theoretical approaches allowed for narrative synthesis through data reduction, display and comparison to draw conclusions in alignment with IRR methodology [12].
The International Prospective Register of Systematic Reviews (PROSPERO) was searched to identify if any similar systematic review is currently being conducted. The literature search was guided by a clearly formulated clinical question, ‘In individuals receiving a blood product transfusion, what frequency of vital sign measurement should be used to monitor for a reaction?’ to find all relevant articles addressing the clinical issue of interest by reviewers. Specifically, the structured and organized method of identifying and evaluating the body of peer-reviewed literature was framed using a population, intervention, comparison, outcome (PICO) statement.
Search methods
Six frontline nurses, four academic partners (nurse scientists, research librarian and Doctor of Nursing Practice) and two institutional Doctor of Nursing Practice leaders evaluated and synthesized evidence to identify transfusion reaction screening best practices using keywords determined by the research team. To increase consistency among reviewers, all reviewers vetted publications, independently weighed levels of evidence and rated the quality of the same sample of articles before coming together to discuss results as a group. Articles and data included in the final sample were agreed upon through consensus after dialogue.
A research evidence table was compiled to amalgamate significant discoveries and aid in synthesizing published findings. The Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) 2020 checklist guided researchers through the literature appraisal process [14]. The PRISMA Statement assisted investigators in evaluating effectiveness and unintended consequences of the healthcare intervention of blood product transfusion reaction screening to assure transparency of literature review reporting.
Search strategy
The Boolean search strategy included using the key search terms of ‘blood transfusion’ AND ‘vital signs’ OR ‘nurse assessment’ AND ‘reaction’ as MeSH (Medical Subject Headings) major word terms. The databases and clinical sources searched included Cumulative Index to Nursing and Allied Health Literature, Cochrane Library of Systematic Reviews, National Institutes of Health U.S. National Library of Medicine (Medline) and The National Library of Medicine database (PubMed).
A research librarian conducted the preliminary and final searches; cross-verified the search strategy and confirmed that all applicable existing publications were included. All existing peer-reviewed articles, not limited to English or research, through June 2022 were included. Applying manual searches of reference lists for included studies allowed for expansion of the search coverage. After removing duplicates, a nurse scientist and research librarian screened titles and abstracts for potential relevancy. Eight additional reviewers met as a group to further examine the remaining full-text manuscripts for inclusion or exclusion.
Inclusion criteria applied for literature selection of studies, guidelines and reports used for this review were qualitative, quantitative, mixed methods and clinical practice guidelines (CPGs). Non-empirical studies such as reviews, letters, commentaries and governmental documents were not included. Because no studies were found to match the clinical question, reviewers agreed the findings from continuing nursing education, reports with a focus on detection of blood transfusion reactions and manuscripts discussing strategies and methods for nurses to identify blood transfusion reactions in patients were helpful in informing the consensus opinion of the group. Publications without relevance to blood product transfusion reaction detection or associated adverse events were excluded.
Search outcome
Published articles identified through database searching were initially evaluated and selected by screening titles and abstracts. Inclusion criteria required selecting publications involving individuals being screened for blood product transfusion reactions using vital signs. Additional application of inclusion criteria involved selecting articles relevant to answering the clinical question or validating blood transfusion reaction detection criteria. Manuscripts were reviewed separately before conducting discussions to make the final selection decisions. Discrepancies in reviewer perspectives led to discussing the disputed manuscripts for selection determination by the team.
Quality appraisal and risk of bias assessment
The 11 reviewers independently read, evaluated and appraised each article. Investigators grouped publications by key contexts reported and summarized settings, populations and study designs on a standardized research table. Research tables including author(s), date, purpose(s), reported outcome(s), sample size, study design and level of evidence were completed independently by each reviewer to ensure inter-rater reliability of levels of evidence, avoid selection bias and avoid influencing conclusions. Reviewers explored characteristics and methodological quality of each evaluated manuscript's content using the Enhancing the Quality and Transparency of Health Research (EQUATOR)'s Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement guidelines for reporting observational studies according to the studies' designs [15].
Levels of evidence were independently determined by reviewers using the ‘Quick Guide to Designs in an Evidence Hierarchy’ [16]. As a group, a comparison of assigned levels of evidence and quality appraisal tool scores were completed to reach consensus for each article included in the final sample. Collective opinions regarding significance for inclusion to inform the study aims guided final decisions. Due to the low quality of evidence discovered, assessments of risk of bias in studies were addressed by examining pre-intervention, at-intervention and post-intervention elements of each study as a group.
Data extraction and knowledge synthesis
Researchers critically evaluated all physiological parameters and physical assessment findings reported in the literature to validate significance for inclusion in blood product transfusion reaction screening criteria. Gathered data from literature reviewed were independently evaluated, organized and recorded on a standardized evidence table used by all reviewers. Major themes were extracted and documented as they emerged from the final sample of manuscripts and key discoveries were assigned to appropriate major themes to guide synthesis of publicized findings (see Table 1). The authors discussed and reached agreement through consensus of emerging themes and the allocation of manuscripts into the appropriate theme groupings.
| Theme | Description of findings |
|---|---|
| Respiratory assessment importance |
|
| Vital sign measurement frequency |
|
| Inclusion of physical assessment |
|
| Nurse documentation and reporting compliance |
|
| Patient and family inclusion in transfusion reaction recognition |
- Abbreviations: DBP, diastolic blood pressure; SBP, systolic blood pressure.
Data synthesis
Reviewers met as a group to synthesize data from the included studies. Findings were organized by theme in a Microsoft Word document during team meetings. Disagreements were resolved through group discussion. Reviewers noted differences in what vital sign and physical assessment criteria were used to screen for transfusion reactions in reviewed manuscripts. Both reported vital sign components and physical assessment criteria used to detect blood transfusion reactions were consolidated into a table (see Table 2). Main themes emerged including variations in what was included in vital signs, importance of respiratory assessment, vital sign measurement frequency, inclusion of physical assessment, nurse documentation and reporting compliance and patient and family inclusion in transfusion reaction recognition leading to further exploration of subthemes.
| Article | Blood pressure | Heart rate | Respiratory rate | Temperature | Pulse oximetry | Physical assessment |
|---|---|---|---|---|---|---|
| Battard Menendez (2016) | Not specified | Not specified | Not specified | Not specified | Not specified | Chills, pain, nausea and reports of feeling unwell |
| Cortez-Gann (2017) | Systolic blood pressure only | Included | Not included | Included | Not included | Hives, itching, chills, flushing, nausea, pain and cyanosis |
| DeYoung Sullivan et al. (2015) | Not specified (n = 6) | Not specified (n = 6) | Not specified (n = 6) | Not specified (n = 6) | Not specified (n = 6) | Not specified (n = 10) |
| Literature review | Included (n = 4) | Included (n = 4) | Included (n = 1) Only if change noted (n = 1) |
Included (n = 4) | Not included (n = 4) | |
| Mackenzie et al. (2014) | Included | Included | Not included | Not included | Included | Not included |
- Note: Components represent blood component transfusion reaction screening criteria in the four included articles relevant to answering the clinical question.
RESULTS
Peer-reviewed published articles identified through initial search strategies (N = 41) contained duplicate articles (n = 12) which were removed. The remaining 29 articles were then further screened for inclusion by eight research team members using abstracts and titles. Twelve additional articles were excluded through screening of abstracts. After full-text review, nine additional manuscripts were excluded due to lack of relevance to the clinical question or not addressing search terms (Figure 1).
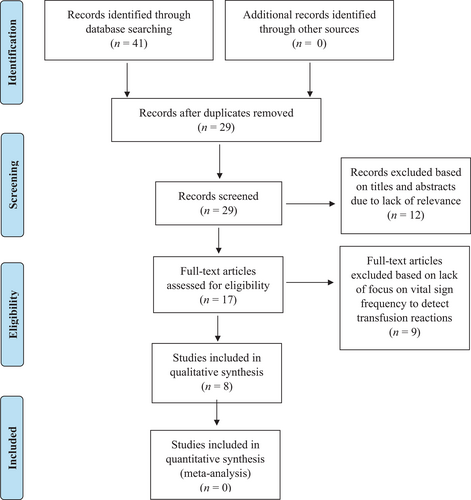
High levels or quantities of evidence were not found to inform investigators' question of best practices in detecting blood product transfusion reactions. Since only four studies were identified to loosely inform vital sign measurement frequency recommendations during blood transfusion, other sources of knowledge were included to further explore the scope of available empirical evidence and guidance. No study was excluded based on quality or methodology. Evidence ratings included 12.5% (n = 1) Level V, 62.5% (n = 5), Level VI and 25% (n = 2) Level VII in the final sample.
Selection of studies
Full-text articles evaluated were compared against clinical question components to determine study inclusion using relevancy to the patient population, intervention of interest, comparison of interest, outcome of interest and timeframe (see Table 3). Two excluded publications focused on predictive computer modelling to detect transfusion reactions [19, 20]. Another excluded article looked at blood component transfusion rate only and included the same sample represented in an included publication [21]. Finally, one excluded study investigated the predictive value of vital signs in geriatric trauma patients for massive blood product transfusion [22]. The final sample used for synthesis included the eight remaining manuscripts.
| Clinical question component | Alignment with literature reviewed |
|---|---|
| P (population of interest) = patients receiving a blood product transfusion | |
| I (intervention of interest) = receiving a blood component transfusion |
|
| C (comparison of interest) |
|
| O (outcome of interest) = frequency of vital signs |
|
- Note: Literature compared to PICO to guide exclusion of articles for relevancy.
- Abbreviation: ICU, intensive care unit.
- using all-aged patients being monitored for blood product transfusion reactions (n = 4) [1-3, 9];
- validating or examining physical assessment component inclusion for reaction detection (especially lung auscultation) (n = 1) [11];
- supporting addition of physical assessment (especially pain) to augment vital sign use (n = 1) [4];
- informing use of oxygen saturation in addition to vital signs for transfusion reaction detection (n = 1) [17]; and
- documentation compliance and completeness by nurses of post-transfusion vital signs (n = 1) [18].
| Authors (year) | Purpose(s) | Reported outcome(s) | Sample size | Study design | Level of evidence |
|---|---|---|---|---|---|
| Battard Menendez (2016) | Continuing nursing education on best practices for monitoring for acute haemolytic transfusion reactions | None | NA | NA | VII |
| Cortez-Gann (2017) | Identify relationship of vital sign changes to signs and symptoms of blood product transfusion reaction | Transfusion reaction rate of 0.15% (n = 116) | Over 77,800 units of blood product transfusions | Retrospective descriptive design | VI |
| DeYoung Sullivan et al. (2015) | Examine the evidence regarding the optimum frequency of vital sign monitoring for haematology patients receiving blood products and to ensure current institutional practice was aligned with the evidence | Vital sign monitoring during blood transfusions | Ten studies evaluated and reported by authors at levels of evidence IV, V and VII | Literature review | V |
| Gehrie et al. (2015) | Evaluate the variation in vital signs observed after blood product transfusion | Pre and post vital signs (temperature, pulse rate and blood pressure) and reported reaction | 3496 blood product transfusions including red blood cells (n = 2359), platelets (n = 476), plasma (n = 659) | Retrospective descriptive design | VI |
| Goodall (2014) | Inform on TACO | TACO | One | Single descriptive case study | VII |
| Hardwick et al. (2013) | Report blood product reaction in a 34-year-old woman with haemophagocytic lymphohistocytosis | Acute pain transfusion reaction | One | Single descriptive case study | VII |
| Mackenzie et al. (2014) | Analyse whether pulse oximeter signals predict blood transfusion and mortality in trauma patients | Continuous vital sign data (heart rate, systolic blood pressure and pulse oximetry), blood product transfusion and mortality | 556 patients | Descriptive design | VI |
| Paiva Dos Santos et al. (2013) | Evaluate nursing documentation of blood transfusion monitoring in inpatient units of a hospital for compliance | Vital signs post transfusion, observation 10 min after transfusion started, transfusion end time and nurses' signature. | 7272 nurses' documentation notes | Retrospective descriptive design | VI |
- Note: Level I evidence indicates systematic review or meta-analysis of all relevant randomized controlled trials (RCTs); Level II evidence indicates evidence-based clinical practice guidelines based on systematic reviews of RCTs; Level III evidence indicates evidence obtained from at least one well-designed RCT; Level IV evidence indicates evidence obtained from well-designed controlled trials without randomization and from well-designed case–control and cohort studies; Level V evidence indicates evidence from systemic reviews of descriptive and qualitative studies; Level VI evidence indicates evidence from a single descriptive or qualitative study, and Level VII evidence indicates evidence from the opinion of authorities and/or reports of expert committees.
- Abbreviations: NA, not applicable; TACO, transfusion-associated circulatory overload.
Characteristics of selected sources of evidence.
None of the four included studies provided evidence regarding how often vital signs should be monitored to detect transfusion reactions or were deemed free of potential bias. No studies included identical screening components for detecting blood product transfusion reactions. ‘Abnormal’ was found frequently as a vital sign finding to indicate a transfusion reaction. Still, parameters to denote what vital sign variation should be considered ‘abnormal’ were not found in the literature except for an increased body temperature of 1°C without supporting evidence [1, 6].
Practical considerations of vital sign assessment
Reviewed literature recommended vital signs be obtained before, after 15 min and at transfusion completion [2, 3, 9]. Variations in physiological screening criteria incorporated into ‘vital signs’ across reviewed publications made metanalysis of quantitative data, such as blood transfusion reaction rates, impossible and presented challenges to meta-synthesis. Investigators identified evidence indicating more than the frequency of vital signs at prescribed intervals was needed to detect blood transfusion reactions.
Only four articles were included for synthesis due to relevance to the asked clinical question. Four additional articles introduced evidence related to themes emerging during the literature review. Instead of discovering what vital sign assessment frequency best detects blood product transfusion reactions, reviewers identified gaps in evidence and practice. Emerging themes guided informative and actionable synthesis of discoveries to guide clinical nursing practice.
Vital signs defined
Vital sign components used to detect transfusion reactions differed across reviewed literature where specific components were reported. The only vital sign elements consistently included to detect transfusion reactions were systolic blood pressure, heart rate and body temperature (Figure 2). Respiratory rate was often excluded from ‘vital signs’ employed to identify reactions to blood product transfusions.

Pain inclusion in vital signs
Pain was repeatedly absent from vital sign assessment across manuscripts despite being frequently mentioned as clinically significant to transfusion reaction detection [2-4, 6, 9]. Only one reviewed manuscript supported including pain in vital sign monitoring to detect a blood product transfusion reaction [4]. In two studies, vital signs included SaO2 to screen for transfusion-related adverse events [1, 4]. Investigators found lack of a standard definition of what constitutes ‘vital signs’ led to inconsistency in physiological components included across studies used for blood transfusion reaction detection.
Physical assessment inclusion
One discovery emerging from reviewed literature was the importance of nurses completing physical assessments (especially lung auscultation) to detect transfusion reactions. Results from two publications implied the importance of including oxygen saturation (SaO2) when monitoring for blood transfusion reaction in addition to periodic vital sign assessment [11, 17]. Another manuscript supported adding physical assessment, pain and respiratory rate to blood reaction monitoring [4]. Literature findings underscored physical assessment inclusion detected indications of transfusion reaction missed by vital signs alone [6]. Clinical assessments incorporating vital sign and physical exam components were found crucial for detecting acute or delayed transfusion reactions.
Documentation of vital signs
Three studies highlighted the importance of nurse documentation compliance and completeness. Reviewed evidence revealed documentation deficiencies of vital sign results during blood product transfusions ranging from 57.3% to 88% of the time [1, 2, 18]. Post-transfusion vital sign documentation was the least documented transfusion-associated assessment by nurses (83.8%) [18]. Nurse incomplete documentation of vital sign assessments alludes to difficulties complying with even the bare minimum standards for transfusion reaction monitoring.
DISCUSSION
Vital signs defined
Across reviewed studies, including more components for blood transfusion reaction detection led to a higher rate of reported transfusion reaction events. These findings suggest comprehensive vital signs with the addition of physical assessment components detect blood transfusion reactions better than incomplete vital sign monitoring with limited vital sign components lacking physical assessment inclusion. Lack of derangement definitions to distinguish ‘abnormal’ vital sign components or to indicate transfusion reactions also prevent triggering blood reaction recognition and interventions by nurses [6].
The Joint Commission standards direct nurses to record respiratory rate during transfusions only if a significant change from baseline occurs, while notably excluding respiratory rate in vital signs to be monitored [2, 6]. How can clinicians detect ‘significant’ changes in the respiratory rate during a transfusion without checking a respiratory rate or knowledge of what shift from baseline represents a ‘significant’ deviation?
Reviewers found a paucity of evidence to guide what components should be included in ‘vital signs’ to identify transfusion reactions. While some clinicians consider SaO2 and pain vital signs, other practitioners may consider these components of a physical assessment [6]. Adding pain to vital sign monitoring for transfusion reaction detection aligns with the recommendation by the U.S. Department of Veterans Affairs to add pain as the fifth vital sign in 1999 and The Joint Commission's condition for accreditation to include pain with vital signs in the year 2000 [6, 23, 24]. Standardizing the inclusion of SaO2 and pain into vital sign assessments would ensure consistency in monitoring for blood transfusion reactions using the same screening criteria globally.
Frequency of vital sign measurement
In correlation with literature findings, the National Institute for Health and Care Excellence (NICE) Blood Transfusion CPGs only state ‘vital signs’ should be completed before, during and after blood transfusions without specifying what elements constitute vital signs or what results should raise clinician concerns [25]. Notably, the NICE Blood Transfusion CPG recommendations for vital sign monitoring were based only on the opinion of guideline developers without supporting evidence evaluating the clinical effectiveness or hazards [25]. In one study, 40% of the sample had vital sign changes after participants reported symptoms [2]. Consequently, vital sign monitoring at the start, after 15 min and at the conclusion of a transfusion may not detect transfusion-related events and do not detect delayed transfusion reactions.
One 921 bed U.S. hospital added vital signs 1-h post-transfusion to identify delayed reactions due to the study's outcomes [3]. The average blood product transfusion reaction time was over 1.5 h, with a wide variation in times from transfusion to reaction onset in the sample. Adverse transfusion events would go undetected with only 1-h post vital sign completion. These inconsistencies highlight the importance of standardizing vital signs across healthcare settings.
Partnering with patients and family
Only 9% of transfusion reactions in one study were discovered by routine vital sign monitoring alone [2]. Engaging patients and families as partners in detecting transfusion reactions led to earlier identification and management of transfusion-related events [2, 9]. Educating patients and families on what condition changes warrant immediate reporting to caregivers creates partnerships in care to improve recognition, timely management and clinical outcomes. The NICE Blood Transfusion CPG only informs nurses to provide verbal and written information to patients and family members on a list of transfusion-specific information that does not include guidance on detecting or monitoring for acute or delayed transfusion reactions or what warrants contacting clinicians immediately [25].
Respiratory assessment
Including respiratory rate monitoring due to deterioration predictive ability represents a key finding important to incorporate into nursing practice [6]. Oxygen saturation, respiratory rate and lung sound monitoring augment transfusion-related reaction detection. Pulmonary changes may lead to decreased SaO2, increased respiratory rate and alterations in lung sounds stemming from transfusion-associated circulatory overload (TACO), transfusion-related acute lung injury (TRALI) and transfusion-associated dyspnea (TAD) [2, 3, 6, 11, 17, 26]. The leading cause of transfusion-associated deaths prior to 2016 in the United States was TRALI [8]. Fiscal years 2016 through 2018 saw TACO grow to become the leading blood transfusion-associated cause of death in the United States [8].
National Healthcare Safety Network Hemovigilance Module data from 18,308 reported adverse reactions included 15 (65%) fatalities involving pulmonary complications of TACO, TAD and TRALI [26]. Sixty-one of the reactions involving TRALI included 44 (72.1%) deemed serious and 6 (10%) deaths [26]. Transfusion-reaction assessments including SaO2, respiratory rate and lung sounds throughout the monitoring period may detect these top transfusion-related conditions leading to death better than mere vital signs.
One U.S. Veterans Affairs hospital study reported that blood transfusion was not associated with significant changes in recipient vital signs [1]. Notably, neither respiratory rate nor SaO2 was included in the study's definition of ‘vital signs’ or applied by practitioners to detect a reaction in 3496 blood component infusions used to determine study results. Had researchers included respiratory rate and oxygen saturation in patient assessments, vital sign changes may have been found significant to detecting transfusion reactions in participants.
Inclusion of physical assessment findings
Focusing only on vital signs may overcloud physical symptoms of a transfusion reaction (Figure 3). Transfusion reaction signs and symptoms include physical findings only detected with a clinical assessment. Acute pain transfusion reaction supports inclusion of pain assessment throughout blood transfusion [4, 9]. Recommendations to add assessments for hives/itching, chills, dyspnea, flushing, nausea and back pain were made to detect transfusion reaction sooner [3]. For example, immediate febrile transfusion reactions not only result in a rise in body temperature but also include the symptoms of chills and malaise [9]. Haemolytic transfusion reactions require immediately stopping a blood transfusion. The haemolytic transfusion reaction may be detected through assessment of chills, pain, nausea, vomiting, shock and dark urine in addition to a rise in body temperature [2, 11]. Allergic reactions may only be detected through detection of urticaria, pruritis or hives. Vital sign monitoring frequency may be less critical than a thoughtful physiologic assessment to transfusion reaction detection at the earliest point of clinical deterioration.
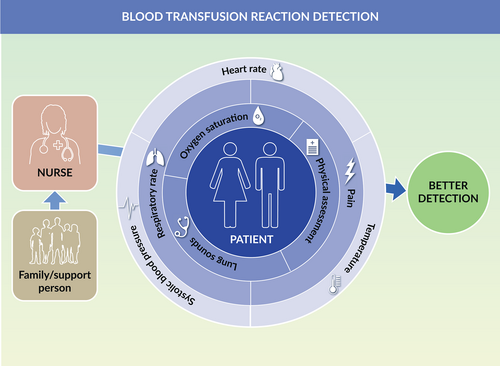
Limitations
Selection and sample biases were controlled through methodological use of independent review and evaluation by research team members before reaching a consensus on inclusion or exclusion of an article or evidence for synthesis of findings. As previously noted, limited evidence and a lack of high-level evidence were found to answer the clinical question being asked. Few relevant studies were identified using the selected search strategy to inform synthesis of discoveries from published literature. Applying search terms as free text words in addition to MeSH terms may have provided additional evidence to address the clinical question. The quality of research identified relevant to this literature review established new gaps in scientific literature needing further investigation. As a final point, varied study designs, definitions and methods used for calculating transfusion reaction rates in reported publications prevented meta-analysis of quantitative results.
CONCLUSION
While blood transfusion reaction detection remains fundamental for optimal patient outcomes through intervention at the earliest point in care delivery, no high-level evidence was found. Few studies have reported on patient screening criteria to detect transfusion reactions. Due to the lack of high-level evidence and guidelines in peer-reviewed literature to inform nurses of how frequently vital signs should be monitored and what components should be included in ‘vital signs’ to identify transfusion reactions, an evidence-based answer to the clinical question was not found by researchers. The absence of evidence to support conventional recommendations on the frequency of vital signs during a blood product transfusion led reviewers to more questions than answers.
Relevance for clinical practice
Reviewers explored what should be assessed by nurses to best detect transfusion reactions according to published evidence. Limited evidence and absence of guidance regarding measurable changes in assessment findings indicative of possible transfusion reactions led our team to more quandary than answers. Guidelines defining what deviations from vital sign baselines should raise alarms during transfusions are needed. Without evidence-based advice of what vital sign findings represent ‘abnormal’, vital signs may have limited usefulness in triggering transfusion reaction detection by nurses. More research needs to be completed to answer how often vital signs should be performed to detect blood transfusion reactions and to develop validated vital sign standards for reaction detection.
Adopting strategies to improve reaction detection such as employing patients and families to help recognize transfusion reaction signs and symptoms during transfusion and post-discharge improve detection of acute and delayed reaction events. Healthcare systems should compare evidence summaries and CPGs to current policies to ensure evidence-based practices are employed by nurses monitoring for blood transfusion reactions and other procedures. When quandaries arise during the investigation of evidence, these difficulties provide unique perspectives for consideration by clinicians and scholars alike. Evidence-based nursing practices may only be introduced to nurses at the point of care delivery with thorough investigation of published literature, synthesis of findings and global application of evidence-based findings.
ACKNOWLEDGEMENTS
J.K.R. designed the review. J.K.R., L.J., M.D., J.R. and B.W. collected and analysed the literature. J.K.R., L.J., M.D., J.R. and B.W. reviewed the manuscript. J.K.R. and B.W. edited the manuscript.
CONFLICT OF INTEREST
All authors have no conflicts of interest to disclose.




