The use of contrast-enhanced ultrasonography for the detection of active renal hemorrhage in a dog with spontaneous kidney rupture resulting in hemoperitoneum
The authors declare no conflict of interest.
Abstract
Objective
To describe the use of contrast-enhanced ultrasonography (CEUS) for the detection of active renal hemorrhage in a dog with spontaneous kidney rupture resulting in hemoperitoneum.
Case summary
A 9-month-old, sexually intact male Boxer dog presented for acute collapse, abdominal pain, and tachycardia. Physical examination findings were consistent with hypovolemia and acute abdomen. B-mode ultrasonography revealed peritoneal effusion and a right kidney mass. Subsequently, a CEUS study was performed on the right kidney, which demonstrated active hemorrhage from that kidney resulting in both hemoretroperitoneum and hemoperitoneum. At exploratory surgery, ultrasonographic findings were confirmed and a right nephrectomy was performed. Histopathology demonstrated severe parenchymal alterations along with the presence of nematode larvae. Fecal and urine testing for the presence of parasitic ova were negative. Identification of the larvae was inconclusive. At 30 days postoperatively, repeat B-mode ultrasound and clinicopathologic testing was unremarkable. The dog was alive at 1 year postsurgery with no ill effects.
New or Unique Information Provided
To the authors’ knowledge, this is the first report of CEUS for the detection of active hemorrhage from a kidney resulting in hemoretroperitoneum and hemoperitoneum in a dog. Although rare, the finding of nematode larvae within the renal parenchyma may have been the cause of kidney rupture. Importantly, surgical removal of the kidney was curative. Benign processes causing kidney rupture such as parasitic infestation should be considered in the working diagnosis as related to geographical location.
Abbreviations
-
- AFAST
-
- abdominal focused assessment with ultrasonography for trauma
-
- AFS
-
- abdominal fluid score
-
- CEUS
-
- contrast-enhanced ultrasonography
-
- FAST
-
- focused abdominal sonogram for trauma
Introduction
Ultrasound imaging is often used in the evaluation of dogs with suspect hemoperitoneum for both diagnosis and for identifying sources of bleeding before surgery. Splenic masses are the most commonly reported source of hemorrhage in dogs with tumors; however, other abdominal organs may be involved, including the liver, adrenal glands, and kidneys.1-3 Previous reports have shown that dogs with splenic masses and concurrent hemoperitoneum have high rates of malignancy.4 Other causes of nontraumatic causes of hemoperitoneum include coagulopathy.2 Thus, a thorough workup is important in deciding whether the case of hemoperitoneum should be managed medically or surgically. When a case is managed surgically, the identification of the source of bleeding is helpful prior to surgery in determining the degree of expertise needed for definitive ablation of ongoing hemorrhage. Standard B-mode ultrasonography can indirectly screen for the source of hemorrhage. More advanced ultrasound techniques such as contrast-enhanced ultrasonography (CEUS) when available can identify the source of intra-abdominal bleeding. In both human and veterinary medicine, CEUS increases the sensitivity and specificity of conventional ultrasonography with color flow Doppler, and might be a valid alternative to anesthesia and costs associated with performing computed tomography or magnetic resonance imaging.5 In veterinary medicine, although the use of CEUS holds promise,6, 7 reports of CEUS of the kidney are still limited.8, 9 To the best of the authors’ knowledge, the CEUS evaluation of injured renal parenchyma in a dog with spontaneous kidney rupture and subsequent hemoperitoneum has not previously been reported in the veterinary literature. In addition, the presence of nematode larvae within the renal parenchyma, which may have been cause of the kidney rupture, is discussed.
Case Summary
A 9-month-old, 20 kg, sexually intact male Boxer dog presented to an emergency hospital in northern Italy for evaluation of acute collapse and abdominal pain. The dog had appeared normal up until 3 hours previously when the owner observed weakness and an increased heart rate. There was no known trauma or toxin exposure. Vaccinations were current, monthly heartworm preventative therapy had been given since 2 months of age, and the prior health history was unremarkable. Abnormalities on physical examination included pale mucous membranes, a prolonged capillary refill time, tachycardia (200/min) with weak peripheral pulses, generalized weakness, and diffuse abdominal pain on palpation. Dorsal metatarsal arterial pulses were palpable. An over-the-needle catheter was placed in the right cephalic vein and blood for a CBC, serum biochemical profile, and coagulation profile was collected. A sample of urine was also submitted for dipstick evaluation and urine specific gravity assessment. A 400 mL IV bolus (20 mL/kg) of warmed lactated Ringer's solution was delivered over 15–30 minutes. Methadone1 was administered at 0.1 mg/kg IM for analgesia.
Blood work abnormalities are shown in Table 1. A normocytic normochromic nonregenerative anemia, mature neutrophilia, and thrombocytopenia were demonstrated on CBC. Prothrombin time and partial thromboplastin time were normal. Urinalysis revealed a specific gravity of 1.035 with trace hematuria on dipstick evaluation. These results were considered consistent with acute hemorrhage and subsequent decreased renal perfusion. A second sample of venous blood was collected for blood typing and the result confirmed the presence of dog erythrocyte antigen 1.1.
| Variable (reference range) | Patient values |
|---|---|
| Hct (0.37–0.55 L/L [37–55%]) | 0.25 (25.2%) |
| Hemoglobin (120–180 g/L [12–18 g/dL]) | 82 (8.2 g/dL) |
| RBC (5.5–7.9 × 1012 cells/L [5.5–7.9×106 cells/μL]) | 3.84 (3.84 × 106 cells/μL) |
| WBC (6.00–17.00 × 109 cells/L [6,000–17,000/μL]) | 27.20 (27,200/μL) |
| Neutrophils (3.00–11.50 × 109 cells/L [3,000–11,500/μL]) | 22.90 (22,900 cells/μL) |
| Platelets (120–350 × 109 cells/L [120–350 × 103 cells/μL]) | 45 (45 × 103 cells/μL) |
| TP (55–77 g/L [5.5–7.7 g/dL]) | 52 (5.2 g/dL) |
| Albumin (25–40 g/dL [2.5–4.0 g/dL]) | 22 (2.2 g/dL) |
| Creatinine (35.4–106.1 μmol/L [0.4–1.2 mg/dL]) | 229.8 (2.6 mg/dL) |
| BUN (1.8–10.7 mmol/L [5–30 mg/dL]) | 23.9 (66.8 mg/dL) |
| Phosphorus (0.7–2.1 mmol/L [2.3–6.6 mg/dL]) | 2.3 (7.02 mg/dL) |
| Fibrinogen (7.3–17.6 μmol/L [250–600 mg/dL]) | 3.2 (110 mg/dL) |
- Htc, hematocrit; RBC, red blood cells; WBC, white blood cells; TP, total protein; BUN, blood urea nitrogen.
Ultrasonographic examination of the abdomen revealed a moderate amount of echogenic free fluid and an irregular mass approximately 6 cm × 5 cm involving the cranioventral pole of the right kidney. The majority of the mass was isoechoic to the adjacent renal cortex, with multiple internal hypoechoic foci devoid of distal acoustic enhancement (Figure 1). The ultrasonographic appearance of the remainder of the renal cortex and the corticomedullary distinction were unremarkable in the right kidney and throughout the left kidney. No other abnormalities were observed. No pleural or pericardial fluid was detected by the focused abdominal sonography for trauma (FAST) examination on the subxiphoid view. Hemoperitoneum was confirmed by comparative packed cell volumes of the abdominal fluid (23%) to peripheral venous blood (25%), and the finding was that that the abdominal sample was nonclotting. Bacterial culture of the abdominal fluid was not performed.
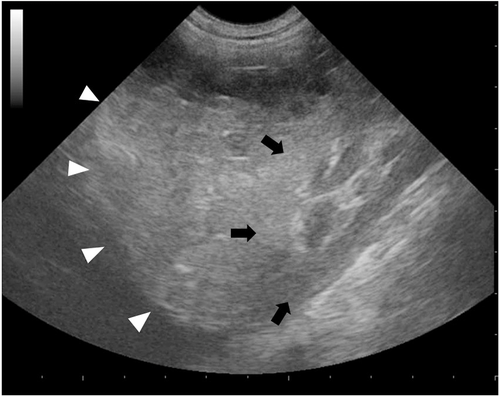
Based on the ultrasonographic findings, the main differential diagnoses included renal hematoma, granuloma, abscess, or neoplasia associated with intra-abdominal hemorrhage. Since color flow Doppler was inconclusive, CEUS was performed in effort to determine if the kidney was actively bleeding. A total volume of 0.6 mL (0.03 mL/kg) of sulfur hexafluoride filled microbubbles2 was injected intravenously in the right cephalic vein as a bolus, immediately followed by a 5 mL flush with sterile saline. CEUS was performed with a dedicated machine3 equipped with contrast-specific imaging technology4 for selective visualization of the contrast bubbles. Continuous scanning with a low mechanical index (0.07) and an acoustic pressure of 30 kPa during both early arterial) and late corticomedullary phases started immediately. The early arterial phase was defined as the time during which only the arterial vessels were enhanced (5–10 s after injection). The late corticomedullary phase began with homogeneous enhancement of the renal cortex followed by the medulla, which gradually became isoechoic with the cortex (10 s to maximum 2 min after injection). Total recording time was 120 seconds.
Qualitative evaluation of the contrast-enhancement pattern of the right kidney revealed a large, nonvascularized mass involving its cranioventral pole. The mass was surrounded by a thin, vascularized rim in direct continuity with the adjacent isoechoic renal cortex. Both the early arterial and the late corticomedullary phases were characterized by a small area in the renal cortex without enhancement and by two hyperechoic linear to fountain-like foci with strong enhancement extending from the renal cortex into the center of the mass (Figure 2). The presence of the hyperechoic linear to fountain-like foci was considered supportive of an active renal hemorrhage causing extravasation of the contrast agent from the ruptured kidney into the center of the mass. Based on this finding an exploratory celiotomy was performed with high suspicion, the source of hemorrhage was from the right kidney.
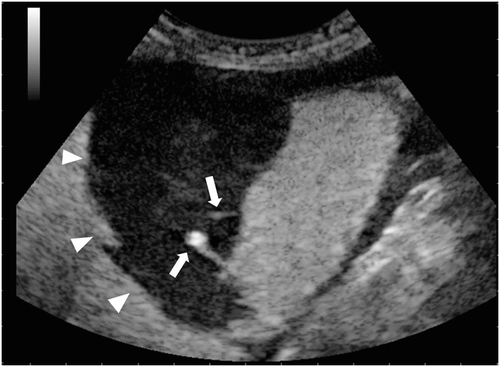
Preoperative stabilization included a transfusion of 300 mL (15 mL/kg) fresh whole blood dog erythrocyte antigen 1.1 positive in the left cephalic vein and warmed lactated Ringer's solution at 4 mL/kg/h in the right cephalic vein. Preoperative mean arterial pressure measured by oscillometric methods5 was 80 mm Hg and oxygen saturation measured by pulse oximeter was 99% in room air during spontaneous ventilation. Approximately 200 mL of hemorrhagic fluid were removed from the abdominal cavity. Active hemorrhage was observed from an irregular mass involving the cranioventral pole of the right kidney. The remainder of the exploratory celiotomy was unremarkable with no other sources of hemorrhage being identified. A right nephrectomy was routinely performed.
The postoperative course was uncomplicated and monitoring included vital signs (temperature, pulse, respiratory rate), mean arterial pressure, urine output, and serial monitoring of cell blood count and azotemia. Injectable antibiotic therapy (enrofloxacin, 5 mg/kg, IV, q 24 h) was given postoperatively along with buprenorphinee (0.01 mg/kg IV q 8 h). The dog was weaned off IV fluids over 72 hours postoperatively and ready for patient discharge; however, the owner elected for the dog to stay another 3 days. The dog was prescribed enrofloxacin (5 mg/kg, PO, q 12 h) and fenbendazole (50 mg/kg, PO, q 24 h) for 2 weeks. At the time of patient discharge, 6 days postoperatively, all clinicopathological values were within the normal limits with the exception of a packed cell volume of 28%.
The right kidney was sectioned mid-sagittally. On the cut surface, the renal cortex was characterized by 2 hemorrhagic, dark red areas present in the craniodorsal and cranioventral pole of the kidney containing clots admixed with necrotic tissue (Figure 3). Light microscopy demonstrated extensive hemorrhagic areas mainly confined to the cortex, but multifocally also involving the medulla. At this level, the renal parenchyma was no longer recognizable and diffuse necrosis was also present. In the remaining parenchyma, the glomerular compartment showed a diffuse global increase in mesangial matrix and focal global sclerosis associated with mild periglomerular fibrosis. Multifocal moderate podocyte hypertrophy with occasional sclerohyalinosis and rarely Bowman's capsule mineralizations were observed. Multifocal eosinophilic amorphous material was occasionally detected in the tubular lumina, and occasional tubular mineralizations were also evident. In addition, numerous multifocal pluricellular and cylindrical structures (at least 160 μm in length and 11–12 μm in diameter) were visible in the lumen of the glomerular capillaries (Figure 4), occasionally in the lumen of the tubuli, and rarely in the interstitium, where they were associated with a mononuclear infiltrate with giant cells. These structures were compatible with nematode larvae. Unstained slides were submitted to a diagnostic laboratory6 for genetic identification.10
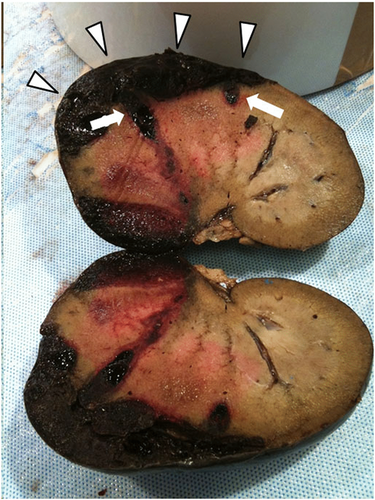
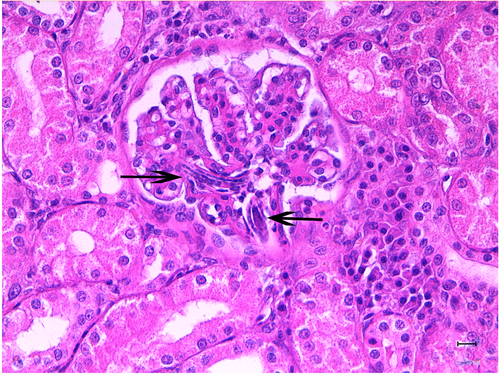
In an attempt to identify the nematode larvae, serial fecal tests, including direct smears, zinc sulfate flotation, and fecal Baermann, and routine urinalysis were performed. No parasite ova or larvae in either the urine or feces were detected. PCR results were negative.
On follow-up examination 1-month postoperatively, B-mode abdominal ultrasound was unremarkable and no clinical pathological abnormalities were found. At 1 year postoperatively the dog was alive with no associated morbidity.
Discussion
The case report documents the more advanced use of ultrasound using CEUS to help localize preoperatively active renal hemorrhage in a dog with spontaneous kidney rupture, resulting in hemoretroperitoneum and hemoperitoneum. Interestingly, nematode larvae were found on histopathology. The parasitic infection may not have been an incidental finding but rather could have been the cause of kidney rupture. Such a curative treatable benign process should be considered in appropriate geographical locations within the world.
B-mode ultrasonography is routinely used for indirectly evaluating for abdominal injuries and intra-abdominal bleeding through the detection of free fluid.11 The FAST examination, by virtue of its higher specificity and sensitivity in the detection of free fluid/hemorrhage as compared with diagnostic peritoneal lavage and computed tomography, has become an increasingly common diagnostic modality in human trauma patients12 and has been reported to improve patient assessment and care.13, 14 In human trauma patients, hemoperitoneum scoring systems based on FAST findings are utilized to semiquantitatively assess the degree of intra-abdominal hemorrhage.15 In veterinary medicine, the abdominal focused assessment with ultrasonography for trauma (AFAST) examination has been applied for the detection of free abdominal fluid, other intra-abdominal pathologies, and possibly pleural and pericardial injuries in patients with and without trauma, and particularly in those with cardiovascular instability.16, 17
Furthermore, because the serial AFAST examination can detect delayed fluid accumulation not apparent on initial AFAST scans, it is used in combination with an abdominal fluid score (AFS) to detect changes in the quantity of abdominal fluid over time.18 By combining the AFS system with initial and serial AFAST scans, the degree of ensuing anemia in acutely hemorrhaging dogs may be anticipated and a semiquantitative measurement of free abdominal fluid obtained. This provides the clinician with a powerful tool for assessing injury severity and a more reliable means than radiographic assessment of abdominal trauma than simply evaluating admission hematocrit and total serum protein.16, 18 However, there are limitations to sonographic detection and FAST examination of parenchymal injuries, especially of lower grade injuries,19, 20 and B-mode ultrasound examination may widen the differential diagnosis for an abnormal finding, as in the present case. To date, the AFAST examination has not been evaluated in veterinary medicine for the detection of solid organ or retroperitoneal injury after blunt abdominal trauma.17 Here, AFAST was used to detect the presence of hemoperitoneum, but it could also have been further applied in combination with the AFS to semiquantitate the degree of bleeding. Also, AFAST and AFS could have been combined to postoperatively screen for the recurrence or resolution of bleeding. Indeed, human and veterinary studies have shown the clinical value of serial examinations.15, 16, 18, 21
B-mode and color flow Doppler ultrasonography provide less detailed blood flow information than either computed tomography or magnetic resonance imaging.22-24 In contrast, CEUS is useful in the evaluation of vascular lesions;25 vascular devices;26 in distinguishing between benign and malignant focal parenchymal lesions;8 and for improving the detection of active, ongoing, and potentially life-threatening hemorrhages.25, 27 Indeed, contrast medium extravasation will appear hyperechoic to adjacent parenchyma, as in the present case, where the presence of 2 hyperechoic linear to fountain-like foci, not recognized with B-mode or color flow Doppler ultrasonography, was presumably due to extravasation of the contrast agent from the ruptured kidney into the large hematoma.27 Although CEUS has been used to visualize blood flow extravasation in human medicine,25, 27 such use in veterinary patients has been reported only in experimental canine and porcine models.28, 29
The most important disadvantages of CEUS include poor stability of ultrasonographic contrast agents and the need for operator expertise and specialized equipment, which increase the cost and reduce the availability of this imaging technique.30 Indeed, sophisticated ultrasound machines must be equipped with Doppler modalities, special low-frequency transducers, software that permits performance of contrast-specific imaging, and, eventually, software for quantitative evaluation of CEUS images.30 Another potential limitation is the time-sensitive nature of the scan; if a diagnostic scan is not acquired or a re-evaluation is necessary, further administration of the contrast agent would be required.31 Moreover, in people, some adverse effects have been reported.32 In general, ultrasonographic contrast agents are well tolerated for abdominal examinations, with minor and self-resolving adverse events (eg, headache, nausea, altered taste, or sensation of heat).32 Generalized allergy-like reactions occur rarely.32 To date, no adverse reactions have been reported in dogs.30
To the authors’ knowledge, spontaneous kidney rupture as a cause of hemoperitoneum is a rare scenario in veterinary medicine,1 while nematode larvae present within the renal parenchyma, as the possible cause of kidney rupture, have never been reported before. Splenic, hepatic, and adrenal masses have been associated with nontraumatic hemoperitoneum in the dog,2, 3 with the spleen reported as the most common source of hemorrhage; however, any mass of the kidney could outgrow its blood supply and result in hemorrhage.33-35 In this case, culture of hemorrhagic fluid was not performed during the initial diagnostic work up because of logistic concerns, while PCR was attempted at a later date10 because the final histological diagnosis of nematode larvae was unexpected. The absence of DNA amplification by PCR might have been due to DNA deterioration after paraffin embedding of the specimens. Furthermore, though the morphological features of the larvae could not be completely reconstructed, not even from serial histological sectioning, the authors may hazard a guess as to their identity. Most of the larvae were found in the glomerular capillary lumen and the periglomerular fibrosis was mild, suggesting a hematogenous route of infestation. In addition, the rare presence of interstitial granulomas does not support the hypothesis for larva migrans visceralis. Numerous larvae were present within the renal parenchyma, but no remarkable eosinophilic infiltration was noted. As eosinophils are known to play an important role in the defense against parasitic diseases, this finding was unexpected, but consistent with previous reports of nematode infestations that describe histopathological lesions without or with only a mild eosinophilic component.36
With regard to the possible involved nematodes, a list with pertinent information is listed in Table 2.36-42 Dioctophyma renale larvae could not be excluded, even if the absence of reports of this nematode infection in Italy make this hypothesis unlikely. Based on the preventative therapy, diameter of the larvae, tissue distribution, or little pathogenic significance in dogs, filarial parasites infection was considered unlikely as well. To the contrary, Angiostrongylus vasorum infection was considered likely based on the hematogenous route of dissemination, the similarity of histological lesions, and the presence of hemorrhage. Indeed, coagulopathies represent a common reported manifestation of this infection,36 even though the severity of bleeding correlates poorly with the extent of detectable coagulation abnormalities.39 The exact pathophysiologic mechanism for the coagulation derangements is not understood; it may be that endothelial disruption by the migrating larvae and adult parasites could result in DIC.39 However, because the PCR results were negative, a definitive confirmation of angiostrongylosis could not be made and remains speculative at best.
| Nematode | Dioctophyma | Dirofilaria | Dirofilaria | Dipetalonema | Angiostrongylus |
|---|---|---|---|---|---|
| species | renale37, 40 | immitis37, 41 | repens37, 41 | reconditum37, 41 | vasorum36-39, 42 |
| Diameter (larvae) | NA | 6.8 μm | 11–12 μm | 4.7–5.8 μm | 0.8–12 μm |
| Route of infection | Ingestion of infected raw fish, frog, or water | Bloodsucking culicids mosquitoes | Bloodsucking culicids mosquitoes | Infected fleas, ticks, lice | Ingestion of infected snail, slug, or frog |
| Preferred tissue localization | Peritoneal cavity, right kidney | Pulmonary arteries, right ventricle of the heart | Subcutaneous or intermuscular tissue | Subcutaneous or perirenal tissue, body cavities | Pulmonary arteries, right ventricle of the heart |
| Ectopic infections | – | Occasionally glomerular capillaries and medullary vessels | – | – | Nervous system, eye, liver, spleen, kidney |
| Miscellaneous | – | Asymptomatic disease or absence of microfilaremia possible | Asymptomatic disease or absence of microfilaremia possible | Asymptomatic disease or absence of microfilaremia possible | Asymptomatic disease, acute bleeding, false negative fecal Baermann test possible |
| Geographical locations | Worldwide | Worldwide (mostly tropical, subtropical, and warm temperate regions) | Europe, Africa, Asia (mostly tropical and subtropical regions) | Worldwide (most prevalent filaroid species infecting dogs in many geographical areas of the Mediterranean Basin, Middle East, South Africa, South America, and Oceania) | Europe, Panama State, Brazil, Columbia, United States, Newfoundland, Australia, Uganda |
- NA, not available; superscripts denote references for information provided.
With regard to the kidney rupture and subsequent hematoma formation, it is authors’ opinion that the presence of the numerous worms in the renal parenchyma could have played a role. Although uncommonly reported, another nematode that can cause kidney rupture is D. renale.40 Other additional hemostasis disorders, such as DIC with consumptive thrombocytopenia, immune-mediated thrombocytopenia, or von Willebrand factor deficiency should also be considered.38, 42
The severe thrombocytopenia observed here could be merely a consequence of acute hemorrhage; however, since disorders of consumption generally result in a mild or moderate degree of thrombocytopenia,43 a combined defect such as hemorrhage and DIC or a concomitant immune-mediated destruction could not be excluded. In this case, however, the presence of an eventually adjunctive hemostasis disorder was not investigated and it was ruled out by the dog having a full recovery with no morbidity at 1 year postoperatively.
In summary, the use of CEUS when available may be advantageously used for the direct detection of active hemorrhage over standard B-mode ultrasonography. Although technically challenging and limited in availability because of specialized equipment, CEUS should be considered as an imaging modality for dogs with hemoretroperitoneum and hemoperitoneum. Furthermore, because nematode larvae were found within the ruptured kidney parenchyma, benign curable conditions should be considered in the differential diagnosis of such cases.




