Mucous membrane pemphigoid in dogs: a retrospective study of 16 new cases
Abstract
enBackground
Mucous membrane pemphigoid (MMP) is a chronic autoimmune subepidermal blistering disease of dogs, cats and humans.
Objectives
The goal of this study was to describe the clinical, histological and immunological features and treatment outcomes of canine MMP.
Animals
Sixteen dogs were diagnosed with MMP based on the presence of mucosal- or mucocutaneous-predominant vesiculation and/or ulceration, histological confirmation of subepidermal clefting and an age of disease onset greater than 6 months.
Results
Six of 16 dogs (38%) were German shepherd dogs and their crosses. The median age of disease onset was 6 years (range: 1–10 years). At the time of presentation, the dogs exhibited erosions and ulcers in the oral cavity (11 of 16; 69%), nasal (nine of 16; 56%), periocular (eight of 16; 50%) and genital (six of 16; 38%) regions. Haired skin lesions were less frequent (six of 16; 38%) and involved mostly concave pinnae. Information on treatment outcome was available for 11 dogs (69%). A complete remission (CR) of lesions was achieved in 10 of 11 dogs (91%). The median time to CR was 33 weeks (range: 6–64 weeks). Treatment regimens varied widely but six of 10 (60%) dogs received a combination of tetracycline antibiotic and niacinamide alone, or with another drug, at the time of CR. Forty percent of the dogs in which CR had occurred experienced lesion relapse upon drug dose reduction.
Conclusions and clinical importance
Canine MMP is a chronic and relapsing disease requiring long term treatment. Combination therapy is often needed to achieve CR.
Résumé
frContexte
La MMP (mucous membrane pemphigoid) est une dermatite d'interface sous-épidermique auto-immune chronique du chien, du chat et de l'homme.
Objectifs
Le but de cette étude était de décrire les données cliniques, histologiques et immunologiques et l'effet des traitements de la MMP canine.
Sujets
Seize chiens ont été diagnostiqués avec MMP basé sur la présence de vésicules et/ou d'ulcères prédominant aux jonctions cutanéo-muqueuses, avec confirmation histologique de clivage sous-épidermique et une apparition des lésions après 6 mois.
Résultats
Six des 16 chiens (38%) étaient des Bergers allemands ou leurs croisés. L’âge moyen de l'apparition de la maladie était supérieur à 6 ans (écart : 1-10 ans). Au moment de la présentation, les chiens présentaient des érosions et des ulcères des régions orale (11 sur 16; 69%), nasale (neuf sur 16; 56%), pério-occulaire (8 sur 16; 50%) et génitale (sic sur 16; 38%).Les lésions des zones velues étaient moins fréquentes (six sur 16; 38%) et impliquaient principalement les faces concaves des pavillons auriculaires. Les informations sur les effets des traitements étaient disponibles pour 11 chiens (69%). Une rémission complète (CR) des lésions a été obtenue pour 10 des 11 chiens (91%). Le temps moyen de CR était 33 semaines (écart : 6-64 semaines). Les protocoles de traitement variaient largement mais six des 10 chiens (60%) ont reçu une combinaison de tétracycline et niacinamide seul ou avec une autre molécule au moment de la CR. Quarante pourcents des chiens ayant atteint une CR présentaient une récidive à la baisse de dose des traitements.
Conclusions et importance clinique
La MMP canine est une maladie chronique et récidivante nécessitant un traitement au long-court. Un traitement combiné est souvent nécessaire pour atteindre une CR.
Resumen
esIntroducción
el penfigoide de membranas mucosas (MMP) es una enfermedad crónica, autoinmune vesicular de perros, gatos y humanos.
Objetivos
el objetivo de este estudio fue describir las características clínicas, histológicas e inmunológicas y resultados de tratamiento de casos de MMP canino.
Animales
16 perros fueron diagnosticados con MMP basado en la presencia de vesículas y/o ulceración predominantemente en mucosas o uniones mucocutáneas, confirmación histológica de separación subepidermal y edad de aparición en animales mayores de 6 meses.
Resultados
seis de 16 perros fueron Pastores Alemanes o sus cruces. La edad media de aparición fue de 6 años (rango: 1-10 años). Al momento de la presentación, los perros presentaban erosiones y úlceras en la cavidad oral (11 de 16; 69%), región nasal (9 de 16; 56%), región periocular (8 de 16; 50%) y región genital (6 de 16; 38%). Las lesiones de la piel fueron menos frecuentes (6 de 16; 38%) y afectaban principalmente a la parte cóncava de pabellones auriculares. Información acerca del resultado del tratamiento estaba disponible en casos (69%). Se obtuvo resolución total de las lesiones (CR) en 10 de 11 perros (91%). La duración media para CR fue de 33 semanas (rango: 6-64 semanas). Los regímenes de tratamiento variaron ampliamente pero seis de 10 perros (60%) recibieron una combinación de tetraciclina y niacinamida solas o con otro fármaco hasta la CR. 40% de los perros con CR presentaron recidiva tras reducir la dosis de medicamentos.
Conclusión e importancia clínica
el MMP canino es una enfermedad crónica recidivante que requiere tratamiento a largo plazo. La terapia combinada es a menudo necesaria para obtener CR.
Zusammenfassung
deHintergrund
Das Schleimhautpemphigoid (MMP) ist eine chronische autoimmune subepidermale blasenbildende Erkrankung bei Hunden, Katzen und beim Menschen.
Ziele
Das Ziel dieser Studie war eine Beschreibung der klinischen, histologischen und immunologischen Charakteristika und der Behandlungsergebnisse des MMP des Hundes.
Tiere
Sechzehn Hunde wurden mit MMP diagnostiziert, die Diagnose basierte auf Bläschenbildung, die auf der Schleimhaut oder an den Übergängen der Haut in die Schleimhaut auftrat und/oder Ulzerierung, die histologische Bestätigung von subepidermaler Spaltenbildung und ein Auftreten der Erkrankung bei einem Lebensalter von mehr als 6 Monaten.
Ergebnisse
Sechs von 16 Hunden (38%) waren Deutsche Schäferhunde und ihre Mischlinge. Das mediane Lebensalter beim Auftreten der Erkrankung lag bei 6 Jahren (Spanne: 1-10 Jahre). Zum Zeitpunkt der Vorstellung zeigten die Hunde Erosionen und Ulzera in der Maulhöhle (11 von 16; 69%), der Nase (neun von 16; 56%), periokulär (acht von 16; 50%) und in der Genitalregion (sechs von 16; 38%). Die Veränderungen in der behaarten Haut waren weniger häufig (sechs von 16; 38%) und betrafen hauptsächlich die konkaven Oberflächen der Pinnae. Eine Information bzgl dem Therapieerfolg gab es für 11 Hunde (69%). Eine vollständige Remission (CR) der Veränderungen wurde bei 10 von 11 Hunden erzielt (91%). Die mediane Zeit bis zur CR betrug 33 Wochen (Spanne: 6-64 Wochen). Die Behandlungsregimes variierten stark, aber sechs von 10 (60%) Hunden erhielten zum Zeitpunkt der CR eine Kombination aus Tetrazyklin und Niacinamid alleine, oder mit einem anderen Medikament zusammen. Vierzig Prozent der Hunde bei denen eine CR auftrat, zeigten bei Dosisreduzierung ein Wiederauftreten der Veränderungen.
Schlussfolgerungen und klinische Bedeutung
Das MMP des Hundes ist eine chronische wiederkehrende Erkrankung, die einer Langzeittherapie bedarf. Eine Kombinationstherapie ist häufig nötig, um eine CR zu erzielen.
要約
ja背景
粘膜類天疱瘡(MMP)はイヌやネコ、ヒトの慢性自己免疫性表皮下水疱疾患である。
目的
この研究の目的はイヌのMMPの臨床的特徴、組織学的特徴、免疫学的特徴、および治療成績を解説することである。
供与動物
粘膜あるいは粘膜皮膚に明らかな水疱および/あるいは潰瘍が存在すること、組織学的に表皮下に裂隙が認められること、および発症年齢が生後6ヶ月以降であることを元に16頭のイヌをMMPと診断した。
結果
16頭中6頭(38%)はジャーマン・シェパード犬とそれらの交雑種であった。発症平均年齢は6歳であった(範囲:1-10歳)。イヌは初診時に、糜爛や潰瘍が口腔内(16頭中11頭;69%)、鼻(16頭中9頭;56%)、眼周囲(16頭中8頭:50%)、ならびに生殖器周囲(16頭中6頭:38%)で認められた。有毛部の皮膚病変はより頻度が低く(16頭中6頭:38%)、ほとんどの症例で耳介内側面に症状が存在した。治療結果に関する情報は11頭(69%)で得られた。病変の完全寛解(CR)が11頭中10頭(91%)のイヌで得られた。CRまでの平均期間は33週間(範囲:6-64週間)であった。治療法は様々であったが、10頭中6頭(60%)のイヌはCRの時にテトラサイクリン系抗菌剤およびニコチン酸アミドの組み合わせのみか、その他の併用薬を投与されていた。CRが得られた40%のイヌでは、薬剤の減量により症状の再発が生じた。
結論および臨床的な重要性
イヌのMMPは長期的な治療を必要とする慢性および再発性疾患である。CRを得るために多くの場合、複数の薬剤を組み合わせた治療が必要である。
摘要
zh背景
黏膜类天疱疮(MMP)是犬、猫以及人的一种慢性、免疫性、表皮下水疱性疾病。
目的
这项研究的目的为描述犬MMP的临床表现、组织学和免疫学特征以及治疗方案。
动物
诊断为MMP的16只犬,其组织学特征主要为黏膜或黏膜皮肤结合处以水疱和/或溃疡,表皮下开裂,发病年龄大于6月龄。
结果
16只犬中的6只(38%)为德国牧羊犬或其杂交犬。该病的中值发病年龄为6岁(范围:1-10岁)。犬只口腔(16只中的11只; 69%)、鼻部(16只中的9只; 56%)、眼周(16只8只中的b; 50%)和生殖器(16只中的6只; 38%)等位置出现糜烂和溃疡。有毛区域病变较少出现(16只中的6只; 38%),包括耳廓凹面。11只犬(69%)治疗有效。11只犬中的10只犬,其症状达到完全缓解(CR)。完全缓解的中值时间为33周(范围:6-64周)。治疗方案多样化,但是10只犬中的6只 (60%)在中值时间内接受了四环素、烟酰胺或另加一种药物的联合治疗方案。随着药物剂量的降低,40%的犬症状出现复发。
总结和临床意义
犬MMP是一种慢性、复发性疾病,需要长期治疗。该病常常需要联合治疗以达到完全缓解。
Introduction
Mucous membrane pemphigoid (MMP), also known as cicatricial pemphigoid, is a chronic autoimmune subepidermal blistering disease (AISBD) recognized in humans, dogs and cats.1-4 In all of these species, it is characterized by a variable combination of vesicles, ulcerations and scarring affecting predominantly mucosae and mucocutaneous junctions. Although exact epidemiological data on canine MMP are unavailable, this disease is considered to be the most common AISBD in this species, with German shepherd dogs appearing to be an over represented breed.2
In humans and animals, MMP is associated with the presence of circulating autoantibodies that target variable antigens of the basement membrane zone (BMZ); these include collagen XVII (BP180), BPAG1e (BP230), integrin α6/β4, laminin-332 and, more rarely, collagen VII.1-4 The demonstration of skin fixed-antibodies and/or activated complement and/or the presence of circulating anti-BMZ antibodies is proposed as one of the diagnostic criteria for MMP in humans.4 Likewise, it has been suggested that, if available, the demonstration of anti-BMZ antibodies and/or complement should be part of the diagnostic criteria for the diagnosis of MMP in dogs.2 Nonetheless, due to autoantigen overlap among the AISBDs, the demonstration of an elevated titre of anti-BMZ antibodies would not help distinguish MMP from other AISBDs.5-7 Furthermore, in humans, especially when appropriate biopsy cannot be obtained, the presence of anti-BMZ antibodies is used predominantly to eliminate other blistering diseases that do not affect the BMZ directly; these include, for example, lichen planus, pemphigus vulgaris, erythema multiforme/Stevens Johnson syndrome and angina bullosa haemorrhagica.6
In humans, the treatment of MMP depends on the “risk group” into which patients are classified. This classification is based on the severity, extent and location of lesions (i.e. “low-risk” group: oral involvement with or without skin lesions; “high-risk” group: eye, genital, respiratory tract and upper gastrointestinal tract lesions).4 Because of the better prognosis in low-risk patients, the treatment is usually more conservative and utilises drugs such as topical glucocorticoids with or without oral low-dose glucocorticoids, tetracycline/niacinamide or dapsone.8, 9 In contrast, patients in the high-risk group are treated with more potent drugs from the onset to avoid, or reduce, the irreversible scarring and strictures associated with this disease. In this group, the use of systemic drugs is mandatory and a combination of adjunctive immunosuppressive medications such as high-dose glucocorticoids, azathioprine, cyclophosphamide and/or intravenous immunoglobulin (IVIG) infusions are often used.8, 9 Unfortunately, because of the chronic and progressive nature of the disease and its tendency to relapse, MMP is challenging to manage in people, and the reported treatment success rates can vary greatly.10
Because of the rarity of MMP in dogs, only limited information about the treatment and outcome of this disease is available. To the best of the authors’ knowledge, there is only a single publication discussing the treatment outcome of 11 dogs with MMP.2 More information about canine MMP will assist to better understand this syndrome and improve our ability to successfully manage this chronic, frequently relapsing disease.
The objectives of this study were as follows: (i) to report information regarding the signalment, clinical presentation, histological and immunological features of additional cases of canine MMP; and (ii) to assess the therapeutic interventions and treatment outcomes in dogs with MMP.
Material and Methods
Case selection
Dogs included in this study were selected from: (i) cases whose sera were tested in our immunodermatology laboratory between 2003 and 2014; (ii) cases diagnosed with MMP by veterinary clinicians at the North Carolina State University (NCSU) between 2003 and 2014, and (iii) cases identified through an e-mail sent to the Vetderm Internet list ([email protected]) between February 2014 and August 2014.
- Clinical observations of mucosal- or mucocutaneous-predominant vesiculation and/or ulceration.
- Histopathological findings of subepidermal vesiculation/separation and exclusion of other pathologies resulting in an epidermal detachment (suprabasal acantholysis, interface dermatitis, epidermal necrotizing diseases) in a biopsy report.
- Disease onset >6 months of age.
Because of the immunological heterogeneity of canine MMP and the lack of ability of direct and indirect immunofluorescence (IF) to distinguish MMP from other AISBDs, positivity for either or both of these tests was not required as additional inclusion criterion.6, 7
In order to obtain information about history, signalment, clinical signs, treatment and outcome, referring veterinarians were asked to complete an ad hoc questionnaire and submit clinical images of the cases whenever available. Medical records, including histopathology reports and clinical images, were reviewed for all cases diagnosed at NCSU and the Vetderm Internet list cases. The treatment outcome was based on findings obtained during the last available physical examination of the affected dog, and focused on reporting information on the number of dogs in which a complete remission (CR) of the disease had been achieved or those with lesions that failed to respond to the treatment. A CR was defined as the absence of new lesions or the complete healing of existing ones, whereas a “failure of therapy” was defined as an inability to control disease activity (i.e. a continuous development of new lesions, an extension of old lesions or a lack of healing). In dogs lost to follow-up, the treatment outcome was based on clinical findings recorded during their final visit.
Histopathology
Sixteen cases had histopathological findings reported of subepidermal vesiculation/separation (the primary histopathological inclusion criterion), based on the assessment of their primary histopathology report. If available, tissue blocks and/or histological sections of biopsies were requested from clinicians that contributed cases to the study. Tissue samples from 11 dogs were available for further review by one of the authors. Histological sections were stained with haematoxylin and eosin and evaluated as described previously.11
Detection of tissue-bound and circulating anti-basement membrane zone autoantibodies
Tissue deposits of IgG, IgM and IgA antibodies and activated C3 complement at the BMZ of the lesional and perilesional skin were detected using direct IF performed on paraffin-embedded skin samples with minor variations from the technique described earlier.12 The source and dilution of immunoreagents had varied over time due to the 11 year span of this retrospective study.
The detection of circulating anti-BMZ IgG and IgA antibodies was performed by indirect IF on salt-split buccal mucosa collected from healthy dogs euthanized at a local shelter or as part of other research protocols using a previously published protocol.13 For the detection of IgE autoantibodies, a secondary monoclonal mouse anti-dog IgE antibody (clone 5.91, Bruce Hammerberg, NCSU Raleigh; NC, USA) was used. Sera from affected dogs were collected during phases of active disease. All patient sera were tested at serial dilutions ranging from 1:10 to 1:2,500 for IgG, 1:10–1:40 for IgA and 1:10–1:50 for IgE and IgM. Sera from normal healthy dogs served as negative controls. A result was deemed positive if a linear fluorescence pattern could be detected at the level of the BMZ. The location of the antibody binding was further specified as dermal, epidermal or mixed depending on whether the fluorescence was detected on the bottom, on the roof or on both sides of the salt-split buccal mucosa section, respectively.
Results
Signalment
Sixteen dogs fulfilled the inclusion criteria and were included in this study. Two dogs were diagnosed with MMP at NCSU, ten dogs were referred for further testing in our immunodermatology laboratory between 2003 and 2014, and four dogs were received as a response to a Vetderm Internet list search performed between February and August 2014. Of these 16 dogs, 12 (75%) were purebreds and four (25%) were crossbred dogs. Six (38%) were German shepherd dogs and their crosses; two (13%) were poodles and their crosses, and there was one each of the following breeds: Shetland sheepdog, English springer spaniel, pug, Rhodesian ridgeback, giant schnauzer and Rottweiler. The female-to-male sex ratio was 1.
The median age of onset of skin lesions was 6 years (range: 1–10 years). Four dogs (25%) developed lesions in early adulthood (between 1 and 3 years of age). Eight of the 16 dogs (50%) developed lesions in mid-adulthood (between 4 and 7 years of age) and the remaining four dogs (25%) had the onset of lesion in late adulthood (8 years and older).
Odds ratios for breed, sex or age predispositions to develop MMP could not be calculated because included dogs were from various continents and a control population was therefore not available.
Clinical summary
Skin lesions were symmetrical in 14 of 16 dogs (88%) and they consisted of erosions and ulcers (16 of 16 dogs; 100% – an inclusion criterion), crusting (nine of 16; 56%), erythema (five of 16; 31%), vesicles/bullae (five of 16; 31%) (Figure 1a), scarring (three of 16; 19%) and hypopigmentation (two of 16; 13%).
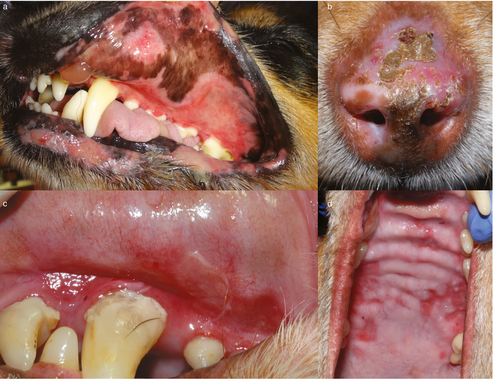
The body regions first reported to be affected by lesions were oral/perioral (12 of 16; 75%), ocular/periocular (six of 16; 38%), nasal (four of 16; 25%) and genital (two of 16; 13%) regions and/or concave pinnae (two of 16; 13%). At the time of presentation to the veterinarian, all dogs (100%) exhibited lesions on mucosae or mucocutaneous junctions assuming the nasal planum to be a modified mucosa. Fifteen dogs exhibited lesions on two or more mucosae or mucocutaneous junctions (median: 3, mean: 3). Haired skin involvement was less common (six of 16; 38%). The distribution of lesions at the time of diagnosis is summarized in Table 1 and representative lesions are shown in Figure 1.
| Affected areas | Number of dogs | Percentage | |
|---|---|---|---|
| Mucosae/mucocutaneous junctions | Oral cavity | 11 | 69% |
| Gingiva | 10 of 11 | 91% | |
| Hard/soft palate | 9 of 11 | 82% | |
| Tongue | 4 of 11 | 36% | |
| Labial/perilabial | 9 | 56% | |
| Nasal planum/perinasal | 9 | 56% | |
| Eyelids | 8 | 50% | |
| Genitalia | 6 | 38% | |
| Anus/perianal | 4 | 25% | |
| Haired skin | Concave pinnae | 5 | 31% |
| Pressure points | 2 | 13% | |
| Periungual | 1 | 6% | |
- Note: For this disease and based on previous experience, the nasal planum was considered to be a modified mucosa; the affected pressure points in both cases were elbows.
Nondermatological complaints reported in six of 13 dogs (46%) were associated with the oral cavity and consisted of malodour (two dogs), pain when eating (two dogs) and excessive salivation/drooling (two dogs). A loss-of-function of affected organs secondary to chronic scarring (e.g. blindness, genital strictures, breathing difficulties) was not reported in any of the dogs.
Treatment and outcome
Information about the treatment and outcome was available for 11 dogs (69%). The remaining five dogs were lost to follow-up immediately after diagnosis. The median time of follow-up was 50 weeks (range: 3–231 weeks). A CR of MMP was achieved in 10 of 11 dogs (91%); the median time to CR was 33 weeks (range: 6–64 weeks). A spontaneous remission of MMP was not seen in any patient.
Treatment regimens varied widely between dogs and they included the following drugs: glucocorticoids, tetracycline antibiotics (i.e. doxycycline or tetracycline), niacinamide, azathioprine, ciclosporin (with or without ketoconazole), dapsone, colchicine, topical glucocorticoids and tacrolimus. These drugs were used either as monotherapy (one of 11; 9%) or in various combinations (10 of 11; 91%) (Table S1). Nine dogs (82%) were treated with two or more drugs throughout the entire treatment period (from initial diagnosis until CR). At the time when CR was documented, the majority of dogs (six of 10; 60%) were receiving a tetracycline antibiotic and niacinamide alone or in combination with another drug (Table 2). Complete remission was also obtained in the single dog treated with oral glucocorticoid monotherapy. The topical use of glucocorticoids and tacrolimus was reported in three (27%) and two (18%) of 11 dogs, respectively. Of these, two of 11 dogs (18%) were treated with topical therapy at the time when CR was achieved.
| Number of drugs | Number of dogs | % (10 dogs total) | Oral | Topical | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GC | Tetracycline antibiotics | NIAC | AZA | CSA | KTZ | DAP | COLC | GC | TAC | |||
| 1 | 1 | 10% | X | |||||||||
| 2 | 2 | 20% | X*† | X | ||||||||
| 1 | 10% | X | X | |||||||||
| 1 | 10% | X | X | |||||||||
| 3 | 2 | 20% | X | X | X | |||||||
| 1 | 10% | X | X | X | ||||||||
| 4 | 1 | 10% | X | X | X | X | ||||||
| 1 | 10% | X | X | X | X | |||||||
- Abbreviations: GC, glucocorticoids; Tetracycline antibiotics (*tetracycline, †doxycycline); NIAC, niacinamide; AZA, azathioprine; CSA, ciclosporin; KTZ, ketoconazole; DAP, dapsone; COLC, colchicine; TAC, tacrolimus.
Oral glucocorticoid monotherapy was the most frequent treatment regimen that failed to induce CR (five of 11; 45%) (Table S2).
No single treatment protocol appeared to be associated with a more rapid disease remission. All 10 dogs in which CR was obtained were maintained on a tapered dosage regimen of their respective drug therapies; none of these dogs had their treatment withdrawn. Four dogs (40%) experienced a recurrence of MMP with a median time to relapse of 12 weeks (range: 1–40 weeks). Of these four dogs, three (75%) relapsed when treatment was tapered (2 dogs) or withdrawn (1 dog), whereas one dog was receiving the initial treatment regimen when the relapse occurred.
Histopathology
For 11 dogs, skin biopsy material was available for further review, with 2–20 histological sections of mucocutaneous junctions, mucosae and/or haired skin per dog. Vesicles were formed by subepidermal clefts (Figure 2a), the majority of which were ruptured and bordered an ulcer. Vesicles ranged from very small to large, occasionally spanning the width of the biopsy, and/or involved follicular infundibula. Most vesicles were empty; however, a rupture of the majority of vesicles limited evaluation of vesicle contents for inflammatory cells, haemorrhage and fibrin. Subepidermal microvacuoles along the BMZ were uncommon and mild (two of 11; 18%) or, less often, marked (one of 11; 9%). A superficial dermal or submucosal band of fibrosis (seven of 11; 64%) was common below vesicles, or below intact epithelium near vesicles (Figure 2b), and ranged from mild to marked. Fibrosis was reorganizing or sometimes had numerous small blood vessels and shared features with a thin band of granulation tissue. Biopsies with and without fibrosis were seen in the same case.
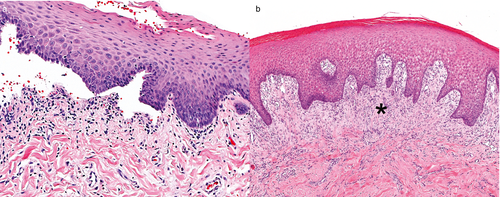
Dermal and submucosal inflammation varied greatly in the extent and type of inflammatory cells present. Perivascular inflammation was occasionally mild and often moderate to marked. Marked inflammation was typically associated with a mucosa, chronic ulceration and/or secondary bacterial infection characterized by neutrophilic crusts, luminal neutrophilic folliculitis and bacterial colonization. Mucosal and mucocutaneous inflammation sometimes formed a band-like infiltrate of lymphocytes and plasma cells just below the epithelium (a lichenoid infiltrate). In at least one biopsy from each dog, mild and occasionally moderate amounts of dermal neutrophils (eight of 11; 73%) and eosinophils (six of 11; 55%) below vesicles or intact epithelium were present. Lymphocytes and plasma cells were common and ranged from mild to marked, when present. However, inflammation did not appear to localize to (target) the BMZ in most cases (nine of 11; 82%). Rowing of individual neutrophils along the BMZ was uncommon (one of 11; 9%) as was rowing of histiocytes along the BMZ (two of 11; 18%), but when present these changes were moderate to marked. Lymphocytic exocytosis was common (nine of 11; 82%) and was mostly mild but sometimes moderate. A few lymphocytes infiltrated infundibula and external root sheath epithelium of hair follicles in one dog.
Regular epidermal or mucosal hyperplasia was common (11 of 11; 100%) and was most often moderate. Although apoptosis of basal, and less often suprabasal, keratinocytes was common (nine of 11; 82%), apoptosis usually only involved rare individual keratinocytes, and only in a few cases was apoptosis numerous enough to be warrant a mild grade. Vacuolation of basal keratinocytes was not a feature. Pigmentary incontinence was absent or ranged from mild to marked, but many sections lacked epidermal pigment to disperse to the dermis.
Detection of tissue-bound and circulating anti-BMZ autoantibodies and complement
The detection of tissue-bound IgG, IgM, IgA antibodies and C3 complement using direct IF was performed in 13 dogs. Twelve of these 13 samples (92%) exhibited a linear deposition of IgG along the BMZ. The only dog with negative direct IF (Case 15) tested positive for circulating anti-BMZ IgG. Basement membrane-bound IgA, IgM (Figure 3a) and C3 were detected in one (8%), four (31%) and two (15%) cases, respectively.
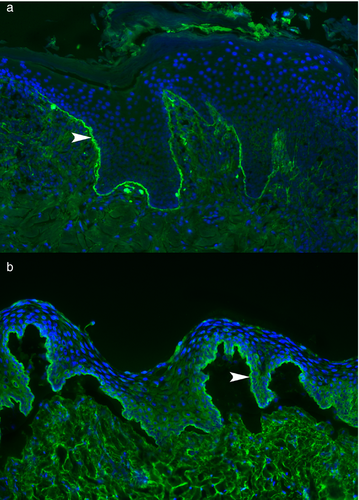
Sera from 11 dogs were available for detection of circulating anti-BMZ IgG, IgA and IgE antibodies. A positive deposition of circulating IgG antibodies in the form of a linear fluorescence deposit at the BMZ of the salt-split buccal mucosa was detected in eight of 11 (73%) sera. The binding of these circulating IgG autoantibodies was nearly always restricted to the epidermal side of the salt-split mucosa (seven of eight; 88%) (Figure 3b), or it was observed on both epidermal and dermal sides (one of eight; 13%). One of the three dogs without detectable circulating anti-BMZ IgG had detectable tissue-bound anti-BMZ IgG (Case 1). Tissues from the other two dogs with negative indirect IF were not available for direct IF staining (cases 3 and 4). Circulating anti-BMZ IgA and IgE antibodies were not detected in any of the 11 dogs.
Discussion
Although MMP is the most common AISBD in dogs, the overall rarity of this condition limits our knowledge about this entity, especially regarding the treatment and prognosis.1, 2, 7 Therefore, the goal of our retrospective study was to provide additional information about the clinical, histological and immunological aspects of this syndrome, with an emphasis on treatment and outcome. This study adds 16 new cases of canine MMP to those previously published.1, 2
The analysis of the signalment of these 16 dogs with MMP is consistent with the previously reported finding that the German shepherd dog is a frequently affected breed.2 A possible breed predisposition for MMP is predictable, as genetics have been linked to the susceptibility of development of many autoimmune diseases in people, including MMP.6 Unfortunately, the relative risk for development of MMP in German shepherd dogs could not be calculated because a control population was not available for such comparison.
The female-to-male ratio in this study was one, which is in contrast to the previously reported data where males were more often affected than females.2 The reason for this disparity is unknown, although the relatively small sample size compared to that of the previous meta-analysis could have prevented the detection of a subtle difference in the ratio.2 Interestingly, in people with MMP, women appear to be affected more often than men by a factor of 1.5 to 2.14
Although the median age of the disease onset reported in this study was similar to that of the previous meta-analysis, most dogs in this study developed the initial signs of MMP in their mid-adulthood (i.e. aged 4–7 years).2 This latter finding was in contrast to the data reported in the previous review in which dogs generally developed the disease during their late adulthood (older than 7 years).2 It is possible that increased awareness of this disease over the past decade has led to an earlier detection of clinical lesions and confirmation of the diagnosis. In humans, MMP is a disease of elderly people with a mean age of onset between 60 and 80 years.6, 14
Most dogs in our study presented initially with oral and/or perioral lesions, an observation similar to that reported in the previous study.2 Over the course of the disease, this region remained the most commonly affected site, although most dogs developed lesions affecting other mucosae or mucocutaneous junctions. Indeed, over 90% of dogs exhibited lesions affecting two or more mucosae or mucocutaneous junctions, a feature also reported in previous cases of canine MMP.2 In a similar way to dogs, oral cavity lesions are present in almost all human patients (85%) with MMP.6 Ninety one percent of dogs with oral involvement had lesions on the gingiva, which early in the course of disease could lead to the misdiagnosis of MMP as another ulcerative gingival disease.15 People affected with MMP often develop blisters on the nasal mucosa, larynx, pharynx or oesophagus. Although none of the dogs in this study exhibited signs suggesting such involvement, an endoscopic examination was not performed in any of the dogs.
The nasal planum appeared to be the second most common area to be affected (nine of 16; 56%); it was also the third most common area in which the initial lesions were reported to develop (four of 16; 25%). Dogs with this lesion distribution could be misdiagnosed clinically as having discoid lupus erythematosus (DLE). However, facial DLE rarely affects the oral cavity and this alone distinguishes MMP from facial DLE.16-18
Our study did not reveal any apparent correlation between the location of lesions, their severity or the number of affected sites and disease prognosis. In contrast, people affected with ocular, laryngeal, genital or oesophageal MMP tend to have poorer prognosis due to the possibility of a functional impairment of these tissues (e.g. blindness in ocular MMP, breathing difficulties in nasal MMP, sexual dysfunction in genital MMP).6 The location of lesions, their severity and the speed of the progression are important factors dictating the nature of the treatment approach in human MMP.4, 9 For example, on one hand, people with severe ocular, genital, oesophageal and/or laryngeal MMP usually are treated with systemic immunosuppressive drugs such as glucocorticoids, cyclophosphamide, azathioprine, mycophenolate mofetil or, in refractory cases, with intravenous immunoglobulins and/or biologics such as chimeric anti-CD20 antibody. On the other hand, the initial treatment proposed to people with mild disease involves topical glucocorticoids, dapsone or tetracycline antibiotics with niacinamide.4, 9
In this study, CR was obtained in 60% of dogs that received tetracycline antibiotics and niacinamide alone or in combination with another immunosuppressive drug. Likewise, five of 11 dogs (46%) included in the previous review of canine MMP had had a partial or complete resolution of clinical signs with a combination of tetracycline and niacinamide;2 combining these data suggests that this combination alone or with additional immunosuppressive drugs should be considered as a first-line treatment for canine MMP. Although the exact pathogenesis of MMP remains unknown, several lines of evidence suggest that antibody-mediated, complement-dependent as well as complement-independent pathways play a role in the dermoepidermal separation occurring in this disease.6, 14 The first pathway is initiated by the binding of autoantibodies to the BMZ antigen(s), which leads to activation of complement, mast cell degranulation, recruitment of polymorphonuclears and BMZ injury by released proteases.6 In addition, there is increasing evidence that complement-independent processes involving keratinocytes, proteases and/or endocytosis with subsequent degradation of the components of the BMZ could be involved in blister formation.19, 20 Similar mechanisms leading to complement-independent blister formation have been investigated in human bullous pemphigoid (BP).21, 22 The inhibitory effect of tetracycline antibiotics and niacinamide on the activity of proteases, along with their wide range of antiinflammatory properties (e.g. inhibition of leukocyte chemotaxis and proinflammatory cytokines), is a logical hypothesis for the observed benefit of these drugs in canine and human patients suffering with pemphigoid diseases, although the mode of action in dogs remains to be elucidated.23-25
Our data indicated that oral glucocorticoid monotherapy was the most frequent treatment regimen that failed to induce CR. Indeed, most dogs reported in this study and in the previous review received a combination therapy including two or more immunosuppressants.2 Although CR was achieved in more than 90% of dogs in this study, a high rate of a disease relapse was observed. The relapsing nature of this disease should be considered when discussing the prognosis and a long-term treatment plan with the owner.
Microscopic findings in this study were similar to those reported previously for canine MMP.1, 2 Subepidermal clefts, an inclusion criterion, were empty or ruptured more often than filled with inflammatory cells. Ulcers were common. Dermal inflammation often contained eosinophils, which is also described in canine epidermolysis bullosa acquisita (EBA) and BP,11, 26 but their numbers were usually low in the MMP. Although neutrophils and eosinophils were common in the dermis, inflammation targeted the BMZ in only few cases, where it overlapped with the inflammatory patterns described for canine EBA.11 Much of the moderate-to-marked inflammation in the dermis and submucosa was considered secondary and it was likely attributable to bacterial infection, ulcers and/or a chronic generic response of mucosal or mucocutaneous tissue to injury; this was often seen as a lymphoplasmacytic (lichenoid) band pattern. These nonspecific inflammatory features could potentially complicate a histological diagnosis.
Occasional basal keratinocyte apoptosis and pigmentary incontinence were observed and were considered nonspecific, as both can be seen with other subepidermal blistering diseases11 and with injured hyperplastic epidermis or mucosa generically. Additionally, the occasional keratinocyte apoptosis in AISBDs could also be the result of anoikis, a form of apoptosis triggered by a loss of the cell attachment to the appropriate matrix.27 Basal cell apoptosis in combination with a generic mucosal lichenoid inflammatory pattern might lead to a misdiagnosis of mucocutaneous lupus erythematosus (MCLE), which shares clinical features with MMP including a propensity to form mucocutaneous ulcers and a breed predisposition for the German shepherd dog. Mucocutaneous lupus erythematosus, however, rarely affects the oral cavity.28 It is possible that some dogs develop both conditions concurrently, because we have observed one case with good histological evidence of both MCLE and MMP.28 Suprabasal apoptosis of keratinocytes might lead to a histological misdiagnosis of the erythema multiforme (EM) group of diseases, but usually EM presents with more suprabasal apoptosis than that observed in this study.29 It is recommended that clinicians collect multiple biopsies to increase their probability of obtaining an accurate diagnosis. Fibrosis is a recognized clinical feature of MMP in humans and dogs.1, 4 In this study, superficial dermal or submucosal fibrosis was seen more commonly histologically than in previous reports of canine MMP or EBA.1, 11 However, the degree of fibrosis varied from absent to marked in biopsies from the same dog and, by itself, fibrosis is not expected to differentiate canine MMP from other subepidermal clefting diseases. Furthermore, chronic secondary bacterial infection might induce fibrosis in some cases nonspecifically, complicating identification of any primary fibrosing features of canine MMP.4
The presence of a continuous deposit of anti-BMZ antibodies and/or C3 complement in the skin of affected people is a diagnostic criterion in human MMP.4 A similar criterion has also been suggested for canine MMP in the past, but, because direct IF is not commercially available, this criterion is not usually and readily fulfilled in veterinary medicine.2 In our study, 13 dogs were available for detection of tissue-bound and/or circulating anti-BMZ antibodies; anti-BMZ antibodies, most frequently IgG, were detected in all dogs using direct and/or indirect IF. Sera from 11 dogs were available for indirect IF using salt-split buccal mucosa, which distinguishes between an autoimmune response directed against antigens localized to the epidermal (collagen XVII, BP230, integrins) or the dermal (laminins, collagen IV, collagen VII) side of the salt-split tissue.30, 31 Canine MMP has been shown to be immunologically heterogeneous, with antibodies targeting various BMZ antigens such as collagen XVII, BP230 or laminin-3322 and, therefore, an antibody deposit can be detected at either the epidermal or the dermal side of the salt-split mucosa. Consequently, although the detection of anti-BMZ antibodies is helpful in confirming the autoimmune nature of the syndrome and narrowing down the differential diagnoses, it is in no means specific for MMP, because other AISBD will often exhibit similar positive results and patterns.11, 26, 32 This opinion is echoed in an editorial that emphasised the importance of the clinician in the diagnostic process.7 Finally, anti-BMZ antibodies and/or complement are not always detected by IF in people and dogs affected with MMP.2, 33, 34 In this study, three of 11 dogs (27%) had undetectable levels of circulating anti-BMZ autoantibodies, a percentage similar to that previously reported in canine MMP.2 Although the exact explanation of the negative indirect IF in the three dogs remains unknown, it is conceivable that these three dogs might have been treated with immunosuppressive medications before blood collection, which could have reduced anti-BMZ autoantibodies below the detectable level. The degradation of autoantibodies over time is an alternative hypothesis.
In conclusion, canine MMP is a chronic, slowly progressive, mucosal/mucocutaneous-dominant AISBD frequently diagnosed in middle-aged German shepherd dogs with histological and immunological findings similar to those reported in a previous canine MMP meta-analysis.2 Treatment with tetracycline antibiotic and niacinamide, often in combination with other immunosuppressive drug(s), was found to be beneficial in more than half of these cases. Unfortunately, the rarity of this syndrome, the low number of dogs with reported treatment outcomes in the literature and the retrospective nature of the reports limits our ability to make definitive conclusions on the best therapeutic approach for canine MMP.
Acknowledgements
The authors thank Barbara Atlee, Kerstin Bergvall, Dorothy Jordan, Rudayna Ghubash, Joel Griffies, Elizabeth Layne, Monika Linek, Nancy Peters, Helen Power, Karen Ross, Sandra Sargent and Stephen White for providing the case material for this study.




