Contact dermatitis: a comparative and translational review of the literature
Abstract
enBackground
Contact dermatitis (CD) is an inflammatory skin condition induced by direct contact with a specific chemical. Irritant CD (ICD) is a nonspecific inflammatory cutaneous reaction to an irritating agent. Allergic CD (ACD) is an immune-mediated antigen-specific skin reaction to an allergenic chemical.
Objectives and methods
The biomedical literature (human, basic science, veterinary) was reviewed to evaluate the current state of knowledge regarding CD.
Results
The incidence of human CD remains unclear, but represents up to 90–95% of all occupational skin diseases. The prevalence of CD has not been established in veterinary medicine. The pathogenesis of CD is not fully understood, but involves a complex cascade of events between resident skin cells, relocated immune cells, pro-inflammatory cytokines and chemokines. The main difference between ICD and ACD is that ACD is an antigen-specific reaction to an allergenic irritating agent whereas ICD is not antigen-specific. To date, there is no fully validated diagnostic test available for CD. Thus, its clinical diagnosis relies on the patient's history, clinical examination, dermatological tests and, in some cases, research-based assays. The most important factor in CD management is the identification and avoidance of the culprit irritant or allergen. In addition, various topical and systemic therapies can be considered.
Conclusion and clinical relevance
CD is a relatively common occupational skin disease in human beings, but the prevalence in veterinary medicine is undefined. It can lead to debilitating clinical signs. Further research in human medicine and even more so in veterinary patients, will be required in order to allow for an evidence-based approach in its diagnosis and management.
Résumé
frContexte
La dermatite de contact (CD) est une dermatose inflammatoire induite par un contact direct avec un agent chimique spécifique. La CD irritante (ICD) est une réaction cutanée inflammatoire non spécifique à un agent irritant. La dermatite de contact allergique (ACD) est une réaction cutanée à médiation immune spécifique d'antigène liée à un agent chimique allergisant.
Objectifs et méthodes
La littérature biomédicale (science fondamentale, humaine, vétérinaire) a été revue pour évaluer l’état actuel des connaissances sur la CD.
Résultats
L'incidence de la CD humaine reste obscure mais représente jusqu’à 90-95% de toutes les dermatoses professionnelles. La prévalence de la CD n'a pas été établie en médecine vétérinaire. La pathogénie de la CD n'est pas totalement élucidée mais implique une cascade complexe d’événements entre les cellules résidentes, les cellules immunitaires migrantes, les cytokines pro-inflammatoires et les chémokines. La principale différence entre ICD et ACD est que l’ACD est une réaction antigénique spécifique à un agent irritant allergisant tandis que l’ICD n'est pas spécifique d'antigène. A l'heure actuelle, il n'y a pas de test diagnostic validé pour la CD. Ainsi, le diagnostic clinique repose sur les commémoratifs du patient, l'examen clinique, les tests dermatologiques et dans certains cas, les tests de recherche. Le facteur le plus important dans la gestion de la CD est l'identification et l’éviction de l'allergène ou de l'irritant en cause. En outre, de nombreux traitements topiques et systémiques peuvent être envisagés.
Conclusion et importance clinique
La CD est une dermatose professionnelle relativement fréquente chez l'homme mais la prévalence en médecine vétérinaire n'est pas connue. Elle peut mener à des lésions cliniques débilitantes. Des recherches supplémentaires en médecine humaine et aussi en médecine vétérinaire seront nécessaires pour déterminer une démarche reposant sur des preuves pour son diagnostic et sa gestion.
Resumen
esIntroducción
la dermatitis de contacto (CD) es una enfermedad inflamatoria de la piel inducida por contacto directo con un compuesto químico específico. La dermatitis de contacto irritante (ICD) es una reacción cutánea inflamatoria no específica a un agente irritante. La dermatitis de contacto alérgica (ACD) es una reacción de la piel inmuno-mediada antígeno-específica a un alergeno químico
Objetivos y métodos
la literatura biomédica (humana, ciencias básicas, veterinaria) fue revisada para evaluar el estado actual de conocimiento en relación a la dermatitis de contacto.
Resultados
la incidencia de la dermatitis de contacto en humanos aún es desconocida, pero representa hasta un 90-95% de todas las enfermedades ocupacionales de la piel. La prevalencia de dermatitis de contacto no esta establecida en medicina veterinaria. La patogenia de la dermatitis de contacto no esta bien conocida, pero implica una cascada compleja de sucesos entre las células residentes de la piel, las células relocalizadas del sistema inmune, citoquinas proinflamatorias y quemoquinas. La principal diferencia entre la dermatitis de contacto irritante y la dermatitis de contacto alérgica es que la dermatitis de contacto alérgica es una reacción específica de antígeno a un irritante alergénico mientras que la dermatitis de contacto irritante no es específica de antígeno. En el momento presente no hay un método validado disponible para diagnosticar la dermatitis de contacto. Por lo tanto el diagnóstico clínico se basa en la historia del paciente, examen clínico, pruebas dermatológicas, y, en algunos casos, pruebas basadas en investigación. El factor más importante en el manejo de la dermatitis de contacto es la identificación y evitar el contacto con el irritante o alergeno. Además se pueden considerar varios agentes tópicos y sistémicos terapéuticos.
Conclusiones e importancia clínica
La dermatitis de contacto es una enfermedad común ocupacional de la piel en humanos, pero su prevalencia en medicina veterinaria está poco definida. Puede producir signos clínicos debilitantes. Se necesita más investigación en medicina humana e incluso más en medicina veterinaria para llegar a un entendimiento basado en evidencia para su diagnóstico y manejo.
Zusammenfassung
deHintergrund
Die Kontaktdermatitis (CD) ist eine entzündliche Hauterkrankung, die durch direkten Kontakt mit einem speziellen chemischen Mittel ausgelöst wird. CD durch ein Reizmittel (ICD) ist eine unspezifische entzündliche Hautreaktion auf einen reizenden Stoff. Die allergische CD (ACD) ist eine immun-mediierte Antigen-spezifische Hautreaktion auf ein allergen wirkendes Reizmittel.
Ziele und Methoden
Die biochemische Literatur (Human, Wissenschaft, Veterinär) wurde reviewed, um den momentanen Wissensstand bezüglich CD zu ermitteln.
Ergebnisse
Die Häufigkeit des Vorkommens von CD beim Menschen ist unklar, repräsentiert aber 90-95% aller beruflich bedingten Erkrankungen. Die Prävalenz von CD ist in der Veterinärmedizin nicht bekannt. Die Pathogenese der CD ist nicht vollständig bekannt, umfasst aber eine komplexe Kaskade von Wechselwirkungen zwischen Hautzellen, abgewanderten Immunzellen, proentzündlichen Zytokinen und Chemokinen. Der hauptsächliche Unterschied zwischen ICD und ACD ist bei ACD eine antigenspezifische Reaktion auf allergene, irritierende Stoffe während ICD nicht Antigen-spezifisch ist. Bis heute gibt es keinen zur Gänze evaluierten Test für CD. Daher beruht die klinische Diagnose auf der Anamnese des Patienten, der klinischen Untersuchung, dermatologischen Tests und, in manchen Fällen, auf durch Forschung erprobteAssays. Der wichtigste Faktor beim Management der CD ist die Identifizierung und die Vermeidung des schuldigen reizenden oder allergenen Stoffes. Zusätzlich müssen verschiedene topische und systemische Therapien in Betracht gezogen werden.
Schlussfolgerungen und klinische Bedeutung
CD ist eine relativ häufige beruflich bedingte Hauterkrankung des Menschen, aber die Prävalenz in der Veterinärmedizin ist nicht definiert. Es kann zu debilitierender klinischer Symptomatik kommen. Weitere Studien in der Humanmedizin sind nötig und noch viel mehr in der Veterinärmedizin, um einen auf Evidenz-basierenden Zugang zu Diagnose und Management zu ermöglichen.
摘要
ja背景
接触性皮膚炎(CD)は特異的な化学物質への直接接触により誘発される炎症性皮膚疾患である。刺激性CD(ICD)は刺激物に対する非特異的な炎症性皮膚反応である。アレルギー性CD(ACD)はアレルギー性化学物質に対する免疫介在性抗原特異的皮膚反応である。
目的および方法
CDに関する情報の現状を評価するために生物医学文献(医学、基礎生物、獣医学)を再検討した。
結果
ヒトCDの発生率は不明であったが、すべての職業性皮膚疾患の90-95%を占めていた。CDの有病率は獣医学では立証できなかった。CDの発病機序は完全にはわからなかったが、固有皮膚細胞、再配置された免疫細胞、炎症後サイトカインおよびケモカインの間の事象の複雑なカスケードが関与している。ICDとACDの主な違いは、ACDはアレルギー性刺激物質に対する抗原特異的反応である一方、ICDはアレルゲン特異的でないことである。データによると、CDに対する有効な診断的試験は立証されていない。それゆえに、臨床的な診断は患者の履歴、臨床検査、皮膚検査および、症例によっては研究による分析を頼りに行われている。CDの管理において最も重要な要因は刺激の原因あるいはアレルゲンの特定および回避である。さらに、複数の外用および全身療法を検討する可能性がある。
結論および臨床的な妥当性
CDはヒトにおいて比較的一般的な職業性皮膚病であるが、獣医学での有病率は不明確である。消耗性臨床症状につながる可能性がある。医学および獣医学での患者において、さらなる研究がエビデンスに基づいた診断や管理に関するアプローチのために必要とされる。
摘要
zh背景
接触性皮炎(CD)是一种炎性皮肤病,由直接接触特殊的刺激化学制剂引起。刺激性CD(ICD)由刺激原引起,是一种非特异性的炎性皮肤反应。过敏性CD(ACD)由致敏化学物质引起,是一种免疫介导的、具有抗原特异性的皮肤反应。
目的和方法
通过回顾生物医学文献(人类医学、基础科学、兽医学),评价当前有关CD的知识状态。
结果
虽然人类接触性皮炎的发生率尚不明确,但代表了近90%-95%的职业性皮肤病。在兽医学上,接触性皮炎的流行率尚不明确。接触性皮炎的发病机理尚未完全明确,但已知包含常驻皮肤细胞、迁移的免疫细胞、前炎性细胞因子和趋化因子,及其间发生的复杂级联反应。ICD和ACD的主要区别在于,ACD是由于对刺激过敏原产生的特异性抗原反应,然而ICD没有抗原特异性。至今为止,没有能确诊CD的诊断实验。因此,该病的临床诊断基于病史、临床检查、皮肤病试验,某些病例还可进行科研性试验。管理CD最重要的是确定和避免刺激原或过敏原。另外,可考虑多种局部和全身治疗。
总结与临床意义
人的CD是一种相对常见的职业性皮肤病,但在兽医学上,其流行性仍不确定。它能够导致衰竭性临床症状。为了寻找有依据的CD诊断和管理方法,需要人类医学、甚至更多在兽医病例中的进一步研究。
Introduction
Contact dermatitis (CD) is an inflammatory response of the skin following contact with a specific substance.1-3 There is no official definition for contact dermatitis in either human or veterinary medicine. This ambiguity is likely a reflection of the complexity of its pathogenesis, the heterogeneity of its clinical pattern and the absence of a fully validated diagnostic tool set.
There are two types of CD in humans and animals: irritant contact dermatitis (ICD) and allergic contact dermatitis (ACD).1, 3-7 ICD could be defined as a nonspecific inflammatory cutaneous reaction following direct contact with an irritating substance (irritant); ICD reactions are dose-dependent and can affect anyone. ACD could be described as an immune-mediated antigen-specific inflammatory skin reaction following contact with a specific allergenic substance (sensitizer); ACD reactions are idiosyncratic and not dose-dependent. It is important to note that most sensitizers are also irritants.1, 4, 8
In general, very little is known in veterinary medicine about contact dermatitis. However, the clinical pattern in dogs and cats appears to be similar to what is observed in people (see ‘Clinical patterns’ section).9 This article will discuss all aspects of CD, especially the similarities and differences between ICD and ACD as they pertain to: epidemiology, clinical patterns, pathogenesis and research models, diagnosis and treatment. Most of the published work relates to human patients and rodent models, but this information will be compared to that available in the veterinary literature whenever possible.
Search strategy
The PubMed MEDLINE and the Veterinary Information Network (VIN) websites (http://www.vin.com) were searched for various combinations of the following keywords: contact dermatitis-related terms (contact sensitization, contact hypersensitivity, contact sensitizers, irritants, atopic dermatitis), keywords related to particular aspects of discussion (e.g. incidence, pathogenesis, models, diagnosis, management) and specific keywords based on the information found in general articles (e.g. lymphocytes, keratinocytes, cytokines, genetics). Human and veterinary textbooks were also reviewed. These searches led to several hundred references. Journal abstracts were screened for relevance to the topic and references of previously published papers were reviewed for additional information. In total 141 articles were utilized for this review.
Skin structure and function
The skin is composed of three layers – epidermis, dermis and hypodermis – which function in thermoregulation, tactile assessment of external environment and protection against foreign substances.10 The epidermis is an avascular and highly cellular portion of the skin containing keratinocytes, Langerhans cells, melanocytes and Merkel cells. The dermis is highly vascularized and contains mainly fibroblasts, but also some macrophages, dendritic cells and lymphocytes. The hypodermis comprises mostly adipocytes and contains larger blood vessels. The skin also hosts various and numerous immune cells, some of which play an important role in the pathogenesis of ICD and ACD. The location and function of these cells are listed in Table 1.
| Cell type | Location | Functions |
|---|---|---|
| Keratinocytes | Epidermis |
• Structural cells of the epidermis • Nonprofessional antigen presenting cells; secrete various cytokines/chemokines that can recruit and activate immune cells; can process and present antigens to lymphocytes |
|
Dendritic cells Langerhan cells Dermal dendritic cells |
Epidermis Dermis |
• Professional antigen presenting cells; uptake antigens in the epidermis and present them to lymphocytes in draining lymph nodes • Has a regulatory role in contact dermatitis by producing IL-10 and switching to macrophage-like cells • Professional antigen presenting cells; uptake antigens in the epidermis and present them to lymphocytes in draining lymph nodes |
|
Lymphocytes T-cells B-cells a |
Draining lymph node Blood/inflamed tissue Blood/inflamed tissue |
• CD4+ (T helper cells): control the type of immune response (Th1 versus Th2 versus Th17) Th1: produces IFN-γ, IL-2, TNF-α, TNF-β Th2: produces IL-4, IL-5, IL-6, IL-10, IL-13 Th17: produces IL-17, IL-21 • CD8+ T-cells (cytotoxic T cells): recognize and kill host cells infected with viruses or other intracellular microbes • Circulating memory T-cells have skin homing skills to ensure skin relocation in cases of cutaneous infections • Resident T-cell population can recruit circulating T-cells • Regulatory T-cells regulate the activation of other T-cells by producing specific cytokines to suppress inflammatory response and maintain tolerance to antigens • Produce immunoglobulins (antibodies) • Responsible for humoral acquired immunity • Circulating memory B cells • Resident B cells • Type 1 B lymphocytes leads to IgM, complement activation leading to vasodilation and transmigration of leucocytes |
|
Natural Killer T-cells (NKT) a |
Blood/inflamed tissue |
• Shares properties of both NK and T cells; expresses IFN-γ and IL-4 • Hypothesized to have a role in allergic contact dermatitis; recognizes glycolipid antigens • Once activated by hapten, they promote the migration of peritoneal B cells to lymphoid organs and the production of hapten-specific IgM |
| Mast cells | Blood/inflamed tissue | • Produces TNF-α and IL-10; involved in induction (maturation and migration of DC) and elicitation phase of contact hypersensitivity |
| Monocytes/Macrophages | Blood/inflamed tissue |
• Phagocytic cell that plays a role in innate and adaptive immune responses • Activated macrophages phagocytose and kill microorganisms, secrete proinflammatory cytokines, and present antigens to helper T cells |
| Neutrophils | Blood/Inflamed tissue |
• Phagocytic cell that is most abundant type of circulating white blood cell and plays a role in innate immune responses • Major cell type mediating acute inflammatory responses to bacterial infections |
| Eosinophils | Blood/inflamed tissue |
• Granulocyte that is abundant in allergic diseases and extracellular parasites • Plays a role in innate immune responses |
| Fibroblasts | Dermis | • Stimulated by TNF-α to produce collagenase and prostaglandin E which contributes to inflammation and tissue destruction |
- a Controversial role in contact hypersensitivity, NK T cells studied in mice (unsure of role in humans).
Epidemiology
The incidence of CD has not been precisely established in human medicine. Human CD is thought to represent approximately 90–95% of all occupational skin diseases11, 12 and approximately 10% of all dermatology office visits.13 ICD is more common than ACD, representing almost 80% of cases.1, 3 There is no official report on the incidence of CD in veterinary medicine and no study has attempted to establish its prevalence in veterinary patients. In dogs and cats, CD is sometimes considered to be uncommon.9, 14 Yet, ACD has been reported to account for approximately 1–10% of all dermatologic cases in small animal medicine.15-17 This apparent contradiction may be a consequence of the difficulty in diagnosing CD and the possible confusion with atopic dermatitis in certain cases.14
Pathogenesis
It is important to note that, at the time of writing, the pathogenesis of ICD and ACD has only been investigated in humans (patients and cell models) and laboratory rodent models. Research in veterinary medicine has only focused on atopic dermatitis thus far.
Irritants versus sensitizers
Common irritants and allergens that have been reported in human and veterinary medicine are listed in Table 2.
| Humans | Dogs | |
|---|---|---|
| Irritants |
Strong acids/alkalis (e.g. calcium oxide, sodium and potassium carbonate, ammonium) ‘Water’ Solvents (e.g. benzene, toluene, xylene, gasoline) Cosmetics Cleansers (e.g. soaps, benzoyl peroxide) Industrial chemicals (e.g. metals, fibreglass) Plants |
Strong acids/alkali Cleansers (e.g. soaps, detergents) Solvents Insecticides (e.g. flea collars, flea shampoos, flea dips) Grasses/pollens Industrial chemicals (e.g. fertilizers, petrolatum) Industrial materials (e.g. paint, carpets, wood preservatives) |
| Allergens |
Metals (e.g. nickel, gold) Cosmetics (e.g. paraphenylenediamine [pPD]) Preservatives (e.g. fragrances, sunscreens) Topical medications (e.g. neomycin, bacitracin) Vehicles for topicals (e.g. propylene glycol) Plants (e.g. Compositae family, Liliaceae family, Alstroemeriaceae family) Industrial materials (e.g. cement, rubber) Clothes (e.g. formaldehyde, pPD Phenylenediamine) |
Metals Topical medications (e.g. neomycin) Vehicles for topicals (e.g. propylene glycol) Skin products: (e.g. shampoos, dips, sprays) Industrial materials (e.g. plastic, cement, wood) Plants (e.g. Commelinaceae family, Asian jasmine, Amarylidaceae leaves and bulbs) |
Irritants (e.g. calcium oxide) are agents that can injure keratinocytes when applied to the skin.3, 5, 6, 18-21 The lesions partially depend on the chemical contact time with the skin, its concentration and its potency.4, 12, 19, 21, 22 When an irritant comes into contact with keratinocytes, it causes cytotoxic damage to keratinocytes and the release of various cytokines, further increasing local inflammation.23-27 Irritants are directly responsible for the cutaneous lesions and local inflammation seen in ICD.4, 6, 21
Sensitizers (e.g. nickel) are irritants that are also immunogenic and can sensitize the immune system.3, 4, 20, 28, 29 These chemicals are thought to be haptens.4, 28 Indeed, sensitizers are believed to be too small (<500–700 Da) to be immunogenic on their own.11, 13, 30, 31 Sensitizers therefore have to covalently bind to epidermal carrier proteins in order to create an immunogenic complex.9, 11, 32 Sensitizers are indirectly responsible for the cutaneous lesions and local inflammation seen in ACD because these clinical signs are induced by a sensitizer-specific immune response.4
ICD pathogenesis
ICD is due to a physical, mechanical or chemical skin injury associated with the direct cytotoxic and inflammatory effects of an irritant (Figure 1).5 Both ICD and ACD involve immune cells, but ICD follows a nonantigen-specific activation of innate immunity and inflammation. Thus, prior exposure and sensitization is not required.4, 12, 33, 34
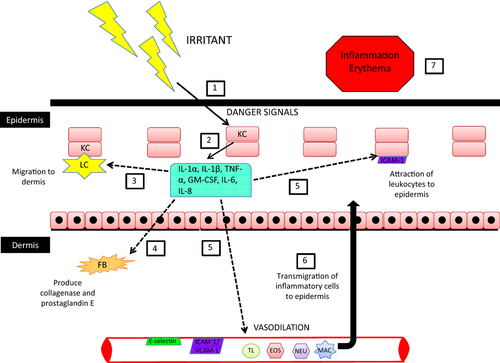
The pathogenesis of ICD starts with the penetration of an irritant through different skin layers, which induces some endogenous ‘danger’ signals (reactive oxygen species, hyaluronic acid fragments, ATP).12, 35-37 Keratinocytes are the main cells in the epidermis (95%) and the first skin cells to be exposed to the irritant. They are therefore the first cells damaged, but they are also thought to play an active role in the development of the overall ICD lesion.4, 12, 18, 19 For instance, irritants have been shown to increase the secretion of various pro-inflammatory cytokines by human keratinocytes (e.g. IL-1α, IL-1β, IL-6, TNF-α, IL-8), both in vivo12, 23, 25, 27 and in vitro.24, 26 The exact cytokine profile of ICD is unknown and likely varies between patients and chemicals; however, IL-1α, IL-1β and TNF-α appear to play central roles in the pathogenesis of ICD.18, 34
The initial step in the inflammatory cascade of ICD is the disruption of the skin barrier by an irritant, which leads to cytotoxic damage of keratinocytes and the release of preformed IL-1α.6, 18, 19, 27, 33, 34, 38-40 The secretion of IL-1α stimulates further production of pro-inflammatory cytokines (e.g. IL-1β, TNF-α, IL-6, IL-8) within the epidermis by keratinocytes.18, 19, 27, 33, 34, 39 In addition to their action in the epidermis, cytokines released by keratinocytes also affect dermal cells. Thus, fibroblasts start producing collagenases and prostaglandin E, further worsening cell injury and local inflammation.18, 34 All of these inflammatory molecules also activate epidermal (Langerhans cells) and dermal immune cells (dendritic cells, lymphocytes).18, 19, 34 The activated immune cells will secrete more cytokines and chemokines, while upregulating adhesion molecules (e.g. ICAM-1, VCAM-1) on keratinocytes, fibroblasts and endothelial cells.12, 18, 34 Chemokines and adhesion molecules will attract more immune cells to the area including neutrophils, macrophages, lymphocytes and eosinophils. This further increases cutaneous damage and inflammation.6, 32
Some authors hypothesize that IL-1α, IL-1β and TNF-α also cause migration of Langerhans cells out of the epidermis into the dermis upon irritant exposure to avoid cell death from the inflammation incurred during ICD.32, 34 In the dermis, Langerhans cells switch into macrophage-like cells; studies have determined that dermal fibroblasts, E-cadherin and IL-10 are required for this phenotypic switch.34, 41-43 These macrophages, in turn, are capable of removing damaged cells.
ACD pathogenesis
ACD is a type IV hypersensitivity reaction that occurs in previously sensitized individuals (Figure 2).3, 7, 44 As such, it is antigen-specific, delayed and involves cytotoxic CD8+ T lymphocytes as the main mediators of skin lesions.
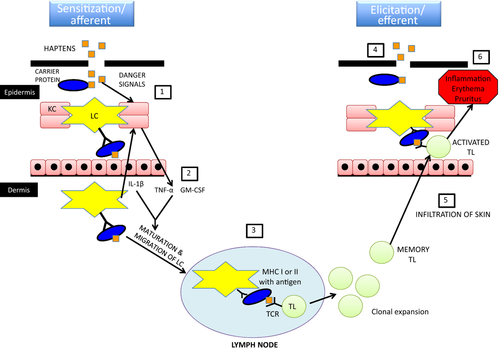
ACD involves two phases in its pathogenesis, which differentiates it from ICD.13, 29, 45 First, ACD requires a sensitization phase wherein the immune system becomes sensitized against a specific antigen (i.e. the sensitizer). An elicitation phase follows, wherein the immune system triggers an antigen-specific response upon re-exposure to this sensitizer.
The sensitization phase develops after a variable period of time following intermittent or continual exposure to the sensitizing chemical (usually weeks to months).6, 9, 14, 20, 46 Sensitizer–protein adducts are thought to represent the true immunogenic agent, rather than the sensitizer itself.28, 29, 47 These chemical–protein complexes are formed in the epidermis when the skin is exposed to the sensitizing chemical. The adducts are then phagocytized by epidermal Langerhans cells or dermal dendritic cells. Following processing, antigen presenting cells can induce the proliferation of sensitizer-specific lymphocytes generated during the sensitization phase.4, 13, 48 The release of pro-inflammatory cytokines and chemokines (IL-1β, TNF-α and GM-CSF especially) after exposure to the sensitizer will further stimulate maturation and activation of local dendritic cells that will migrate towards regional lymph nodes,13, 48 where they will prime T lymphocytes that in turn promote clonal expansion and differentiation into CD8+ and CD4+ cells.13, 20, 32, 48 Although the sensitization phase may remain sub-clinical, most sensitizers are also irritants,1, 4, 8 such that the ACD sensitization phase can also be associated with clinical signs of ICD.
After sensitization has occurred, the elicitation phase may develop within 24 h to several days after re-exposure.44, 49 Memory T cells generated during the sensitization phase mount an immune response when re-exposed to the sensitizer.5, 20, 48, 50 Newly primed effector cytotoxic lymphocytes will then migrate out of the local draining lymph node into the bloodstream until they reach the cutaneous site of re-exposure, which is usually inflamed by the irritating properties of the sensitizer.13, 48 These primed T cells express a skin homing marker named cutaneous lymphocyte antigen (CLA) which allows their migration to the lesional site.6, 13 The sensitizer induces the release of pro-inflammatory cytokines/chemokines and the expression of membrane adhesion molecules by keratinocytes and Langerhans cells, which further attract and activate immune cells.9, 13, 32, 48 Some studies have shown that sensitizer-specific cytotoxic T cells kill keratinocytes that express proteins modified with the hapten (via apoptosis).44, 51, 52 Other studies suggest that these T cells can also induce apoptosis in Langerhans cells.44, 53 Sensitizer-specific cytotoxic T cells also release IFN-γ, which activates keratinocytes resulting in upregulation of their adhesion molecules and cytokines/chemokines, further increasing the recruitment of T cells, NK cells, macrophages, mast cells and/or eosinophils to the site of hapten exposure.13, 44, 49, 52 These immune cells expand the inflammatory process and worsen ACD clinical signs. Th17 lymphocytes appear to play an important role in ACD lesions by perpetuating or even exacerbating the local inflammation.13, 49 In addition to dendritic cells and macrophages, keratinocytes are also capable of uptake, processing and presenting of hapten-protein complexes to effector T cells, playing an active role in the antigen-specific immune reaction.9, 52
The role of Langerhans cells in the elicitation phase of ACD is still debated. Some authors believe that keratinocytes act as the antigen presenting cells during the ACD elicitation phase.13, 29, 52 Furthermore, some researchers believe that only dermal dendritic cells can induce a sensitizer-specific immune response in the skin whereas Langerhans cells – their epidermal counterpart – would tend to induce tolerance instead.54, 55
Interestingly, some markers of regulatory mechanisms have been associated with the resolution of the elicitation phase of ACD. Indeed, despite persistence of the hapten in the skin, ACD reactions appear to be self-limiting in certain patients.50 The spontaneous resolution of this phase involves both keratinocytes and T regulatory lymphocytes.50, 56, 57 Some studies suggest that these T cells and keratinocytes are able to release IL-10, which has an inhibitory effect on CD.48, 50, 56 T regulatory cells are also able to secrete IL-4 and TGF- β, which can suppress the inflammatory process of CD.22, 32, 48, 49 These cytokines are thought to downregulate the ACD reaction by decreasing T cell activation and converting Langerhans cells to a tolerogenic phenotype.49
Genetic predispositions
Contact dermatitis in people is a complex disease that likely involves both genetic and environmental factors. Numerous studies have investigated the role of genetics in human CD and are summarized.58 Some family studies have shown a correlation between CD in parents and their offspring, but this could be secondary to either genetic or environmental factors. Studies conducted in mono- and dizygotic twins have not produced definitive conclusions on the issue to date. Genetic factors thought to be associated with CD include polymorphisms in genes involved in oxidative stress,37, 59 chemical detoxification,60, 61 immunity/inflammation37, 62, 63 and skin barrier function.12, 20, 37, 64-66 More specifically, research on certain genetic polymorphisms within the MHC II and complement pathways in ACD has yielded some significant findings. Genetic polymorphisms in antioxidant pathways have also been linked to CD, such as manganese superoxide dismutase in paraphenylene diamine ACD.59 Additionally, genetic polymorphisms in N-acetyltransferases or glutathione transferases have been found in humans with CD.60, 61 Mutations in the filaggrin gene, a key protein in epidermal homeostasis and barrier function, have been reported to predispose mice to CD and humans to nickel-associated ACD.
Only one study has investigated potential genetic predisposition in canine ICD and the authors did not identify any breed, age or sex predilections.32 No study has evaluated the role of genetic factors (e.g. breed) in canine ACD so far. However, some believe that breeds prone to atopic dermatitis are also predisposed to CD because of a defective skin barrier or continuous trauma and inflammation from atopic dermatitis.2, 14, 67 Interestingly, polymorphisms in canine MHC II have been associated with other diseases, including the immune-mediated skin disease lupus.68 Finally, it is important to note that the N-acetyltransferase pathway, which is thought to be involved in human CD, is completely absent in dogs.69
Models
Models of contact dermatitis have been established and used to study its pathogenesis, but also to predict potential sensitizers. Details behind these different models go beyond the scope of this review and the reader is directed to several published reviews.70-73
Briefly, testing the elicitation phase of contact dermatitis in allergic individuals is routinely conducted using patch tests (see Diagnosis section). In addition to its diagnostic purpose in single cases, this approach has led to important epidemiological studies when extended to larger populations.72 However, studying the sensitization phase in naïve volunteers is considered unethical.
Animal models were originally validated to establish the sensitization potential of a chemical or to study the pathogenesis of contact dermatitis. The first animal models of CD used guinea pigs [Beuhler test and guinea pig maximization test (GPMT)], whereas the most recent ones involve mice [mouse ear-swelling test (MEST) and local lymph node assay (LLNA)]. Although these animal models are all considered to be very reliable in predicting or studying contact dermatitis in humans, the LLNA is now the most commonly used model.72, 74
Growing concerns for animal welfare over the past few decades has led researchers to create nonanimal models. This work was accelerated in the 2000s after the announcement that animal testing would be banned in Europe for cosmetic chemicals, starting in 2013.75, 76 Successful nonanimal-based approaches now combine a battery of tests.72, 73 In silico assays include: Structure–Activity Relationships (SARs), Quantitative structure–activity relationships (QSAR) and some ‘expert systems’ such as the Toxicity Prediction Komputer-assisted Technology (TOPKAT). The glutathione reactivity database and the direct peptide reactivity assay (DPRA) are examples of in chemico assays.72 Nonanimal-based methods also include multiple cell-based assays such as dendritic cell activation tests [e.g. Myeloid U937 Skin Sensitization Test (MUSST), Human Cell Line Activation Test (h-CLAT)], T cell proliferation tests [e.g. chemical-specific lymphocyte transformation test (LTT), T cell priming assay (TCPA)] and, to a lesser extent, keratinocyte activation tests (e.g. IL-18 secretion by NCTC 2544 cells).71, 73
Clinical aspects of CD
Clinical signs of CD
To the best of the authors’ knowledge, little information regarding the general clinical pattern of CD in small animals has been published; however, patterns of CD in dogs and cats appear to be similar to what is reported in humans (Figure 3).9
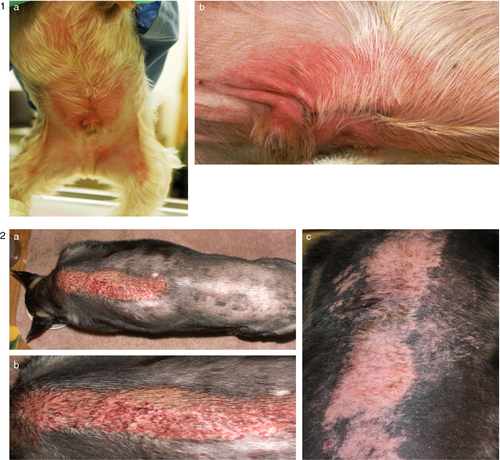
Sites of CD lesions usually include areas of exposed skin and areas that come into contact with the irritant/allergen. In humans common areas depend on the nature of the irritant/allergen; for example, the face and neck are affected with cosmetic-related products, hairline and behind the ears with hair products, and hands with cosmetics or occupational exposure.3, 11, 67 In dogs and cats commonly affected areas tend to be limited to thinly haired or glabrous areas of the skin including the groin, face, ears, plantar interdigital areas, paw pads, perineum, scrotum and ventral tail.9, 14, 15, 32, 77 The distribution of lesions is important as it can help to identify what irritant or allergen may be causing the lesions observed.78 For example, if the lesions are generalized then the cause may be a shampoo. In contrast, if the lesions are localized between the shoulder blades, they may be secondary to the application of a spot-on anti-parasitic product.
Lesions that develop from ICD typically develop only in the area of direct contact and are well demarcated, whereas lesions from ACD may extend beyond the area of contact and therefore be less well defined.47 In both ICD and ACD, acute dermatologic lesions include oedema, erythema and papules, and chronic lesions include scaling, fissuring and lichenification.3, 5, 11, 67, 79 Individuals with ACD are more likely to develop vesicles,1, 11 whereas ICD lesions will more commonly cause ulceration and epidermal necrosis.4, 20 Secondary bacterial pyoderma and/or Malassezia dermatitis secondary to self-trauma may occur in both ICD and ACD.14, 32
ICD usually affects multiple individuals living in the same household, whereas ACD will normally affect a single individual.32 Individuals with ICD are more likely to develop pain and discomfort,1, 4, 20, 67, 79, 80 whereas ACD lesions are more likely to be pruritic.1, 4 Even though certain lesions are more common in ICD versus ACD, overlap is possible and a definitive diagnosis cannot be based solely on lesion character.
Histopathological lesions of CD
It is as difficult to distinguish between ICD and ACD lesions microscopically because it is based on clinical signs.9, 79, 81 Thus, histopathology alone rarely allows a definitive differential diagnosis between the two.32, 79 The degree of dermal inflammation is variable and lesions have often reached advanced stages by the time tissue is sent for analysis. Biopsy samples acquired at <48 h after onset are therefore considered to be more helpful.15, 79 In dogs the histopathology of both types may also show secondary bacterial pyoderma, Malassezia dermatitis and/or seborrhoeic skin disease secondary to self-trauma,32, 79 further decreasing the diagnostic potential of older lesions.
Histopathological lesions of human ICD are divided into acute and chronic features. The most common morphological features of acute ICD include intracellular vacuolation with nuclear pyknosis, cell necrosis and infrequently spongiosis in the epidermis.82-84 The dermis may contain degeneration or disruption of the collagen. Leucocytes (primarily mononuclear cells and occasionally neutrophils) infiltrate the epidermis and dermis.82, 84 In chronic ICD histopathology of the epidermis typically shows some hyperplasia with moderate hyperkeratosis, parakeratosis and acanthosis. There is usually minimal infiltration of inflammatory cells. The dermis, however, may be significantly infiltrated, with mononuclear cells as the dominant cell type.82 Histopathology of ICD should show some degree of epidermal degeneration due to its pathogenesis (direct cellular damage to keratinocytes).79 Acute lesions may be characterized by varying degrees of superficial perivascular dermatitis, neutrophilic epidermal spongiosis, and epidermal ulceration and necrosis.79, 80 The cellular infiltrate may also include lymphocytes and macrophages.80
Interestingly, certain lesions in the histopathology of ACD can be relatively nonspecific such as inflammatory infiltrates, some being mainly lymphocytic and others predominantly neutrophilic.85 Additionally, there may be variability in humans versus dogs, where people will have a predominantly lymphocytic inflammatory infiltrate and dogs will have a predominantly neutrophilic infiltrate.84, 86 The histopathology of ACD in humans is also divided into acute and chronic patterns. In acute ACD, the most common feature is spongiosis and oedema with dilated papillary vessels. In chronic ACD the epidermis is hyperplastic with parakeratosis.87 In dogs ACD often shows varying degrees of superficial perivascular dermatitis32 with epidermal spongiosis that may progress to vesiculation.4, 79 Some studies have shown infiltration with lymphocytes, whereas other studies report eosinophilic or neutrophilic epidermal pustules in patch test sites.77, 79, 80, 88 In one study skin biopsies were collected from 11 allergic dogs with a suspected diagnosis of ACD (based on patch testing) and showed predominantly neutrophilic infiltrates with sparse numbers of histiocytes and lymphocytes.89
Diagnosis of ICD and ACD
The diagnosis of CD is complicated by its complex pathogenesis, similar clinical patterns between ICD, ACD and other inflammatory dermatoses, variable histopathological findings and the lack of fully validated diagnostic tests.3, 4, 20 Thus, the diagnosis is often based on a combination of parameters including the patient's history, physical examination, histopathological examination, ruling out differential diagnoses, restriction and provocative exposure, and patch testing. There is no official algorithm available to weigh out these different factors against each other to reach a final diagnosis.
Obtaining a complete history is crucial for the diagnosis of CD. Relevant information includes clinical evolution of initial lesions, time of onset, potential sources of exposure to irritants/allergens (including occupational exposure for humans) and history of previous CD event.5, 20, 78, 90 Additional information that should be obtained includes the number of affected individuals in the household, recent changes in environment and new topical therapies.16, 32, 78 In humans, differential diagnoses of CD are dependent on whether the patient exhibits signs of acute, subacute or chronic eczematous dermatitis, and include erythema multiforme-like diseases, urticarial papular plaques, lichenoid eruptions, purpuric petechial reactions, granulomatous and pustular reactions, drug eruptions, psoriasis and atopic dermatitis.1, 3, 20, 91 Differential diagnoses for CD in small animals should include atopic dermatitis, adverse reactions to foods, scabies, cutaneous infections (primary infections such as demodicosis and dermatophytosis, or secondary infections with yeasts or bacteria) and cutaneous adverse drug reactions.14, 15, 32, 78
Although the overall history and clinical pattern play an important role in the diagnostic process, it is always important to consider withdrawal/provocation tests (dechallenge/rechallenge).5, 14, 78 For dechallenge, all possible irritating substances should be removed from the skin, hair coat and the environment whenever possible. In cases of CD, this should lead to clinical improvement. After total recovery, the patient could be re-exposed to the suspected irritant (rechallenge). When performing such rechallenge, potential irritants/allergens should be reintroduced one-by-one, starting from the most likely culprit. The patient should be observed for at least 2 days under strict medical supervision. This process is contraindicated for very potent irritants or severe ACD reactions.
ACD is especially difficult to diagnose because the causative allergen may be something that the individual was exposed to for a significant period of time without any adverse event.6, 9, 14, 20, 46 In addition, the exposure associated with lesions might be very small by the time the patient is sensitized.4, 11 Dechallenge/rechallenge can be useful to diagnose ACD; however, there is a risk for lesions to be much worse at rechallenge, even when using small allergen amounts.
Patch testing is considered the gold standard diagnostic test for ACD in humans and animals.2, 20, 45, 46, 92-95 In the open patch test, a drop of the test allergen is applied to clipped skin and the test is left unprotected. Inspection of the skin is made for the next 5–15 days with a positive response characterized by an erythematous skin reaction at the test site.4, 15, 78 The open patch test is rarely used because self-trauma is a common problem. In the closed patch test, the test area is bandaged to prevent the patch from moving and self-trauma (Figure 4). With both approaches it is important to not use excessive concentrations of the allergen because sensitizers can also be irritants. Patches are removed for examination at 48 h, 72 h and sometimes four and 7 days. The skin is examined and graded from 1+ to 4+ based on the degree of lesions (erythema, papules, vesicles) 30 min after removal of the patch.20, 32, 46, 78 The reader is referred for representative images of patch tests in human patients with CD.5, 96
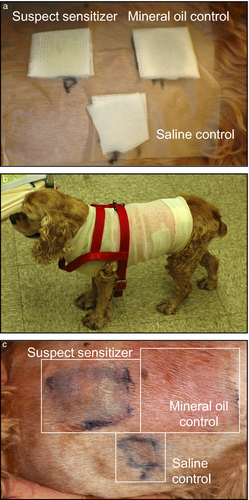
Patch tests present some advantages: they are noninvasive, relatively safe, somewhat cost-effective and do not require the individual to be hospitalized. However, these tests also present some disadvantages: false positive reactions (e.g. pressure and friction from the patch itself; irritation from the tested sensitizer), false negative reactions (e.g. poor contact of allergen to skin; inadequate concentration tested, sensitizing allergen unknown), the impractical aspect of having a bandage around the animal's chest for several days and the risk of severe reactions because it is a form of rechallenge. The sensitivity and specificity of patch testing in veterinary CD is unknown. In human medicine, when patients are carefully selected based on history, clinical pattern and ruling out differential diagnoses first, patch testing has a sensitivity and specificity in the range of 70–80%.2, 91, 97 Currently, a standard veterinary patch test for CD is not available; however, standard human patch tests such as Thin Layer Rapid Use Epicutaneous Test (TRUE®) have been used in dogs (SmartPractice, Phoenix, AZ, USA).15, 46 The TRUE® patch test contains 35 allergens and a negative control. In addition to a standard patch test, Finn chambers may also be used to test individual allergens that are not included in the standard patch test. The North American Contact Dermatitis Group Series is another patch test system commercially available that currently includes 65 allergens.98-100 The North American Contact Dermatitis Group is composed of 14 dermatologists in the United States and Canada, and every 2 years the series is revised. The European Baseline Series recommended by the European Society of Contact Dermatitis is used in Europe and comprises of 30 allergens in its series.
Management of ICD and ACD
General management
The most important factor in CD management is the identification and removal of the culprit irritant/allergen.1, 3, 5, 12, 45, 78, 93 Topical therapy may include bathing with water, with or without a nonirritating shampoo to remove the irritant and soothe the skin. Additionally the prevention and/or treatment of secondary infections with appropriate topical or systemic antibacterial agents and antifungals is required when lesions are deep and/or widely spread.32, 78
Corticosteroids
In both ICD and ACD, topical or systemic symptomatic therapy may be beneficial to relieve some clinical signs.1, 20, 32, 93 Topical corticosteroids can be used for localized lesions to control local inflammation.32, 78 If lesions are generalized, systemic corticosteroids at an anti-inflammatory dose may be helpful for alleviating pruritus and pain associated with inflammation.32, 93 However, human studies have shown conflicting results for the effectiveness of corticosteroids in the treatment of CD.5, 101 An uncontrolled study showed that topical betamethasone 0.1% (applied twice daily for 7 days) improved ICD lesions in humans diagnosed with CD.102 However, a controlled study on sodium lauryl sulfate (SLS)-induced ICD in humans showed no difference between topical corticosteroids (0.1% hydrocortisone or 1% betamethasone, both applied once on the first day and twice daily for the next 4 days), vehicle cream and untreated controls.103 Topical glucocorticoids are the first line of treatment for human patients with ACD90, 104 and are commonly used in veterinary patients as well.14, 32, 46 One human study showed that subjects improved overall compared to vehicle-treated sites when topical steroids (triamcinolone acetonide 0.1% ointment or clobetasol proprionate 0.05% ointment) were applied; however, the improvement was not significant.105 Another study showed that topical 0.05% micronized fluticasone was effective in the management of nickel ACD in humans when applied twice daily for 7 days.106 Systemic corticosteroids (anti-inflammatory dose) are usually reserved for widespread and severe ACD.3, 5, 22, 45
It is important to remember that corticosteroids have numerous and sometimes significant adverse effects, especially when used chronically. Topical corticosteroids may result in atrophy and impairment of the stratum corneum barrier function.19, 22, 104 The most common adverse effects of topical corticosteroids in humans are facial acne, striae and dyschromia.107-109 Systemic corticosteroids may cause polyphagia, polydipsia and polyuria in addition to hypotrichosis, hypertension, diabetes and iatrogenic hypercortisolism.22, 90, 110 Furthermore, it has been reported that the response to topical and systemic corticosteroids seems to decrease over time with chronic use.14, 32
NSAIDs
There is no evidence supporting the use of NSAIDs in CD. Interestingly there are multiple reports of CD reactions to topical NSAIDs in human medicine.111, 112
Antihistamines
No clinical trial has been published to test the efficacy of antihistamine drugs in patients with CD. Some believe that antihistamines could be used in CD patients to decrease pruritus. One study showed that topical antihistamines could potentially decrease inflammation and improve barrier function, possibly because keratinocytes express histamine type 1 and 2 receptors.113 However, in human studies, topical antihistamines, specifically doxepin, have been shown to cause CD and therefore are not recommended as a treatment choice.109, 114, 115 In general these agents usually show limited efficacy.5, 45, 93
Calcineurin inhibitors
Calcineurin inhibitors (e.g. tacrolimus, pimecrolimus, ciclosporin) are part of the standard treatment for atopic dermatitis in both human and veterinary medicine, but there is less information available on their efficacy in CD patients. They have been used off-label in human CD, usually in patients that do not respond to corticosteroid therapy or when steroid sparing agents are required.32, 93, 97, 116 Studies on the efficacy of topical calcineurin inhibitors in murine ACD models have shown that these agents can prevent sensitization and/or elicitation.105, 117 There are only a few studies on the efficacy of calcineurin inhibitors for the treatment of ACD in human patients (topically or systemically) and their results appear conflicting. One study showed only a trend for improvement (no statistical significance) with use of topical calcineurin inhibitors (pimecrolimus 1% cream or tacrolimus 0.1% ointment) and topical steroids (triamcinolone acetonide 0.1% ointment or clobetasol proprionate 0.05% ointment) compared to vehicle-treated sites in people that had previously reacted to nickel.105 Another study showed similar efficacy between a topical steroid (0.05% betamethasone cream) and a systemic calcineurin inhibitor (3 mg/kg/day oral ciclosporin) when used for 6 weeks in humans with hand eczema.118 A double-blinded controlled study evaluated the efficacy of topical 0.1% tacrolimus in people with allergic contact dermatitis to nickel and it was found that 95% (18 patients) experienced clinical improvement.119 Ciclosporin may be beneficial for dogs with ACD at 5 mg/kg/day; however, there are no studies that evaluate the efficacy of calcineurin inhibitors in the management of canine ACD.32
Pentoxifylline
Pentoxifylline is an immunomodulatory agent that is primarily used in humans to reduce blood viscosity and treat vascular disorders.77, 120 It has been shown to suppress the production of TNF-α in vivo in murine models, which is one of the cytokines implicated in the pathogenesis of ACD, and therefore, it may be beneficial in the management of ACD.121 However, two studies that investigated the efficacy of topical pentoxifylline for human ACD produced disappointing results.122, 123 In a veterinary study it was reported that pentoxifylline given orally at 10 mg/kg every 8 h for 2 days effectively prevented the development of lesions in dogs with a history of ACD to plants of the Commelinceae family.77
Hyposensitization
Allergen-specific immunotherapy (ASIT) is controversial in humans with ACD.94, 124 Data published to date do not allow for any definitive statement regarding the effectiveness of oral or injectable ASIT in ACD patients.14, 124 In animals there have been very few evidence-based studies of ASIT for the treatment of ACD. One study with guinea pigs showed that frequent epicutaneous injections of low doses of dinitrochlorbenzene (DNCB), a potent sensitizer, induces systemic hyposensitization within 8 weeks, but discontinuation of these injections resulted in recurrence of lesions.125 Some hypothesize that repeated administration of intralesional steroids directly to the allergen test reaction site will reduce the antigen-specific memory effector T lymphocyte population leading to attenuated T-cell response, and therefore favouring the development of hyposensitivity to the offending allergen.126 In one study, 10 human patients with ACD were given 4 mg of triamcinolone intradermally at positive patch test sites. Results showed that there were significant decreases in patch test reactions, a state persisting for approximately 2–4 months.126 A more recent study performed by the same research group concluded that the percentage of regulatory T lymphocytes increased from week one to seven with weekly injections of triamcinolone at patch test sites.127 There is no information available to date about the potential usefulness of ASIT in canine or feline ACD.
CD versus atopic dermatitis
As described above, there are many similarities between the pathogenesis and the clinical aspects of ICD and ACD.4, 20, 32, 34In addition, differences between CD and atopic dermatitis (AD) are complex and controversial in both human and veterinary medicine.95, 128-132 Atopic dermatitis is an inflammatory skin disorder caused by epidermal barrier defects. It is characterized by a strong Th2 immune response in the acute phase with the addition of Th1 cells and cytokines during the chronic stages.128, 133 These later stages can resemble certain markers involved in ACD.132, 134, 135
The results of human studies which have compared CD to AD have been conflicting.130 Some studies have found that human patients with AD are more prone to CD. They postulate that barrier defects in atopic dermatitis lead to increased probability of sensitizer penetration.95, 135-138 Such a relationship between atopic dermatitis and CD has been demonstrated in atopic dogs as well,9 with AD present in approximately 20% of dogs with CD.32, 132 Conversely, other human studies have shown that AD and CD are independent phenomena.129, 139, 140 Some believe that the balance between Th1 and Th2 immune response is skewed in favour of Th2 in atopic individuals, thereby decreasing the ability of the immune system to develop a Th1-mediated response to contact allergens.95, 135, 138 Importantly, the clinical pattern (e.g. seasonality, lesion type) of CD lesions and AD can be very similar, leading to potential misdiagnosis.95, 141 This could have two significant consequences: first, ACD does not require any long-term treatment, but AD usually does; second, such misdiagnosis impedes research advancement. It has therefore been recommended that extensive patch testing should be performed in any patient that is suspected to have AD, but which is not responding to therapy.141
Conclusions
ICD and ACD develop via a complex pathogenesis that involves both localized and systemic immune response. There is very little evidence-based information available regarding the diagnosis or treatment of CD in people despite decades of research. The paucity of data in veterinary patients or primary cell systems renders diagnosis and treatment even more challenging for veterinarians. Based on the present review of the literature it seems that the following points should be addressed in priority from both researchers and dermatologists. First, a database of allergens that have been associated with CD in dogs should be established, including information about the diagnostic rationale. This would promote networking between clinicians who see dogs with CD and researchers who study it. Such a database would mirror what is already being done in human medicine, such as CAMP database maintained by the American Contact Dermatitis Society.2 Secondly, diagnostic and therapeutic guidelines for canine CD need to be established. Beyond allowing the profession to establish the prevalence of this important skin disease in dogs, guidelines might promote further research including investigations of potential genetic predispositions and clinically relevant pathogenic biomarkers.
Acknowledgements
The authors would like to thank Sandra Grable for helping find the photographs within our picture archives and Andrea Hasbach for providing some feedback on our manuscript.




