Cytologic features of oral squamous cell carcinoma in an Indo-Pacific bottlenose dolphin (Tursiops aduncus): Papanicolaou stain and immunocytochemistry using liquid-based cytology
Abstract
Although oral cytology using Papanicolaou stain is useful for the early detection of oral premalignant lesions and squamous cell carcinoma (SCC) in people, little work has been conducted on this topic in veterinary medicine. This paper describes the features of oral cytology using Papanicolaou stain and immunocytochemistry on liquid-based cytology slides in a case of oral SCC in an Indo-Pacific bottlenose dolphin (Tursiops aduncus). In this case, dysplastic cells with koilocyte-like changes and SCC cells were identified using the Papanicolaou stain. These cells were positive for p53 using an immunocytochemistry analysis. A cytologic diagnosis of SCC was made. We believe that the early detection of premalignant oral lesions and SCC in dolphins can be significantly improved with cytology using liquid-based cytology, Papanicolaou staining, and immunocytochemistry.
1 CASE PRESENTATION
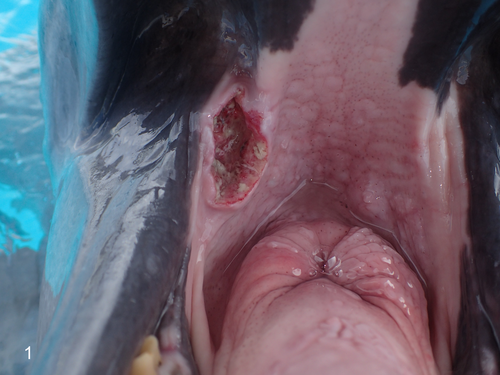
A captive male Indo-Pacific bot, tlenose dolphin (Tursiops aduncus) aged >45 years was reared in Okinawa Churaumi Aquarium (Japan). The animal had a history of a hemorrhagic ulcered mass (4 cm × 2 cm) on the right buccal mucosa that grew over a 2-year period (Figure 1) for which cytology and biopsy of the mass were performed to make a diagnosis.
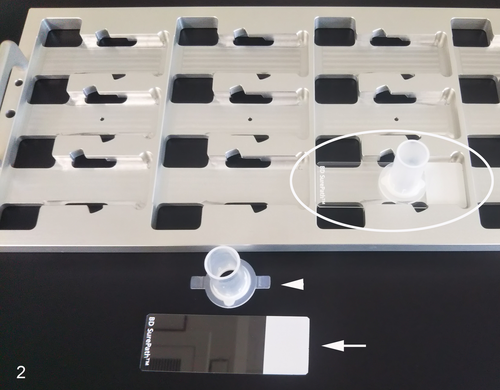
The oral lesion was scraped with a toothbrush to collect cells for the cytologic analysis, and the cells were immediately immersed and stirred in a large test tube containing 20 mL of CytoRich Red Preservative Fluid (Becton Dickinson). A liquid-based cytology (LBC) slide of the above sample was prepared using a modified SurePath method (Becton Dickinson) (Table 1; Figure 2). Three different SurePath slides were stained with Papanicolaou stain (Table 2), and p53 and p16 immunocytochemistry. For the p53 immunocytochemistry, antigen retrieval was performed with a citrate buffer (pH 6.0) heated at 90°C for 20 minutes. Then, a commercially available mouse, anti-human wild-type p53 protein antibody (clone DO-7, dilution 1:200) (Dako) was applied. The p16 immunocytochemistry was performed using a CINtec p16 kit (clone E6H4; mouse anti-human p16 antibody;Roche Diagnostics GmbH). For the p53 and p16 immunocytochemistry, counterstaining was performed using Mayer's hematoxylin.
| Step | Procedure |
|---|---|
| 1 | Fix the sample with CytoRich Red Preservative Fluida (30 min) |
| 2 | Centrifuge the specimens at 3,000 rpm (2 min) and remove the supernatant |
| 3 | Add distilled water (1.5 mL) to the sediment and resuspend it |
| 4 | Transfer the specimen (0.5 mL) to a settling chambera (3 times) and mount it on the positively charged slidea (15 min) |
| 5 | Invert the slide racka and discard the supernatant |
| 6 | Rinse the inside of the settling chamber with 95% ethanol |
| 7 | Invert the slide racka and discard the supernatant |
| 8 | Remove the settling chamber and immediately soak in 95% ethanol (30 min) |
- a Becton Dickinson.
| Step | Procedure |
|---|---|
| 1 | 95% ethanol |
| 2 | Running water (1 min) |
| 3 | Gill's hematoxylina (5 min) |
| 4 | Running water (5 slow dips) |
| 5 | 0.5% HCl in 70% ethanol (10 slow dips) |
| 6 | Running water (5 min) |
| 7 | 95% ethanol (10 slow dips) |
| 8 | Orange G-6a (2 min) |
| 9 | 95% ethanol (10 slow dips) |
| 10 | EA-50a (5 min) |
| 11 | 100% ethanol (20 slow dips) |
| 12 | 100% ethanol (20 slow dips) |
| 13 | Xylene (30 slow dips) |
| 14 | Xylene (30 slow dips) |
| 15 | Mount coverslip |
- a Muto Pure Chemicals, Tokyo, Japan.
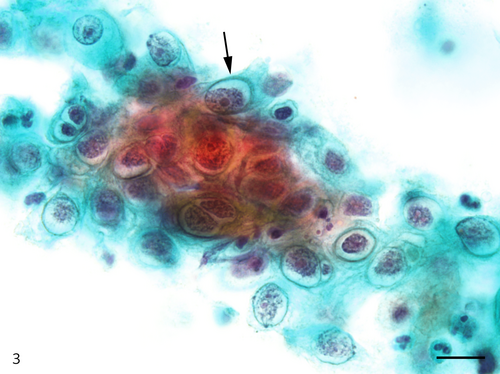
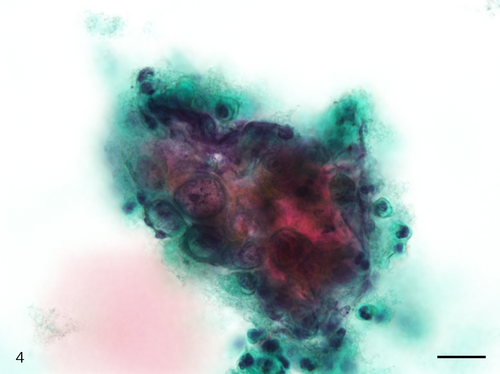
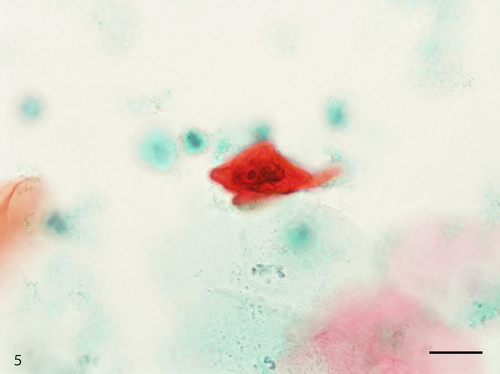
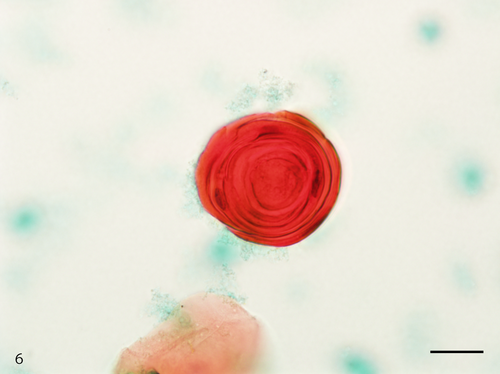
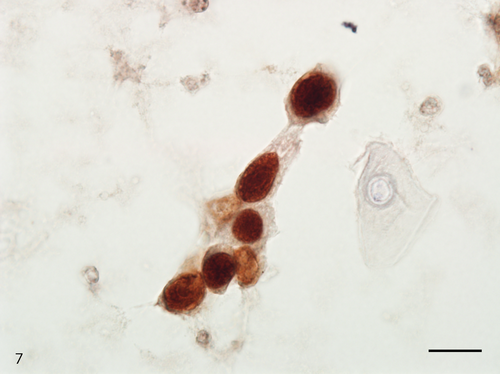
The Papanicolaou stained slide contained a moderate number of cells with an even cell distribution; neutrophils and necrotic debris were present in the background. An air-drying artifact was not evident on this slide, and there were few erythrocytes even though the sample was bloody. The dysplastic cells with koilocyte-like changes and squamous cell carcinoma (SCC) cells were observed. These dysplastic cells were arranged in sheets, and although the nuclei were generally mononuclear, bi-nuclear nuclei were also observed (Figure 3). The intranuclear features of the dysplastic cells were commonly characterized by granular nuclear chromatin and prominent eosinophilic nucleoli. In SCC cells, both keratinized and nonkeratinized cells were observed. Although the nonkeratinized SCC cells mainly appeared as an overlapping structure (three-dimensional) (Figure 4), the keratinized SCC cells tended to appear singly. The keratinized SCC cells displayed a polygonal or fiber-like morphology (Figure 5) and occasional onion-like clusters (keratin pearl) (Figure 6). The nuclei of the keratinized and nonkeratinized SCC cells were characterized by fine or granular nuclear chromatin and prominent eosinophilic nucleoli. According to the immunocytochemistry analysis, dysplastic cells with koilocyte-like changes and keratinized and nonkeratinized SCC cells were positive for p53 (Figure 7) but negative for p16. Based on the above cytomorphologic and immunocytochemical findings, a cytologic diagnosis of SCC was made.
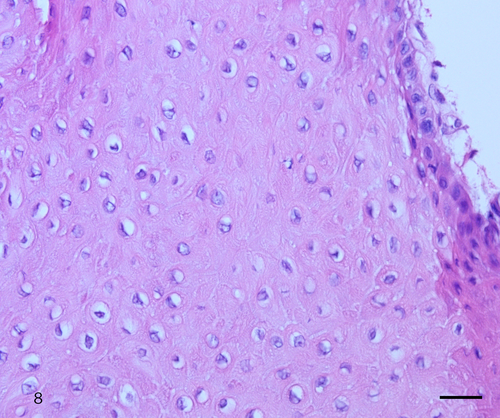
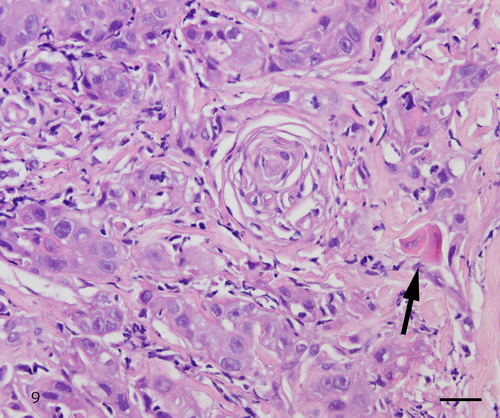
The histologic examination of the biopsy, similar to cytology, showed dysplastic cells with koilocyte-like changes and keratinized and nonkeratinized SCC cells. The dysplastic cells with koilocyte-like changes contained vacuolated cytoplasm and eosinophilic nucleoli (Figure 8). Keratinized and nonkeratinized SCC showed fine and granular nuclear chromatin and prominent eosinophilic nucleoli (Figure 9). These histologic findings confirmed the diagnosis of conventional SCC.
2 DISCUSSION
Human papillomavirus (HPV) has been reported to be etiologically associated with oral SCC in people.1 Similarly, oral SCC believed to be caused by papillomavirus has been reported in bottlenose dolphins; however, there are few reports on the oral cytology in such cases.2-4 Although the gold standard for diagnosing oral SCC is histology, cytology has the advantage of being minimally invasive and can be used to test a wide area. Therefore, oral cytology in people is suitable for screening tests and is useful for the early detection and management of oral dysplasia and SCC.5 Research was recently conducted in people to determine whether LBC developed for cervical cytology can be applied to oral cytology.5, 6 The SurePath method is one of the primary LBC methods and has reported advantages over conventional methods, including a better cell recovery rate, cell preservability, and a reduced number of inadequate samples.5, 7 Additionally, the SurePath method allows for immunocytochemistry and HPV genetic testing using the residual material left after cytomorphologic examinations.5, 8 The intranuclear accumulation of p53 protein that results from p53 gene mutations can be detected using immunocytochemistry, and it is often observed in human oral dysplasia and SCC. Moreover, p16 immunocytochemistry is regarded as a surrogate biomarker of high-risk HPV infection in people.9
In veterinary cytology, the Romanowsky-type stains (Wright stain, Giemsa stain, Diff-Quick stain) are most commonly used.10 Although Giemsa staining is a simple stain procedure excellent for observing hematologic tumor cells, the cytomorphology cannot be observed when the smear is thick or the cells are clustered.10, 11 Conversely, because the Papanicolaou stain was developed to detect dysplasia and SCC of the uterine cervix, it is excellent for observing intranuclear structures, keratinized cytoplasm, and cell clusters.11
This report is the first case of oral SCC in an Indo-Pacific bottlenose dolphin diagnosed by cytology using LBC (SurePath method), Papanicolaou stain, and immunocytochemistry. In the present case, dysplastic cells with koilocyte-like changes and keratinized and nonkeratinized SCC cells were observed. These cytologic findings are consistent with the histologic findings of oral papilloma and SCC in Atlantic bottlenose dolphins.2 Koilocytosis is a cytomorphologic feature characterized by a clear perinuclear area and is considered the viral cytopathic effect of papillomavirus infection.12 In people, koilocytic cells have slightly increased N/C ratios, mono- or bi-nuclei, granular chromatin, and basophilic nucleoli. Meanwhile, in the present case, the nucleoli of koilocyte-like cells were larger than those in people and were also eosinophilic. Chun et al reported that more prominent nucleoli were present in the tumor cells of the SurePath staining method compared with the conventional staining methods.13 Therefore, the large nucleoli might be a result of the SurePath method, although it is not evident whether the eosinophilic nucleoli of koilocyte-like cells are a characteristic of dolphins or a result of the staining conditions. To the best of our knowledge, there are no previous reports of koilocyte-like cells in veterinary cytology, potentially because Papanicolaou staining has not been widely used in veterinary cytology, making it impossible to observe detailed cytomorphology of these cells.14 Oral SCC in people displays a multistep process of development from premalignant lesions such as papilloma and leukoplakia.15 We inferred that detecting these premalignant lesions with cytomorphology using Giemsa staining is difficult because the cellular atypia of these lesions is likely mild in dolphins as well. Thus, it is possible that oral cytology using Papanicolaou stain, which is excellent for observing intranuclear structures and keratinized cytoplasm, leads to the early detection of premalignant oral lesions and SCC in dolphins.
The SurePath method is one of the primary LBCs approved by the Food and Drug Administration. In addition, because the SurePath method can also be used manually if a slide rack and centrifuge are available, it can be performed in veterinary hospitals. The SurePath method principally functions through gravity sedimentation and electrical adhesion. Briefly, the settling chamber is clamped to a positively charged SurePath slide, and a cell suspension treated with CytoRich RED is added. Gravity then settles the cells on the slide, and a positively charged slide is combined with negatively charged cells.7 In the present case, air-dry artifacts were not present on the SurePath slide because the toothbrush used to collect cells was immediately immersed in CytoRich RED. Additionally, there were only few erythrocytes on the SurePath slide despite bleeding at the time of cell collection because the erythrocytes were hemolyzed by CytoRich RED. Therefore, the SurePath method can improve the slide quality (no air-dry artifacts and a clear background). Moreover, this method provides a standardized protocol, and the residual material left over after Papanicolaou staining can be used for immunocytochemistry and a papillomavirus genetic test.5 Although not assessed in the present case, the SurePath method could also be theoretically applied to veterinary genetic testing.
More than 100 types of HPV have been identified in people, including HPV types 16 and 18, which are known to be strongly associated with the carcinogenesis of oral and cervical SCC.1 HPV infection within the oral cavity can result from oral sex.16 Conversely, although only a limited number of sequenced and characterized papillomavirus genomes have been reported in dolphins, multiple papillomavirus types have been identified in the genital and esophageal lesions of cetaceans.17-19 Additionally, papilloma and SCC were simultaneously observed in three free-ranging Atlantic bottlenose dolphins.2 These findings suggest that progressive pathologic processes involving the malignant transformation of papillomavirus oral cavity infection can occur in dolphins. Although the causes of oral papillomavirus infection in dolphins are uncertain, a recent study reported that the novel papillomavirus was detected in the feces of a wild bottlenose dolphin; thus, there could be fecal-mediated oral infections.20 Therefore, oral papillomavirus infection might be facilitated by the ability of the virus to maintain its viability in aquatic environments. In people, it has been reported that low-risk HPV causes primarily koilocytosis and has a minimal relationship with high-risk HPV (HPV types 16 and 18).21 However, in the present case, the dysplastic cells with koilocyte-like change and SCC cells were present on the same slide. This discrepancy could be caused by a carcinogenic mechanism of the papillomavirus that differs between people and dolphins.17-20, 22 Second, a coinfection of high-risk papillomavirus and low-risk papillomavirus could have caused this discrepancy. In addition, although coinfection with herpesvirus and papillomavirus has been reported in genital tumors of free-ranging Atlantic bottlenose dolphins,23 no findings such as intranuclear inclusion bodies, indicating herpesvirus infection, were found in the cytologic and histologic specimens of the present case.
The p53 immunocytochemistry was reported to detect early alterations associated with oral carcinogenesis in people.6 In this study, not only SCC cells but also dysplastic cells of premalignant lesions were positive for p53. Therefore, the immunocytochemistry of p53 is likely to be useful for detecting premalignant lesions with slight cellular atypia, which could be overlooked during microscopic observations. Cervical and oropharyngeal SCCs in people have been reported to have a high positivity rate for p16 immunostaining (100% and 90.2%, respectively).9, 22 The HPV E7 oncoprotein binds to the retinoblastoma (Rb) protein and degrades it, and the oncogenic stress of the functional Rb loss leads to p16 overexpression. Therefore, positive p16 immunostaining is a well-accepted surrogate biomarker of transcriptionally active HPV in the cervix and oropharynx SCC.9, 22 However, in the present case, all dysplastic cells and SCC cells were negative for p16 immunocytochemistry. This discrepancy could be caused by the absence of p16 cross-reactivity in dolphins compared with people. Another potential cause for the negative p16 immunoreactivity is a lack of the E7 gene in the currently identified cetacean papillomavirus.17-20 Thus, p16 immunostaining is not likely to detect papillomavirus in oral premalignant lesions and SCC of cetaceans, including dolphins.
In conclusion, oral cytology using the LBC (SurePath method), Papanicolaou staining, and p53 immunocytochemistry is a useful diagnostic method and a promising tool for the early detection, management, and research of oral SCC in dolphins.
ACKNOWLEDGMENTS
The authors thank Ms Kaori Enomoto and Ms Akane Furukawa for their technical assistance. This research was supported by a grant from JSPS KAKENHI (Grant number: 20K20664).
DISCLOSURE
The authors have indicated that they have no affiliations or financial involvement with any organization or entity with a financial interest in, or in financial competition with, the subject matter or materials discussed in this article.




