Influence of preanalytical factors on feline proteinuria
Abstract
Background
To date, little information is available about the effect of preanalytical factors on the urinary protein-to-creatinine (UPC) ratio in cats.
Objectives
We aimed to evaluate the effect of a commercially available cat litter, creatinine measurements at three different dilutions of urine, and different storage conditions on the UPC ratio in cats.
Methods
Feline urine specimens were prospectively collected. Twenty-two whole-urine specimens were placed uncovered and in contact with cat litter for 1 hour; 25 urine supernatants were diluted 1:10, 1:20, and 1:100 for creatinine measurements. The correlation, difference, agreement, and concordance in classifying specimens according to International Renal Interest Society staging were determined. Storage effects on UPC ratios were assessed in specimens stored for 6 hours at +20℃ (n = 20), 1 week at +4℃ (n = 20), and 3 months at −20℃ (n = 25). Specimens were also subjected to four freeze-thaw cycles (n = 20). Results were compared, and clinical significance was assessed by comparing each UPC ratio to the inter-assay range of the baseline value.
Results
Exposure to cat litter did not affect UPC ratios. A positive proportional bias was found in the 1:100 dilution compared with the 1:20 dilution; however, concordance was high for all comparisons. At +20, +4℃, and after four repeated freeze-thaw cycles, UPC ratios were stable. Compared with baseline values, UPC ratios decreased (P < .01) after 8 and 12 weeks at −20℃. However, all UPC ratios were within the inter-assay variability of the baseline value.
Conclusions
Exposure to cat litter did not affect UPC ratios, but further studies are necessary to evaluate other potential variables. The effects of the dilutions and storage conditions were clinically acceptable, although the 1:20 and 1:100 dilutions were not perfectly comparable.
1 INTRODUCTION
The development of proteinuria in cats with chronic kidney disease (CKD) has been linked to disease progression1, 2 and increased risk of mortality.3, 4 The quantification of proteinuria is, therefore, a necessary step in the diagnosis and staging of feline CKD, as recommended by the current guidelines.5 Although the gold standard for detecting proteinuria is the quantification of urine proteins during a 24-hour urine collection,6, 7 urinary protein-to-creatinine (UPC) ratio measurements in spot urine specimens is currently considered a reliable estimation of daily protein excretion in cats.8 The diagnostic reliability of UPC ratios is based on the assumption that the test is minimally influenced by preanalytical and analytical variables.9 To our knowledge, little available information regarding the effects of preanalytical variables on UPC ratios in cats is present in the literature.
Cystocentesis and manual compression of the bladder are the most frequent methods of urine collection in cats. The two methods yielded comparable and reliable UPC results in a previous study, and both are recommended in feline practice.10 However, these methods could be invasive and impractical in some cats, especially during serial monitoring of nephropathic and proteinuric patients. In particular, cystocentesis could present some risks11 and could cause iatrogenic hematuria, which, in turn, might falsely increase the UPC ratio.12 Thus, the UPC ratio in spontaneously voided urine collected in cat litter by the owner in a home environment could be useful in some clinical situations. To our knowledge, the preanalytical influence of the litter in cats was previously evaluated only in urinary glucose and phosphate measurements.13, 14 However, no information is available regarding the preanalytical influence of the litter on feline UPC ratios.
Urinary creatinine is commonly measured using the same assay used for serum creatinine measurements. Since creatinine concentrations are 25-100 times higher in urine than in plasma, it is necessary to dilute urine specimens before measuring, which represents a potential preanalytical source of error. In dogs, differences in the magnitude of urine dilutions before measuring creatinine concentrations was shown to affect creatinine measurements, leading to different UPC ratios.15
Specimen handling, such as different storage temperatures and storage durations before urine creatinine measurements, is another preanalytical factor that can affect UPC ratios. In dogs, it has been demonstrated that UPC ratios increased significantly 12 hours after storage at room temperature and 4℃,15 but we found no information about this subject in cats.
Therefore, the purpose of this study was to determine whether preanalytical factors can affect proteinuria evaluations in cats. Specifically, we studied the effects of preanalytical factors on urinary protein (UP) and urinary creatinine (UC) concentrations, and UPC ratios. The preanalytical factors included (a) a litter used to collect spontaneously voided urine, (b) the preanalytical dilution of urine for creatinine quantification, and (c) the storage of urine specimens at different temperatures and times.
2 MATERIALS AND METHODS
2.1 Specimens
Urine specimens were prospectively collected from client-owned cats presented for routine diagnostic investigations from January 2015 to February 2016 and from July 2017 to January 2018. The specimens were collected at the Internal Medicine Unit of the Veterinary Teaching Hospital (University of Milan) during routine health screens, under informed consent signed by the owners. According to the University of Milan ethics committee statement (number 2/2016), biological specimens collected in this setting could be used for research purposes. Due to the analytical nature of this study, we enrolled cats irrespective of age, sex, and breed or underlying disease, and whether the cats had diseases that could affect urine composition (eg, CKD, lower urinary tract inflammation, and neoplasia) were included to increase diversity of the sample data.
Eight to ten milliliters of urine was collected from each cat by ultrasound-guided cystocentesis. Urinalysis and supernatant collection were performed as previously described.15
2.2 Analytical methods
Urinary protein was measured using a colorimetric method based on pyrogallol red molybdate (Urine proteins, Sentinel Diagnostics), and urinary creatinine was measured with the modified Jaffe method (Creatinina, Real-Time Diagnostics). Both methods were validated in feline urine in a previous study.16 All tests were performed with an automated biochemical analyzer (Cobas Mira, Roche Diagnostics) on urine supernatants. Methods were controlled daily with quality control material (UniChem Level 1 and Level 2; Instrumentation Laboratory), and calibrations were performed when the Westgard rule 12s was violated on the control solutions. To reduce the effect of intra-assay variability on results, all the tests were performed in triplicate, and the mean values were used for the data analysis. The UPC ratio was calculated by dividing the UP concentration by the UC concentration.
2.3 Effect of the litter
Twenty-two specimens were included in this analysis. Six milliliters of uncentrifuged urine was split into two parts. Three milliliters was placed into a conical 10 mL tube and stored tightly sealed at room temperature, and the other 3 mL was placed in an 85 mm diameter Petri dish. Petri dishes were filled with plastic nonabsorbent spherical pellets (ProfiCat Litter, Dlm srl) until a monolayer was present on the bottom of the Petri dish; this cat litter was a commercially available material, specifically designed to collect urine. The same amount of cat litter was placed in each Petri dish. Urine was left uncovered in the Petri dish for 1 hour at room temperature to mimic the urine collection of an owner at home. Then, urine was collected with the pipette provided by the litter manufacturer and placed into a 10mL tube. Urine supernatants placed for 1 hour in a tube were labeled “SpecimenTUBE,” and those placed for 1 hour in the litter were labeled “SpecimenLITTER.” These supernatants were collected and analyzed for UP and UC concentrations and UPC ratios. UP and UC concentrations and UPC ratios measured in the SpecimenTUBE supernatants were considered the base values.
2.4 Effect of urinary creatinine dilutions
Twenty-five urine specimens were included in this analysis. Three aliquots of each urine supernatant were diluted 1:10, 1:20, and 1:100 (final volume: 200 μL). UC was measured at three dilutions (UC1:10, UC1:20, and UC1:100). UP concentrations were determined in the undiluted supernatant since all the concentrations were within the range of the linearity of the method (ie, 0.2 to 2.10 g/L).16 The UPC ratio corresponding to each creatinine dilution (UPC1:10, UPC1:20, and UPC1:100) was calculated.
2.5 Effect of storage
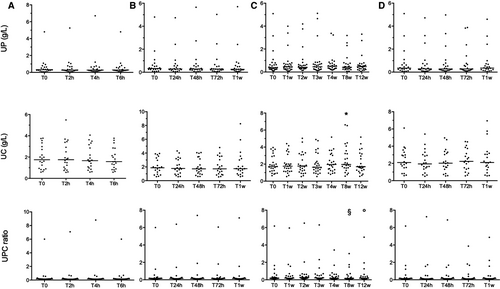
A total of 37 specimens were included in this analysis. Some specimens were included in two or more storage conditions, and all sets of specimens in each storage condition differed from one another. To assess the short-term stability at room temperature, 20 specimens were assayed immediately after collection (T0) and then divided into three aliquots (300 μL each). The aliquots were stored at room temperature (~20℃) and analyzed after 2 (T2h), 4 (T4h), and 6 (T6h) hours.
To assess the effect of refrigeration, 20 specimens were assayed at T0, then divided into seven aliquots (300 μL each). Aliquots were stored at 4℃ and analyzed after 24 (T24h), 48 (T48h), and 72 (T72h) hours, and after 1 week (T1w).
To assess stability in frozen specimens, 25 specimens were tested immediately at T0, then divided into six aliquots (300 μL each) and stored at –20℃. Aliquots were analyzed at 1 (T1w), 2 (T2w), 3 (T3w), 4 (T4w), 8 (T8w), and 12 weeks (T12w) after storage. Each specimen was allowed to warm up at room temperature (for ~1 hour) and thoroughly vortexed before the analysis.
To assess the effect of repeated freeze-thaw cycles, 20 specimens were used. One aliquot (1 mL) for each patient was tested immediately at T0 and frozen at –20℃. Specimens were thawed, analyzed, and frozen again after 24 (T24h), 48 (T48h), and 72 (T72h) hours, and 1 week (T1w) for a total of four freeze-thaw cycles. At each measurement time, all frozen specimens were thawed at room temperature for ~1 hour until completely thawed and then vortexed thoroughly before analysis; immediately after, the specimens were refrozen at –20℃ for the next run. All samples were stored in 1.5-mL Eppendorf tubes.
2.6 Statistical analysis
Statistical analysis was performed using two commercially available software packages (MedCalc Statistical Software, version 16.8.4, Ostend, Belgium; GraphPad Prism version 5.0; GraphPad Software). A P < .05 was considered statistically significant. The distribution of the variables was assessed using the Kolmogorov–Smirnov test.
For the tests focused on determining the effect of the cat litter, UP, UC, and UPC obtained in SpecimenTUBE and SpecimenLITTER supernatants, results were compared using the Wilcoxon signed-rank test, and correlations were assessed with the Spearman correlation test. Agreement between results was assessed via the Deming regression and Bland-Altman tests; and concordance in classifying specimen as nonproteinuric (NP), borderline proteinuric (BP), or proteinuric (P) according to the International Renal Interest Society (IRIS) guidelines in cats5 was tested with Cohen k test. Cohen's k coefficient was used to label concordance as “very good” (k = 0.8-1), “good” (k = 0.6-0.8), moderate (k = 0.4-0.6), “fair” (k = 0.2-0.4), “poor” (k = 0.0-0.2), or “absent” (k < 0).17
Similarly, for the evaluation dilutional effects of urine supernatants, the results (UC and UPC) obtained from the three dilutions were compared with the Friedman test, followed by Dunn's multiple comparison test as a post hoc test to compare results of each dilution; the agreement between results was tested with Deming regression tests and Bland-Altman tests; concordance in classifying the specimen according to IRIS guidelines was tested with the Cohen k test.
To assess the influence of storage, the results obtained at different time points were compared using the Friedman test, followed by Dunn's multiple comparison test. To further evaluate the potential significance of the storage, UPC changes over time were compared with an arbitrary and conservative range of values calculated, considering the analytical variability of the UPC measured at T0. Specifically, the UPC at each time point (measured in triplicate) was compared with the range of UPCs calculated, applying the inter-assay coefficient of variation (CV) of the method (16.4%, determined in a previous study)16 to the respective UPC measured at T0. The lower and upper limits of this range were calculated as follows: lower limit of the range =UPC at T0 − (UPC at T0 × 0.164); upper limit of the range = UPC at T0 + (UPC at T0 × 0.164). The UPC variation was considered of clinical importance if its value fell outside the calculated range at any time point.
3 RESULTS
3.1 Effect of litter
Individual UPC values recorded in the SpecimenLITTER and SpecimenTUBE supernatants are shown in Table S1. UP, UC, and UPC measured in SpecimenLITTER compared with those measured in SpecimenTUBE were not statistically different (UP, P = .770; UC, P = .074; UPC, P = .169; Table 1). UP, UC, and UPC between SpecimenTUBE and SpecimenLITTER were significantly correlated with one another (P < .001) with high correlation coefficients (Table 1).
| Litter | Tube | Correlation | Deming | Bland-Altman | ||
|---|---|---|---|---|---|---|
| Median (min to max) | Median (min to max) | Spearmana | Slope (95% CI) | Intercept (95% CI) | Bias (95% CI) | |
| UP (g/L) | 0.312 (0.001 to 1.293) | 0.318 (0.004 to 1.349) | 0.97 | 1.05 (0.91 to 1.19) | –0.66 (–8.45 to 7.13) | 1.35 (–19.7 to 22.4) |
| UC (g/L) | 2.408 (0.178 to 6.500) | 2.137 (0.144 to 5.792) | 0.98 | 1.07 (0.94 to 1.21) | –4.13 (–46.81 to 38.54) | –14.9 (–85.2 to 114.9) |
| UPC | 0.15 (0.01 to 0.89) | 0.14 (0.01 to 0.92) | 0.96 | 1.02 (0.93 to 1.12) | –0.01 (–0.03 to 0.02) | 0.01 (–0.09 to 0.10) |
Note
- Spearman correlation coefficients, intercepts, Deming regression slopes, and biases recorded with Bland-Altman testing are also shown.
- Abbreviation: CI, confidence interval.
- a All the P-values were <.001.
The Deming regression test showed neither constant nor proportional errors (Table 1 and Figure S1), and the bias determined with the Bland-Altman test for the UP, UC, and UPC was not statistically significant (Table 1 and Figure S2).
Agreement in classifying specimens according to the IRIS staging system was considered “very good” (Cohen k coefficient = 0.955). Only two IRIS staging shifts were recorded; one from stage BP (UPC ≥ 0.20 and <0.40) to stage NP (UPC < 0.20) and one from stage NP to stage BP.
3.2 Effect of urinary creatinine dilutions
Median and min-max values for UC1:10, UC1:20, UC1:100, UPC1:10, UPC1:20, and UPC1:100 are shown in Table 2, and individual UPC values are shown in Table S2. No significant differences were found among the three UC (P = .468) and UPC (P = .540) dilutions, and a high correlation was seen across the three dilutions (Table 2). When comparing dilutions with the Deming and Bland-Altman tests, only proportional error between UPC1:20 and UPC1:100 was found (Table 2). Agreement in classifying specimens according to IRIS stages was labeled “very good” for all the three comparisons (1:20 vs 1:10, k = 0.83; 1:20 vs 1:100, k = 1; 1:10 vs 1:100, k = 0.83). Only two specimens within the 1:10 urine dilutions yielded a disparate IRIS stage (one NP instead of BP and one BP instead of NP) compared with the other dilutions.
| UC1:10 | UC1:20 | UC1:100 | Spearman correlationa | Deming | Bland-Altman | |||
|---|---|---|---|---|---|---|---|---|
| Slope (95% CI) | Intercept (95% CI) | Bias (95% CI) | ||||||
| 1:10 vs 1:20 | 0.95 | 1.08 (0.96 to 1.21) | −16.69 (−48.27 to 14.90) | 1.98 (−62.81 to 66.78) | ||||
| UC | 1.914 (0.877 to 5.315) | 1.881 (0.896 to 5.438) | 1.803 (0.893 to 6.037) | 1:20 vs 1:100 | 0.89 | 1.06 (0.84 to 1.28) | −20.50 (−74.70 to 33.69) | −6.13 (−109.29 to 97.02) |
| 1:10 vs 1:100 | 0.94 | 0.97 (0.79 to 1.16) | −2.99 (−51.16 to 45.19) | −8.12 (−109.46 to 93.21) | ||||
| 1:10 vs 1:20 | 0.92 | 0.99 (0.93 to 1.05) | 0.01 (−0.01 to 0.02) | 0.01 (−0.06 to 0.06) | ||||
| UPC | 0.16 (0.07 to 1.25) | 0.17 (0.06 to 1.21) | 0.16 (0.07 to 1.31) | 1:20 vs 1:100 | 0.90 | 1.08 (1.03 to 1.12) | −0.01 (−0.02 to 0.01) | 0.01 (−0.05 to 0.07) |
| 1:10 vs 1:100 | 0.84 | 1.07 (0.99 to 1.15) | −0.01 (−0.03 to 0.02) | 0.01 (−0.07 to 0.09) | ||||
Note
- Spearman correlation coefficients, intercepts, Deming regression slopes, and biases recorded with Bland-Altman testing are also shown.
- Abbreviation: CI, confidence interval
- a All the P-values were <.001
3.3 Effect of storage
Results of the different storage conditions are summarized in Figure 1, and individual UPC values are shown in Tables S3-S6. No significant differences were found for UP, UC, and UPC when stored at room temperature for 6 hours and +4℃ for 1 week. During long-term storage at −20℃, UP did not change during 12 weeks of storage, whereas UC was significantly higher (P = .02) at T8w compared with T2w. UPC was significantly lower (P < .01) at T8w compared with T1w, T2w, and T3w, and at T12w compared with T2w. No significant differences were found for UP, UC, and UPC after four freeze-thaw cycles within 1 week. All UPCs at each time point were within the inter-assay variability of T0.
4 DISCUSSION
In this study, different preanalytical variables were evaluated to assess the potential effects of these variables on urinary protein and creatinine quantification and UPC measurements in cats. We also assessed what effect the results might have on clinical decision-making.
To our knowledge, the possible effects of litter on feline UPC was not previously evaluated. Our results demonstrated that UPC was not affected when whole urine was in contact with plastic litter for 1 hour at room temperature. The only two cases that showed a shift of the IRIS stage (from BP to NP and from NP to BP) had mean UPCs close to the cutoff of 0.2 (UPC = 0.22 and = 0.17); the recorded variability was interpreted as analytic inter-assay variability.16
The procedure evaluated in this study did not consider many other possible variables occurring in daily practice scenarios. For example, feces or detergent contamination could occur and, in turn, affect urinalysis.18 Furthermore, a delay in collecting urine from the litter (over 1 hour) could have resulted in a marked evaporation of the specimen and an alteration in the UPC. However, evaporation would more likely have a major effect on UP and UC concentrations and less of an effect on the UPC ratios, given the similar theoretical concentrating effects on proteins and creatinine (both of which are not volatile compounds at room temperature). Given that we did not record a significant increase in UC concentrations, specimen evaporation was considered unlikely. Therefore, the results of our study suggested that UPCs measured in urine collected at home from cat litter can be considered reliable only when the specimen is collected within 1 hour, and a new dedicated litter or an uncontaminated litter cleaned with distilled water is used. These preliminary results need to be confirmed with further studies involving more variables that occur in clinical practice.
The dilution of urinary supernatants to measure UC was shown to be a source of variability in dogs.15 Feline urine has a generally higher creatinine concentration (compared with dogs) due to the higher concentrating ability of the feline kidney. Thus, we did not expect the lower supernatant dilution (1:10) to dilute urine enough to lower the creatinine concentration within the linear range of the method. Conversely, we expected the 1:100 dilution to decrease the creatinine concentration too close to the lower quantification limit of the method. Therefore, we expected that the 1:20 dilution would be the best dilution to lower the creatinine concentrations within the linear range of the method in most specimens. Although the range of linearity of the Jaffe method was exceeded in many specimens with the 1:10 dilution and creatinine concentrations were close to 0.01 g/L in some specimens with the 1:100 dilution, no differences were found among the three dilutions for the UC or UPC ratio. The only recorded difference was a positive proportional bias of the UPC1:100 compared with the UPC1:20. Thus, according to this result, the 1:20 and 1:100 dilutions should not be considered perfectly comparable. The use of a calibrator with a higher creatinine concentration could be a possible solution to improve accuracy. However, accuracy could be lower in serum and diluted urine specimens using this approach.
In clinical practice, other methods can be used to measure serum and UC concentrations. Different methods could yield different accuracies between dilutions. According to a previous study,19 benchtop analyzers using dry chemistry methods underestimated creatinine concentrations compared with the two wet chemistry methods (Jaffe and enzymatic methods). Moreover, in that study, the inaccuracy between the Jaffe and enzymatic methods was <5%, and both methods had similar linearity ranges.19 Therefore, based on the similar analytical performances of the two wet chemistry methods, the results of our study could also likely be applied to laboratories using enzymatic methods, although further evaluations are needed.
In this study, storage at room and refrigeration temperatures and in repeated freeze-thaw cycles lacked statistical differences for the periods evaluated. We set these conditions because they were the common storage conditions that occur in clinical practice during overnight or weekend times when laboratory services are not immediately available or when samples are shipped to referral laboratories. Thus, we can surmise that feline UPC results should be considered reliable in these settings. However, we used supernatants in this study, whereas whole urines are commonly stored in practice. Therefore, further investigations are needed to determine if similar results occur in whole-urine specimens.
Conversely, some significant differences were found when specimens were stored at −20℃, where the UC was higher, and the UPC was lower at some storage time compared with others. These differences are difficult to explain from a biochemical point of view. A combination of specimen evaporation15 and protein degradation over time20 could theoretically produce these patterns. Determining the source of these changes was beyond the scope of this study and; therefore, further research is needed to elucidate this biochemical mechanism.
A previous canine study suggested that specimen dehydration likely contributed to UPC reduction over time in stored urine specimens.15 Thus, the increase in UC (and decrease in UPC) detected in our study could be explained by this hypothesis. Although the UP concentrations did not differ over time, a low protein modification rate over time could also have occurred. Proteins have been shown to fragment with decreasing measurements shown in frozen human urines.20-22 Moreover, urinary albumin fragments were shown to be less able to react with wet biochemistry methods in people.23 Another potential reason for UP concentrations to decrease without altering UC values is the freezing-induced sedimentation of total solids.24 This residual sediment was generated in urinary supernatants after freezing, mainly in the presence of higher urinary calcium concentrations and acidic urines, and was also shown to lower UP concentrations.24 Residual sediments were noted in some specimens of this study, but specimens were always vortexed after thawing, and thus, the effect of such residue on our results was probably minimal.
Taken together, the differences found at −20℃ in this study were more important from a statistical standpoint than from a practical standpoint, and the results should be considered stable. However, in rare cases, the UPC ratio could change enough to result in a shift in the IRIS stage of proteinuria (see Table S6). Therefore, the clinical relevance of analytical variation needs to be evaluated with further studies.
Although specimens included in this study covered a wide range of UP and UC concentrations and UPC ratios, most values were within the reference interval or within the range of proteinuria commonly observed in most cats with kidney disease.3, 4, 25 Therefore, in this study, we investigated which preanalytical factors could affect the results of samples with UPC ratios commonly observed in practice; however, we remain unsure as to how these factors might affect samples with extremely high proteinuria. Moreover, it is worth noting that all measurements were carried out in triplicate, a routine rarely performed in clinical settings. Therefore, the variability reported in this study is likely lower than would be obtained with individual measurements.
ACKNOWLEDGMENTS
Some of the specimens included in this study were part of a study funded by the Winn Feline Foundation (grant number WZ14-009). The authors thank Dr Alessandro Stranieri for the English language editing.
CONFLICT OF INTEREST
None of the authors of this paper has a financial or personal relationship with other people or organizations that could inappropriately influence or bias the content of the paper.




