Hepatozoon canis infection causing a strong monocytosis with intra-monocytic gamonts and leading to erroneous leukocyte determinations
[Corrections added on August 16, 2019, after first online publication: The title has been changed from ‘Hepatozoon canis infection resulting in a marked monocytosis with intra-monocytic gamonts and erroneous leukocyte differential counts’ to ‘Hepatozoon canis infection causing a strong monocytosis with intra-monocytic gamonts and leading to erroneous leukocyte determinations’ and the second sentence in abstract has been changed from ‘The dog had a prominent monocytosis (14.0 × 109 /L) with H canis gamonts detected in approximately 40% of the monocytes, but none were found in the neutrophils.’ to ‘The dog had a prominent monocytosis (14.0 × 109/L) with H canis gamonts detected in most monocytes, but none were found in the neutrophils.’]
Abstract
In this case report, a Swedish flat-coated retriever was diagnosed with an extensive Hepatozoon canis infection. The dog had a prominent monocytosis (14.0 × 109/L) with H canis gamonts detected in most monocytes, but none were found in the neutrophils. On the hematology system ADVIA 2120 peroxidase (PEROX) cytogram, most leukocytes were seen as a distinct cell population above the lymphocytes, which indicated that most of the cells were larger than lymphocytes and had weak myeloperoxidase staining. This distinct cell cluster appeared to be of a single cell type but was incorrectly divided by the ADVIA 2120 into lymphocytes, monocytes, and large unstained cells (LUC). The total leukocyte counts on the ADVIA 2120 WBC basophil (BASO) channel were much higher than that on the WBC PEROX count. The WBC BASO cytogram appeared abnormal with two parallel cell populations, so the BASO WBC count was considered erroneous. Polymerase chain reaction and DNA sequencing verified H canis infection. The dog was treated with subcutaneous imidocarb dipropionate (6 mg/kg) injections every other week. Post-treatment hematology analyses indicated that the percentage of parasitized leukocytes decreased from 40% to 5% about 4 weeks after the start of treatment and were not found in any monocytes 6 weeks after the beginning of the treatment. In conclusion, H canis infection in this dog was associated with a strong monocytosis, and gamonts were present in many monocytes, which caused aberrant automated leukocyte counts to occur.
1 CASE PRESENTATION
A 10-year-old, female, Swedish flat-coated retriever was admitted to a veterinary clinic in Stockholm, Sweden, with a 2-week history of poor appetite, polydipsia, occasional vomiting, and lethargy. The owner had found occasional ticks on the dog during the previous month. The dog had a fever (39.6°C) and appeared slightly depressed at the initial examination. Anaplasma phagocytophilum infection was suspected because of the fever, thrombocytopenia (40 × 109/L), and an increased C-reactive protein (CRP) concentration of 218 mg/L (RI < 7 mg/L). Although no Anaplasma inclusions were detected upon examination of the blood smear, the dog was treated with doxycycline.
One week later, the dog was presented again to the clinic for a second examination. At this time, the dog had slightly pale mucous membranes and a temperature of 39.3°C, but no other clinical findings were noted. The CRP concentration continued to be elevated at 135 mg/L and parasites were seen in many of the leukocytes; therefore, EDTA blood samples were sent to the Clinical Pathology Laboratory at the Swedish University of Agricultural Sciences (SLU) for evaluation. Results from the ADVIA 2120 (Siemens Healthcare, Erlangen, Germany) at SLU and the referring clinic's Medonic (Boule Diagnostics, Spånga, Sweden) impedance-based veterinary hematology analyzer are shown in Tables 1 and 2, and Figure 1A and Figure S1. The manual differential leukocyte WBC count showed Hepatozoon canis gamonts in about 40% of the leukocytes of the blood smear monolayer (Figures 2 and 3). In a sample taken 16 days later, about 54% of the leukocytes in the monolayer were infected with H canis, and an even higher percentage of cells contained the parasite at the feathered edge of the smear. A Polymerase chain reaction using primers Hep forward (5′-AAA CGG CTA CCA CAT NTA AGG A-3′) and Hep reverse (5′-AAT ACA AAT GCC CCC AAC TNT-3′) to amplify 504 bp of the 18S rRNA gene of Hepatozoon spp. was followed by DNA sequencing (Diagnostic Laboratories, Langford Veterinary Service, Bristol, UK) as previously described.1 Polymerase chain reaction confirmed the intracellular parasite to be H canis.
| First sampling | Reference interval | |
|---|---|---|
| WBC counts | 19.3 × 109/L | 6.0-17.0 |
| Granulocytes | 16.0 (83%) | 3.5-12 |
| Lymphocytes | 2.5 (13%) | 0.9-5.0 |
| Monocytes | 0.8 (4.1%) | 0.3-1.5 |
- Abbreviation: WBC, white blood cell.
| First samplinga | Second samplinga | RI | |||
|---|---|---|---|---|---|
| ADVIA 2120 | Manual | ADVIA 2120 | Manual | ||
| 109/L (%) | 109/L (%) | 109/L (%) | 109/L (%) | 109/L | |
| WBC BASO | 24.6b | 38.0b | 5.8-16.0 | ||
| WBC Perox | 18.2b | 22.2b | 5.8-16.0 | ||
| Neutrophils | 7.1 (29%) | 6.2 (34%) | 8.3 (22%) | 4.4 (20%) | 3.3-10.4 |
| Lymphocytes | 11.9 (49%) | 2.1 (12%) | 19.3 (51%) | 2.4 (11%) | 1.5-4.7 |
| Monocytes | 2.6 (11%) | 9.5 (52%) | 4.9 (13%) | 14.0 (63%) | 0.1-1.0 |
| Eosinophils | 0.6 (2.4%) | 0.4 (2%) | 2.0 (5.3%) | 1.1 (5%) | 0.2-1.6 |
| LUC | 2.3 (9.3%) | 2.9 (7.5%) | 0-0.1 | ||
- Abbreviations: LUC, large unstained cells on the ADVIA 2120; RI, reference interval; WBC BASO, white blood cell count from basophil channel; WBC PEROX, white blood cell count from the peroxidase channel.
- a The second sample was taken 17 days after the initial presentation and sampling. Both blood samples were mailed; the first sample was 1 day old and the second sample was 4 days old.
- b The instrument leukocyte alarms were WBC comparison error and peroxidase no-valley. ADVIA 2120 absolute leukocyte differential counts were based on the total leukocyte counts from the ADVIA BASO channel. Manual leukocyte subpopulations were calculated from the total leukocyte counts of ADVIA PEROX channel, which were considered more correct than those from the ADVIA BASO channel.
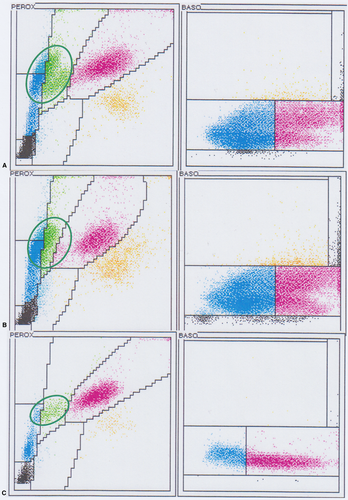
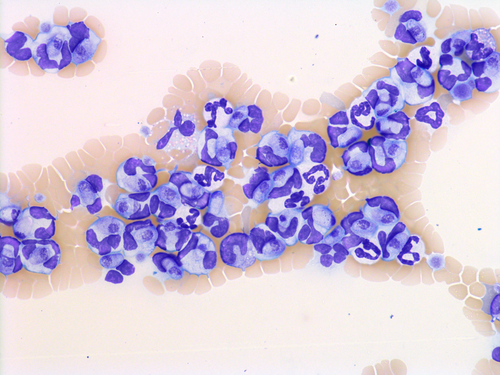
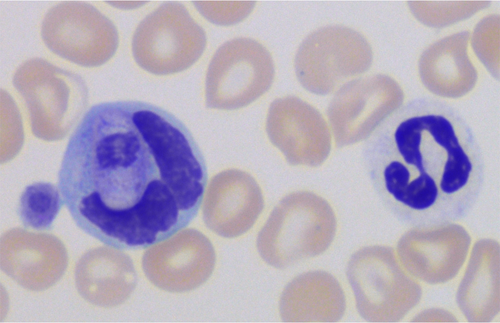
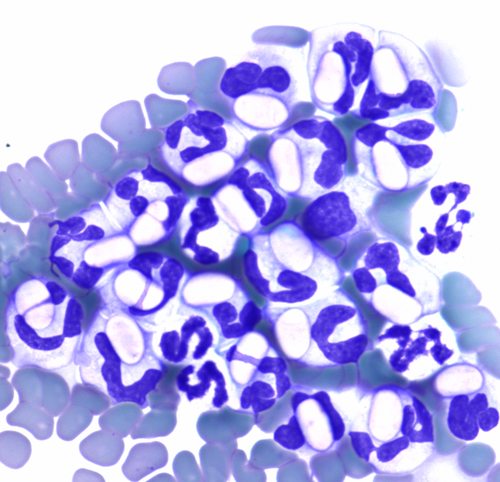
The infected leukocytes were considered to be monocytes based on morphology during blood smear review (Figures 2 and 3) and the location of these cells on the ADVIA WBC cytograms (Figure 1A, 1B). In the ADVIA PEROX cytogram, a single, large, and unusual leukocyte population appeared above the lymphocyte cluster on the y-axis, which indicated that these cells were larger than lymphocytes. This leukocyte population was quite far to the left along the x-axis, indicating weak peroxidase staining. Monocytes are often found in this area. The infected leukocytes often had very lobulated nuclear shapes but, based on the darker blue cytoplasm and more open chromatin pattern, these cells appeared to be monocytes. The initial Hemacolor-stained blood smears from the referring clinic had palely stained the H canis gamonts and leukocytes, which made differentiation of neutrophils and monocytes difficult (Figure 4).
The nuclear and cytoplasmic neutrophil morphologies appeared normal in both the ADVIA WBC PEROX cytograms and blood smears (Figures 1-3). The manual neutrophil count (34%) was similar to the one made with the ADVIA (29%), and the neutrophil and eosinophil cell clusters were present at the expected locations in the ADVIA PEROX cytograms.
The manual monocyte count in the monolayer area of the blood smear was 52% (9.5 × 109/L) in the first sample and 63% (14.0 × 109/L) in the next sample 17 days later, indicating a strong monocytosis. Both samples had a distinct large leukocyte population with weak peroxidase staining in the ADVIA PEROX cytogram near the location where monocytes are commonly found (Figure 1A,B). However, the ADVIA classified them into three cell-types: lymphocytes, monocytes, and large unstained cells (LUC). The second sample (17 days after initial presentation) was judged to contain 60.5% monocytes on the basis of: (a) the sum of ADVIA monocytes (13%); (b) the ADVIA LUC percent (7.5%); (c) an estimated portion (40%) of the larger cells in the lymphocyte counting area, which were likely monocytes; and (d) the subtraction of 11% lymphocytes identified with the manual count. This percentage (60.5%) was similar to the manually-derived count of 63%.
The ADVIA basophil (BASO) cytograms were difficult to interpret (Figure 1A,B). There were two long, linear, and parallel cell populations. The upper population was more abnormally positioned relative to the lower one and resembled a “mirror image” of the lower population. The lower population looked quite typical in form and location. The densest leukocyte cluster, representing the largest number of cells, was seen in the mononuclear cell area (to the left in the lower cell population) and not in the neutrophil area in the BASO cytogram. The BASO x-axis histogram confirmed that the greatest number of cells were in the mononuclear cell area (Figure S2). The second upper population appeared quite abnormal and unusual. This cell cluster was diffuse and elongated. It was located higher on the y-axis, indicating that these particles were larger than what would be seen with normal leukocytes after the addition of the ADVIA BASO reagent to remove cytoplasm. The WBC BASO count was much greater than the WBC PEROX count. The PEROX cytogram appeared more typical; therefore, the total WBC count from the PEROX channel was judged to be the more accurate of the two measurements. Using the same sample, the Medonic impedance WBC count was 19.3 × 109/L, which was more similar to the ADVIA WBC PEROX count than the WBC BASO count. The Medonic WBC histogram also showed an unusual cell population that was larger than neutrophils and found more to the right in the histogram (Figure S1) and likely contained parasitized leukocytes.
After three treatments with imidocarb dipropionate every other week, the number of infected leukocytes had decreased to 5% of all leukocytes. Two weeks thereafter, none were seen in blood smears. The dog remained healthy until a tumor developed under its tongue, which led to euthanasia 1 year after the H canis infection.
2 DISCUSSION
Hepatozoon canis infection is infrequently seen in Swedish dogs; it is usually only reported to occur in dogs from other countries or in dogs that have traveled to endemic countries.2 Dogs become infected by ingesting an infected tick. The tick vectors for H canis—Rhipicephalus sanguineus, R turanicus, and Amblyomma ovale—are not naturally present in Sweden. Sporadic findings of R sanguineus have been described, probably originating from ticks that have been imported by dogs, people, or birds.3 Foxes are suspected vectors, but Hepatozoon sp. infection has not been reported in Swedish foxes. Furthermore, Ixodes ricinus, the common tick that infests Swedish dogs, is not a vector for H canis.4 The 10-year-old dog in this study was born in Sweden and, according to the owners, had not been abroad, so the finding of Hepatozoon gamonts in the blood sample was highly unexpected. The source of infection, in this case, is unknown.
Hepatozoon canis gamonts were found in the blood monocytes of this dog but were not detected in neutrophils. In most reports, H canis gamonts are described in neutrophils,5-8 although in one study H canis infection in both neutrophils and monocytes.9 Canine monocytes have been reported to be coinfected with H canis and Ehrlichia canis.10 In another study, the infected leukocytes were described as myeloperoxidase-negative neutrophils.11 Although some studies do not specify which leukocyte type is infected,12 other studies have tried to identify which type of leukocyte is infected. Makimura et al stained infected dog leukocytes with α-naphthyl acetate esterase (ANAE) and chloroacetate esterase (CAE).13 The infected leukocytes were ANAE positive and CAE negative, which is the characteristic staining pattern of monocytes.
Another study examined the ultrastructure and cytochemical characteristics of H canis-infected leukocytes in 58 canine blood samples.14 The infected leukocytes were ANAE, CAE, peroxidase (PER), and leukocyte alkaline phosphatase (LAP) negative, which does not agree with the characteristics of neutrophils (CAE, PER, and LAP positive, and ANAE negative) or monocytes (CAE, PER and LAP negative, and ANAE positive). In dogs, myeloperoxidase is present in the primary granules from the promyelocyte stage to the segmented neutrophil stage, and CAE activity is present at all of the canine neutrophil maturation stages.15 We do not expect that infected leukocytes would be neutrophils that have lost PER and CAE activity. The American canine hepatozoonosis, Hepatozoon americanum was described to infect macrophages in skeletal muscle cysts and granulomas, as well as in peripheral blood based on morphology and immunohistochemical reactions.16
The extent of parasitemia can vary considerably in dogs with H canis infection.17 Leukocyte counts are often within RIs in dogs with low parasitemia and increased in dogs with many infected leukocytes.17 The dog in our case study had Hepatozoon gamonts in most of its monocytes, and the infection induced a profound monocytosis; however, this report describes only a single case.
Hepatozoon canis gamonts (Figures 2 and 3) showed great morphologic detail with May-Grunwald Giemsa staining. However, H canis in a blood smear stained with Hemacolor (Figure 4) had much less detail, and the oval gamonts appeared empty; the morphological differences between neutrophils and monocytes were less apparent when the blood was stained with Hemacolor. Diff-Quick staining has also been reported to reveal fewer morphological features of Hepatozoon sp. gamonts than a Giemsa stain in another canine case.18
The H canis infection in our case study caused several errors on both hematology instruments. On the Medonic cell counter, the infected monocytes were classified as granulocytes, but many appeared to be larger than the neutrophils and were located further to the right than where neutrophils normally appear on the WBC histogram. The ADVIA automated differential WBC count indicated a lymphocytosis, but the manual differential WBC counts indicated normal numbers of lymphocytes. A common error with the ADVIA automated differential counts on canine blood is that a single monocyte cluster in the cytogram is divided into lymphocytes, monocytes, and LUC.19, 20 Monocytes are usually present in such low numbers that this error goes unnoticed. And, because this dog had a very prominent monocytosis that comprised most of the cells in this cluster, the error was observed. Basophils, mast cells, immature lymphocytes, and macrophages can all be present in this area, but these cells were not seen in this dog's blood smears.
The second upper population in the ADVIA BASO channel was considered abnormal, and this pattern has not been previously reported. Leukocytes in the ADVIA BASO channel are pretreated with a lysing reagent that removes the cytoplasm. The remaining particles in canine blood are mainly due to the nuclei, the size of which is shown on the y-axis. Increasing optical density or “complexity” of that nucleus is shown along the x-axis. The unusual upper population was located higher on the y-axis, indicating that these structures were larger than normal leukocytes lacking cytoplasm. These abnormal cells/particles could have been free of the gamonts or parasites in what remained of the leukocytes after the lysing reagent was applied.
In conclusion, this Swedish dog had an extensive infection with H canis gamonts, apparently in the monocytes. The high level of parasitemia and strong monocytosis induced several errors in the readings on two hematology analyzers.
DISCLOSURE
The authors have indicated that they have no affiliations or financial involvement with any organization or entity with a financial interest in, or in financial competition with, the subject matter or materials discussed in this article.




