Hematologic and biochemical variables of hedgehogs (Erinaceus europaeus) after overwintering in rehabilitation centers
Abstract
Background
Information about laboratory reference intervals (RIs) of European Hedgehog (Erinaceus europaeus) hospitalized at rehabilitation centers is scarce.
Objective
The purpose of this study was to establish hematologic and biochemical RIs for rehabilitated hedgehogs before the release into the wild, and to assess whether sex and management of the center influence laboratory results.
Methods
Blood was collected from 50 hedgehogs at 3 centers. Thirty-eight animals were included in the study based on normal body weight, absence of clinical signs of disease, Bunnell index > 0.80, and absence of hibernation during overwintering. CBCs were performed using an automated laser cell counter followed by morphologic analysis of blood smears. Clinical biochemistry was performed using an automated spectrophotometer. RIs were determined as recommended by the ASVCP guidelines.
Results
Hematology profiles revealed a prevalence of lymphocytes, a constant presence of nucleated RBCs, Howell–Jolly bodies and basophils, and bilobed nuclei in neutrophils and eosinophils. Biochemistry profiles were characterized by higher creatinine and urea concentrations, and higher ALP and GGT activities compared with other domestic species. The sex did not influence the results. Conversely, numbers of eosinophils, activated and large granular lymphocytes, and concentrations of total protein, glucose and cholesterol were different among the centers, likely due to different management practices (eg, antiparasitic treatments, environmental exposure to microorganisms, diet).
Conclusion
The RIs established in this study can be used to monitor the health status of hedgehogs in rehabilitation centers. As management practices appeared to influence some variables, it is recommended to standardize the management protocols to minimize their influence on laboratory data.
Introduction
The European Hedgehog (Erinaceus europaeus) is a widespread wild insectivorous mammal in Italy and the rest of Europe. The absence of natural predators and the protection by law contributed to a wide distribution of these animals. Nevertheless, due to environmental changes such as the extensive use of pesticides resulting in a reduced availability of insects or other natural food resources for hedgehogs, together with a continuing elimination of rural areas, many hedgehogs are now living in human residential areas where they are exposed to risks such as respiratory and neoplastic diseases probably associated with environmental pollution, and traumas due to getting hit by cars. The breeding and detention of hedgehogs is prohibited by law in Italy, thus rescued sick hedgehogs must be rehabilitated in officially recognized centers for wild animal rescue (eg, WWF oasis or veterinary clinics), and, after hospitalization and overwintering in such centers, the animals must be released back into the wild.
One of the main causes for hospitalization are weak and underweight young animals born in autumn, which will probably not survive hibernation.1, 2 Other common problems at admission are heavy infestation by ecto- and endoparasites, particularly fleas and ticks,3 potentially causing blood loss anemia,4 and parasitic bronchopneumonia due to Capillaria aerophila and/or Crenosoma striatum. Moreover, hedgehogs can harbor zoonotic agents such as Salmonella spp., Yersinia pseudotubercolosis, Rhabdovirus, and Trychophyton mentagrophytes.5 In general, any deviation from normal and healthy can result in hematologic and biochemical changes. However, despite the high number of hedgehogs that are hospitalized in private clinics or in rehabilitation centers, it can be challenging for practicing veterinarians to assess the basic health status, and to diagnose or monitor specific diseases of rescued hedgehogs, not last to assess whether previously hospitalized animals can be safely released. These problems are in part due to the lack of information about hematologic and biochemical reference intervals (RIs) of normal healthy hedgehogs.
In the literature, the majority of studies published on hedgehogs refers to parasitic disease,3, 5-7 and only 2 studies report laboratory variables.1, 8 The goal of this study was to establish RIs for European hedgehogs following the guidelines published by the American Society of Veterinary Clinical Pathology (ASVCP).9 These RIs should be useful for future assessment of laboratory results of sick and hospitalized hedgehogs. In addition, we investigated whether sex or management of the rehabilitation centers show an influence on hematologic and biochemical variables in hedgehogs overwintering there.
Materials and methods
Animals and samplings
Demographics of the reference population
All animals included in this study came from 3 different rehabilitation centers located in Northern Italy, in a region characterized by a mild climate at the beginning of autumn (mean temperature up to 20°C) influencing the reproductive cycle to a late parturition at the end of the summer.
Initially, blood was collected from a total of 50 hedgehogs (all belonging to E europaeus species). Specifically, 2 groups of hedgehogs came from 2 different rehabilitation centers (center 1, n = 14 and center 2, n = 24), where the animals were hospitalized at the beginning of autumn. Although it was not possible to exactly determine the age of these hedgehogs, they were likely juveniles (less than 3 months old when captured in autumn), as they were found during daytime, while normal adults are active at night only. The third group of hedgehogs were born in captivity in a veterinary clinic (center 3; n = 12) at the beginning of autumn, where they were also overwintered.
Upon arrival at the rehabilitation centers, the 38 animals in centers 1 and 2 underwent a complete physical examination, in addition fecal samples were collected for parasitologic examination. Out of 50 animals, 41 were sexed in the 3 centers. All animals were weighed to assess their chances to survive hibernation. Hedgehogs weighing < 650 g are considered at risk to survive hibernation.10 The Bunnell index (BI), a measurement of the ratio between longitudinal and latitudinal circumferences measured when animals are tightly curled, was also assessed. Hedgehogs with a BI > 0.80 are considered fit for survival of hibernation after release.10 Underweight hedgehogs with a BI < 0.8 were prevented from hibernation by maintaining them indoors at temperatures > 8°C. All animals were maintained in cages in groups of 4 or 5 animals, or, if necessary, they were kept alone to facilitate the administration of treatments. In all 3 institutions, they were fed a mixture of dry and wet commercial cat food (Adult fit 32; Royal Canin, Aimargues, Camargue, France, or Le Chat; Monge, Monasterolo di Savigliano, Cuneo, Italy) at approximately 20–30 g/day. In addition, apple slices and water ad libitum were offered at variable intervals according to different caretakers.
Hedgehogs from center 3 received a complete physical examination at birth. They were bottle-fed with commercially available powdered milk substitutes designed for kittens (Esbilac, PetAg; Hampshire, IL, USA) from days 2–28 every 3 hours, followed by a gradual weaning period coupled with the introduction of adult food. After artificial weaning, hedgehogs from center 3 were managed as in the other rehabilitation centers, with the only difference that they received a preventive anti-parasitic treatment with 25 mg/kg levamisole (Ascarilen; Teknofarma, Torino, Italy) by hypodermic injection, and 100 mg/kg oral febendazole (Panacur; Intervet/Schering-Plough Animal Health, Milton Keynes, UK). As mentioned earlier, underweight hedgehogs or animals with a BI < 0.8 were prevented from hibernation. Starting spring season each animal was weighed and given a thorough additional physical examination, including determination of BI.
Inclusion and exclusion criteria
Establishing the health status and exclusion criteria in a wildlife species is particularly challenging, given the lack of contact with the animals and the limited access to a clinical history. The inclusion criteria for this study were (1) the absence of external pathologic signs or evidence of disease at the second visit starting spring season just before release, (2) a target bodyweight between > 0.650 < 1.5 kg (hedgehogs weighing > 1.5 kg are considered obese,7 a frequent effect of artificial feeding and an exclusion criteria), (3) a BI > 0.80 (defined cut-off for release of hedgehogs to the wild),10 and (4) the absence of hibernation during overwintering.
Preanalytical methods
Blood samples were collected in 2009 before the hedgehogs were released into the wild. To avoid any possible influence of circadian changes, all the samplings at a given center were performed at the same time of day. Unfortunately, it was not possible to consistently fast hedgehogs before blood collection. Blood samples were collected at center 1 and 2 on different days of the same week in March. In center 2, samples were collected during 2 monthly work sessions in March and April, and in center 3, samples were collected in 2 monthly work sessions in May and June.
Blood sampling was performed under general anesthesia, induced and maintained with 2% of isofluorane (Isoflurane Vet FL VT 250 mL; Merial, Duluth, GA, USA), mixed with oxygen and administered through a facemask. Blood samples were taken from the jugular vein using a 2.5 mL syringe with a 22 ga needle, following a modification of the indications by Lewis et al.1 Immediately after sampling, the blood was divided into 2 aliquots, appr. 0.5 mL were placed into an EDTA tube (Miniplast; LP Italiana S.p.A., Milano, Italy) for the CBC, and approximately 1 mL was transferred into a plain tube (Vacuette; Greiner Bio-One GmbH, Kremsmuenster, Austria) to obtain serum by centrifugation for the biochemical analyses. Tubes were refrigerated at +4°C and then transported to the laboratory within 24 hours. Immediately after arrival, the CBC was performed, and plain tubes were centrifuged at 453g for 15 minutes. Serum was transferred to plain conic tubes (Eppendorf, Hamburg, Germany) and frozen at −20°C to be analyzed within 3 months.
Analytical methods
Hematology
The CBC was performed using an automated laser hematology analyzer (Sysmex XT-2000iV; Sysmex Co., Chuo-ku, Kobe, Japan) equipped with a multispecies software for veterinary use. The following variables were measured: HGB, RBC count, MCV, WBC count, platelet (PLT) count (by both an impedance method, PLT-I, and an optical method, PLT-O). Plateletcrit (PCT), HCT, MCH, and MCHC were calculated automatically from the impedance counts.11 Quality control and calibration were periodically performed with e-check Xe (Sysmex Co.). As the Hedgehog is not included in the multispecies software of the instrument, the “other species” setting was used. Using this approach, the differential WBC count was based on a non-species-specific gating that could be manually re-gated to identify WBC subpopulations. In addition, manual differential WBC counts were performed by 2 independent observers on at least 200 WBC on blood smears stained with May–Grünwald Giemsa.
During the microscopic analysis of blood smears, all blood cells were examined, namely the percentage of nucleated RBCs (nRBCs), the presence of Howell–Jolly bodies, the presence of pleomorphic RBC (eg, anisocytosis or poikilocytosis), quantity and morphology of PLT (size, shape, granularity), WBC morphology (eg, bilobed nuclei of neutrophils and eosinophils, activated lymphocytes or macrophages). In addition, relative reticulocyte counts/per 1000 RBC were determined on smears made with 50 μL of blood mixed with 50 μL of brilliant cresyl blue, and after incubation at 37°C for 20 minutes.
Clinical chemistry
Biochemistry profiles were performed using an automated chemistry analyzer (Cobas Mira; Roche Diagnostics, Basel, Switzerland) and reagents provided by Real Time Diagnostic System (Viterbo, Italy). Quality control was performed with 2 levels of human control serum samples (Precinorm U and Precipath U; Real Time Diagnostic System) before each assay, and calibration was performed with human based calibrators (Calibrator; Real Time Diagnostic System). The serum concentration or activity of the following analytes was measured: GGT kinetic IFCC method), urea (urease method), glucose (GOD-POD method), total protein (biuret method), albumin (bromochresol method), ALP (kinetic IFCC method), creatinine (Jaffè method), ALT (kinetic IFCC method), calcium (ortho-cresophtalein method), inorganic phosphate (molibdate method), CK (CK-NAC method), triglycerides (GOD-PAP method), and cholesterol (CHOD-POD method).
Statistical analysis
RIs were determined using an Excel (Excel; Microsoft Corp., Redmond, WA, USA) spreadsheet with the Reference Value Advisor (version 2.0) set of macroinstructions12 that performs computations following the IFCC-CLSI recommendations as also suggested by ASVCP guidelines.9 The computations include common descriptive statistic (eg, sample size, mean, median, SD, and minimum and maximum values), test of normality according to Anderson–Darling with histograms and Q-Q- plots, and Box–Cox transformation. To detect outliers, 2 different tests were used: Dixon–Reed and Tukey's test. Following the ASVCP guidelines,9 outliers classified as “suspected” by the software were retained. Conversely, far outliers were removed from the analysis. Calculation of RIs was based on the assumption about data distribution and multiple RIs are reported, obtained by using standard and robust methods on both nontransformed and transformed data. When needed (ie, when based on the number of animals per group and on the distribution of data, the software was unable to calculate the RI), data were log-transformed before analysis. With the sample size 20 ≤ x < 40, a nonparametric bootstrap method was used to calculate the 90% confidence interval (CI).
Variables in males were compared with females using an Excel spreadsheet with the Analyse-it set of macroinstructions (Analyse-it software, version 2.21; Analyse-it Software Ltd, Leeds, UK). Specifically, a nonparametric t-test was used for unpaired data (U Mann–Whitney), while the comparison between the results recorded in the 3 centers was assessed using a nonparametric ANOVA test (Kruskall Wallis) followed by a Bonferroni test.
Results
Thirty-eight hedgehogs fulfilled the inclusion criteria described above. For 4 animals, EDTA blood was unavailable or inadequate; therefore, hematologic RIs were determined for 34 samples. In 2 cases, there was no blood smear available, thus WBC differential counts were performed on 32 samples. Biochemical analyses were performed in 30 hedgehogs, as 8 samples were excluded due to insufficient sample size or preanalytical factors such as hemolysis or lipemia. Calcium and phosphate were measured in the 26 hedgehogs from centers 1 and 2, but not in the animals at center 3. Among the 12 excluded hedgehogs (8 from center 2 and 4 from center 3), 3 animals were not underweight at admission and went into hibernation, and 9 animals were sick after overwintering (eg, cranial trauma, bone fracture, respiratory disease, neurologic deficit, cachexia, and bleeding disorders), had para-physiologic conditions such as obesity (2 cases) or were pregnant.
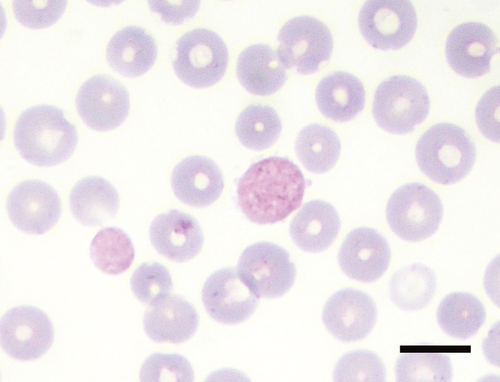
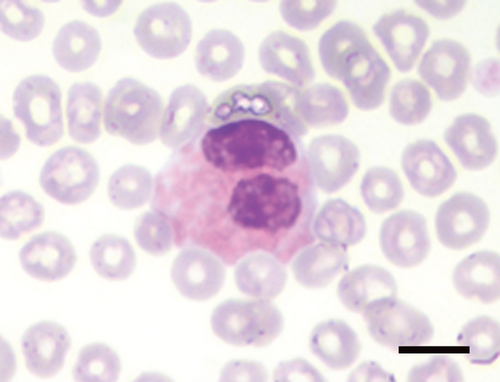
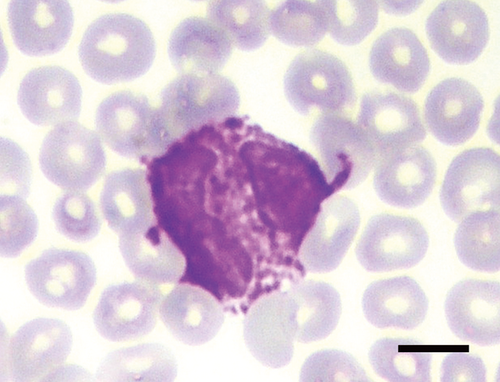
Precision data of the Sysmex variables are reported in the supplemental Table S1. Blood smear examination revealed that almost all hedgehogs had up to 6% nRBCs, and between 1 and 14 Howell–Jolly (H–J) bodies per 20 high power fields (HPF, Table 1, Figure S1). PLT-O counts were higher than PLT-I counts (data not shown). In this study, > 8–10 platelets per HPF were considered adequate. In addition, large pleomorphic hypergranular platelets (compared to platelets in common domestic species) were noticed on smears (Figure 1), while actual platelet clumps were rare (0–2/smear). The differential WBC counts revealed a prevalence of lymphocytes, which occasionally appeared as large granular lymphocytes (LGL) or as lymphocytes with hyperbasophilic cytoplasm (activated lymphocytes). Up to 10% basophils were counted (Figure 2), and up to 10% of neutrophils and eosinophils had bilobed nuclei (Figure 3).
| Analyte | SI Units | N | Mean | SD | Median | Min | Max | RIa | Lower Ref Lim 90% CI | Upper Ref Lim 90% CI | Dist.b | Methodc |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HCT | L/L | 34 | 0.32 | 0.04 | 0.33 | 0.24 | 0.39 | 0.25–0.41 | 0.23–0.27 | 0.39–0.42 | G | R |
| RBC | 1012/L | 34 | 8.1 | 1.1 | 8.2 | 5.6 | 10.2 | 6.0–10.4 (S) | .5.4–6.5 | 9.8–10.9 | G | R |
| Hemoglobin | g/L | 34 | 115.6 | 16.1 | 116.5 | 83.0 | 146.0 | 83.7–149.3 | 76.1–92.3 | 141.8–156.3 | G | R |
| MCV | μm3 | 34 | 40.0 | 2.7 | 39.9 | 34.7 | 45.8 | 34.5–45.3 | 33.5–35.8 | 44.0–46.6 | G | R |
| MCH | pg/cell | 34 | 14.2 | 0.7 | 14.3 | 12.5 | 15.8 | 12.7–15.8 (S) | 12.4–13.1 | 15.4–16.1 | G | R |
| MCHC | g/L | 34 | 357.0 | 20.7 | 356.5 | 299.0 | 401.0 | 312.9–400.0 (S) | 302.0–323.8 | 385.8–412.0 | G | R |
| Reticulocytes | % | 34 | 1.8 | 1.3 | 1.5 | 0.2 | 5.2 | 0.2–5.0 (S) | 0.0–0.3 | 3.8–6.3 | NG | RT |
| Reticulocytes | 109/L | 34 | 144.6 | 111.2 | 122.1 | 15 | 438.7 | 10.7–423.8 | 3.2–23.4 | 318.4–539.6 | NG | RT |
| nRBCs | % | 32 | 2.2 | 1.7 | 1.8 | 0.0 | 6.0 | 0.1–6.5 (S) | 0.0–0.3 | 4.4–8.6 | NG | RTd |
| nRBCs | 106/L | 32 | 0.18 | 0.15 | 0.13 | 0.00 | 0.76 | 0.01–0.63 | 0.00–0.03 | 0.40–1.11 | G | RT |
| WBC | 106/L | 34 | 8.4 | 2.9 | 8.1 | 3.0 | 14.7 | 2.2–13.9 | 1.1–3.5 | 12.3–15.7 | G | R |
| Neutrophils | % | 32 | 35.1 | 9.0 | 34.6 | 19.7 | 57.3 | 16.2–53.1 | 11.6–20.6 | 47.6–58.0 | G | R |
| Lymphocytes | % | 32 | 50.0 | 9.4 | 50.4 | 29.1 | 69.3 | 31.2–69.6 | 26.7–36.4 | 65.0–75.0 | G | R |
| Monocyte | % | 32 | 2.7 | 1.6 | 2.6 | 0.0 | 6.3 | 0.1–7.5 | 0.0–7.6 | 5.9–9.1 | G | RTd |
| Eosinophils | % | 31 | 7.5 | 4.9 | 6.7 | 0.0 | 20.9 | 0.0–12.0 (S)(R) | 0.0–0.0 | 8.5–16.3 | NG | RTd |
| Basophils | % | 32 | 3.5 | 2.1 | 3.3 | 0.4 | 10.0 | 0.4–8.7 | 0.2–0.9 | 7.2–10.6 | NG | RT |
| Neutrophils | 106/L | 32 | 2.7 | 1.2 | 2.5 | 0.9 | 6.1 | 0.9–5.9 | 0.8–1.2 | 4.8–7.2 | NG | RT |
| Lymphocytes | 106/L | 32 | 4.0 | 1.6 | 3.9 | 1.5 | 7.8 | 0.6–7.2 | 0.0–1.4 | 6.2–8.2 | G | R |
| Monocyte | 106/L | 32 | 0.2 | 0.1 | 0.2 | 0.0 | 0.6 | 0.0–0.6 | 0.0–0.0 | 0.5–0.7 | NG | RT |
| Eosinophils | 106/L | 31 | 0.6 | 0.0 | 0.5 | 0.0 | 2.4 | 0.0–2.2 (S)(R) | 0.0–0.9 | 1.6–3.0 | NG | RTd |
| Basophils | 106/L | 32 | 0.3 | 0.2 | 0.3 | 0.0 | 0.6 | 0.0–0.7 | 0.0–0.1 | 0.6–0.8 | NG | RT |
| Platelets (optical) | 106/L | 34 | 230.7 | 102.6 | 224.2 | 48.0 | 462.0 | 48.9–469.4 (S) | 12.4–86.1 | 403.1–523.7 | NG | RTd |
- Ref Lim indicates reference limit, Dist., distribution; nRBCs, nucleated RBCs.
- a (S) indicates that suspected outliers were present; (R) indicates that far outliers were identified and removed.
- b G, Gaussian, NG, non-Gaussian.
- c Method: R, robust; T, transformed, data transformed to Gaussian prior to applying parametric or robust methods.
- d Log-transformed before analysis of reference intervals.
Precision data of biochemical variables are reported in the supplemental Table S1. They were characterized by high activities for ALP and GGT, and high concentrations of creatinine and urea, when compared with common domestic species (Table 2, supplemental Figure S1).
| Analyte | SI Units | N | Mean | SD | Median | Min | Max | RIa | Lower Ref Lim 90% CI | Upper Ref Lim 90% CI | Dist.b | Methodc |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Calcium | mmol/L | 30 | 1.7 | 0.7 | 1.8 | 1.1 | 3.3 | 1.3–3.2 (S)(R) | 0.8–1.7 | 2.9–3.3 | NG | RT |
| Phosphate | mmol/L | 26 | 2.2 | 0.5 | 2.1 | 1.5 | 3.5 | 1.5–3.5 (S) | 1.4–1.6 | 3.0–4.1 | NG | RT |
| Urea | mmol/L | 30 | 14.7 | 3.1 | 14.3 | 10.1 | 23.2 | 7.9–20.7 (S) | 6.3–10.0 | 18.8–22.7 | G | R |
| Creatinine | umol/L | 27 | 57.8 | 36.2 | 48.6 | 20.3 | 187.4 | 19.9–175.3 (S)(R) | 16.4–25.5 | 117.9–265.8 | NG | RT |
| GGT | U/L | 30 | 17.7 | 9.6 | 16.6 | 6.8 | 41.9 | 5.2–47.5 | 4.1–6.9 | 38.0–64.2 | NG | RT |
| ALP | U/L | 29 | 141.4 | 48.0 | 135.2 | 77.0 | 253.0 | 68.3–255.7 (R) | 60.6–82.2 | 215.8–292.6 | NG | RT |
| ALT | U/L | 29 | 81.0 | 37.5 | 78.0 | 46.0 | 174.0 | 43.3–194.3 (S)(R) | 39.4–49.5 | 153.9–247.0 | NG | RT |
| Glucose | mmol/L | 30 | 5.9 | 0.8 | 5.9 | 4.3 | 7.3 | 4.3–7.5 | 7.0–4.7 | 7.0–7.8 | G | R |
| Triglyceride | mmol/L | 29 | 0.36 | 0.15 | 0.34 | 0.11 | 0.72 | 0.12–0.75 (R) | 0.09–0.17 | 0.62–0.91 | G | RT |
| Cholesterol | mmol/L | 30 | 3.7 | 0.8 | 3.5 | 2.4 | 5.3 | 2.0–5.3 | 1.6–2.4 | 4.8–5.7 | G | R |
| Total protein | g/L | 30 | 68.8 | 10.2 | 67.4 | 54.1 | 100.5 | 44.4–88.3 (S) | 39.3–52.9 | 81.5–95.9 | G | R |
| Albumin | g/L | 30 | 35.0 | 3.4 | 34.7 | 30.3 | 45.7 | 27.3–41.7 (S) | 25.1–29.4 | 39.3–43.9 | G | R |
| Globulin | g/L | 30 | 33.8 | 7.6 | 32.7 | 23.7 | 54.8 | 17.1–48.3 (S) | 13.3–21.7 | 43.2–53.7 | G | RT |
| A:G ratio | 30 | 1.07 | 0.17 | 1.06 | 0.73 | 1.44 | 0.71–1.41 | 0.63–0.80 | 1.31–1.51 | G | R |
- Ref Lim indicates reference limit, Dist. Distribution.
- a (S) indicates that suspected outliers were present; (R) indicates that far outliers were identified and removed.
- b G, Gaussian; NG, non-Gaussian.
- c Method: R, robust; T, transformed, data transformed to Gaussian prior to applying parametric or robust methods.
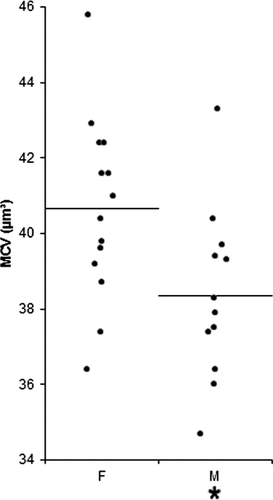
There were no significant differences between males and females, with the exception of a higher MCV in females than in males (Figure 4). Conversely, significant differences related to the management were observed (Figure 5). Specifically, the MCH was significantly higher in hedgehogs at center 3 than at center 1, and nRBCs were more numerous in animals at center 3 than at centers 1 and 2. Relative eosinophil counts were lower in animals at center 3 than at centers 1 and 2.
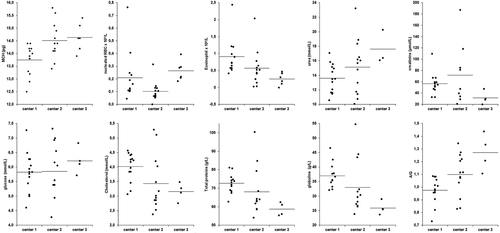
Among biochemical analytes, cholesterol concentrations were significantly lower in hedgehogs at centers 2 and 3 compared with animals at center 1, urea concentration was significantly higher in animals at center 3 than at center 1, and glucose concentration was significantly higher in hedgehogs at center 3 compared with animals at centers 1 and 2 (Figure 5). Creatinine concentration was higher in animals at center 2 than at the other 2 centers, although only the difference in comparison with animals at center 3 was statistically significant. The concentrations of total protein and total globulin were higher and the A/G ratio was lower in animals at center 1 compared with hedgehogs at the other 2 centers (Figure 5).
Discussion
The establishment of RIs in wild animals like hedgehogs is difficult, due to the potential influence of poorly controlled parameters even in captivity. Often, exact age determination is impossible. In this study, all hedgehogs were likely juveniles (probably less than 3 months old when captured and then approximately less than 9 months old when sampled before release into the wild) and the age differences between animals at the 3 centers were balanced by the deferred blood collection. Conversely, circadian fluctuations should not have affected the results as blood was collected approximately at the same time of day in all 3 centers.
Stress associated with captivity and subclinical diseases can potentially affect the results. Therefore, in this study, samples were only collected from clinically healthy animals with normal physiologic parameters such as body weight and BI. Finally, a variable that could have influenced the results is the effect of general anesthesia. However, as anesthesia was performed in all groups, possible artifacts due to anesthesia (hypoglycemia, increased RBC mass) are unlikely to explain the differences reported in Figures 4 and 5.
Only a few reports about RIs of hedgehogs are available.1, 8 However, published hematology data were obtained using instruments and sampling sites (medial saphenous vein) different from that of our study; the only report on clinical chemistry does not provide information about the reference population or about the method employed to calculate the RI. Due to these limitations, it is difficult to statistically compare our results with those reported in literature. Nevertheless, hematologic RIs recorded in this study were similar to those of previous reports.1, 8
The most interesting findings were the high number of nRBCs, H–J bodies, and reticulocytes. In most species, these findings are suggestive of erythroid regeneration in response to erythropoietin release or of marrow-blood barrier damage and inappropriate erythroblastosis in the case of nonregenerative anemia.13-15 Hedgehogs have a nonsinusoidal spleen16 and this may explain the presence of H–J bodies, as demonstrated for horses and cats.13 Although these findings may depend on the juvenile age of the animals included in the study (in most species young animals have an intense erythropoiesis) or on splenic contraction due to stress, it is possible that RBCs in hedgehogs are released to blood earlier than in other species. Additional studies on mechanisms of erythropoiesis in hedgehogs are needed for a better understanding of these observations.
The automated PLT-I count, used in many veterinary diagnostic laboratories, underestimates the real number of platelets, likely due to the overlapping between small RBC and large PLT, a well-described phenomenon in cats.17 This hypothesis is confirmed by the detection of large hypergranular, polymoprhic platelets on blood smears, in the absence of excessive platelet clumping, and by the higher PLT-O (that differentiate large PLT from small RBCs), which provided counts similar to those reported for other species.18-20 The platelecrit (PCT) would probably provide a more accurate estimate of platelet mass in hedgehogs, as demonstrated in dogs.21 However, automated instruments do not accurately count large PLTs.22 In our hedgehogs, the instrument reported a flag of “abnormal” platelet morphology instead of the actual mean platelet volume or PCT in almost all cases; therefore calculation of an RI for this variable was not possible. Consequently, in the absence of PLT-O counts, the microscopic assessment of platelets on blood smears may represent the best method to estimate PLT numbers in hedgehogs.
WBC counts were similar to those previously reported1 and the differential count revealed a relative prevalence of lymphocytes, as established in other species, such as rats, mice,23 and ruminants.24 There were some LGL, which have been considered cytotoxic lymphocytes, at least in cattle;24 alternatively, the granulation could also indicate immune stimulation.25
An interesting finding were the relatively high relative and absolute basophil counts, which, in other species, have been associated with allergic conditions26 or helminthiasis.27 Whether this is a reasonable explanation in these study animals is questionable in the face of negative fecal examinations and regular antiparasitic treatments.28
In most species, the number of nuclear lobes increases with the maturation stage of neutrophils and eosinophils.29 Cells with bilobed nuclei could thus be erroneously interpreted as immature cells. Nevertheless, their morphology in hedgehogs was different from that of nonsegmented immature granulocytes. Therefore, they could be considered as normal mature cells with an atypical nuclear shape as it occurs in Pelger–Huet anomaly.30
The MCV was higher in females than in males, as already reported,1 but the RIs from both genders were largely overlapping, suggesting that this difference is significant from a statistical standpoint but not biologically relevant, as it does not affect the interpretation of the hemogram.
The RIs for biochemical values were generally wider than those of previous reports8 and had higher upper reference limits. In other species, high creatinine and urea concentrations are consistent with renal insufficiency.31 Nevertheless, based on the lack of symptoms or of other laboratory changes, the high values recorded in hedgehogs may depend on prerenal azotemia due to the protein overload associated with the use of commercial food for carnivores instead of a species-specific diet. Similarly, in spite of the high ALP and GGT activity, cholestasis is unlikely, as no other clinical or laboratory abnormalities were found. No information about ALP isoenzymes in hedgehogs is available. The bone isoenzyme, which is usually high in young animals due to bone remodeling,32 may be responsible for the high ALP activity. Alternatively, this may depend on the intestinal isoenzyme that mostly contributes to total ALP activity in rodents, especially in the case of fasting or damage.33
Significant differences were determined between animals managed in the 3 different centers, such as highest eosinophil counts in centers that did not practice anti-parasitic treatments, and lower total protein and globulin concentrations in center 3, which may depend on an overall cleaner environment, and consequently, less immune stimulation. Similarly, the dietary regimen may have influenced some metabolites, such as urea, glucose, and cholesterol concentrations induced by lipomobilization by a non-species-specific diet. Other differences were slight and probably biologically irrelevant (eg, nRBC, MCH) or attributable to the high individual variability (eg, creatinine).
In conclusion, the RIs determined in this study should be helpful in the monitoring and assessment of the health status of non-hibernized male and female hedgehogs in rehabilitation centers. Future studies on a larger number of animals with selected diseases are needed to assess the clinical diagnostic usefulness in routine practice of the RIs established in this study.
Acknowledgment
This study has been supported by the University grant PUR 2008 and by the European Social Fund (Fondo Sociale Europeo, Regione Lombardia), through the grant “Dote Ricerca.”
Disclosure: The authors have indicated that they have no affiliations or financial involvement with any organization or entity with a financial interest in, or in financial competition with, the subject matter or materials discussed in this article.




