Allogeneic peripheral blood haematopoietic stem cell transplantation for the treatment of dogs with high-grade B-cell lymphoma
Abstract
Autologous peripheral blood haematopoietic stem cell transplantation (HCT) cures 33%–40% of dogs with high-grade B-cell lymphoma. We hypothesized, based on human allogeneic bone marrow transplantation literature, that transplanting dogs using canine donor leukocyte-matched CD34+ cells would lead to fewer relapses and increased cure rates. We retrospectively reviewed medical records of dogs diagnosed with high-grade B-cell lymphoma who received an identical allogeneic HCT. A total of 15 dogs transplanted at four facilities were identified. Five of fifteen dogs relapsed before transplant. The mean number of donor CD34+ cells/kg harvested and infused into recipient dogs was 8.0 × 106/kg (range: 2.08 × 106/kg–2.9 × 107/kg). The median disease-free interval and overall survival of all dogs was 1095 days (range: 9–2920 days) and 1115 days (range: 9–2920 days), respectively. Two of five dogs, not in remission at transplant, died in the hospital. The median disease-free interval and overall survival of the remaining three dogs was 25 days (range: 15–250 days) and 1100 days (range: 66–1902 days), respectively. The median disease-free interval and overall survival of the 10 dogs who had not relapsed was 1235 days (range: 19–2920 days) and 1235 days (range: 19–2920 days), respectively. One dog died soon after discharge of presumed gastric-dilatation-volvulus. Eight of nine remaining dogs lived >4 yrs post-alloHCT, leading to a cure rate of 89%. Acute graft versus host disease was seen in three dogs. These results suggest that allogeneic HCT can cure ~50% more dogs than those treated with autologous HCT.
1 INTRODUCTION
An important early discovery, using the canine preclinical haematopoietic transplant research model, was that dogs have major histocompatibility complex (MHC) antigens, called dog leukocyte antigens (DLA), coding for class I and II genes, similar to human leukocyte antigen (HLA) expression.1, 2 Further studies in research dogs supported the notion that engrafted DLA-matched donor T-cells cause significant graft-versus-host-disease (GvHD), leading to a beneficial secondary graft-versus-lymphoma (GvL) response.3, 4 The subsequent research elucidating molecular techniques to accurately characterize these polymorphic loci5, 6 laid the groundwork for defining the genetics of HLA and the importance of HLA typing in human allogeneic transplants (alloHCT).
The use of haematopoietic stem cell transplantation (HCT) to treat client-owned dogs in a clinical setting with high-grade B-cell lymphoma (BCL) is still in its infancy. We've shown that 33% and 40% of dogs with BCL in the first remission are cured of their disease (defined as living >2 yrs post-autologous HCT [post-autoHCT]) and not dying from lymphoma) using either autoHCT or autoHCT in conjunction with adoptive T-cell therapy (ACT), respectively.7, 8 As such, relapse remains a significant problem. Since early studies showed client-owned dogs with BCL remained disease-free using identical alloHCT4 (using donor derived DLA-matched stem cells), provided GvHD complications could be managed, and the publication of a case report describing the cure of a client-owned dog with T-cell lymphoma using an alloHCT protocol,9 we began offering identical alloHCT to treat client-owned dogs with BCL. This procedure is made possible by the commercial availability of extremely accurate DLA-genotyping. In this case series we describe the feasibility, safety, and outcome of 15 identical alloHCT transplants in dogs diagnosed with BCL using peripheral blood CD34+ cells isolated from mobilized DLA-identical donors.
2 MATERIALS AND METHODS
2.1 Recipient dogs
Between 2006–2018, 15 dogs diagnosed with BCL underwent identical alloHCT at one academic veterinary hospital (North Carolina State University College of Veterinary Medicine) and three private practice specialty hospitals (Bellingham Veterinary, VCA West LA, MedVet Medical & Cancer Centers for Pets). One dog included in this study was previously described in separate case report.10 Most dogs were diagnosed and treated by the referring veterinarian and presented to the transplant facility for alloHCT. At diagnosis, staging of recipient dogs was performed according to the World Health Organization staging system for dogs with lymphoma. Immunophenotype assignment was determined using flow cytometry of LN FNAs, PARR, or both. Per the referring clinician's discretion, all recipient dogs received a multiagent chemotherapy protocol, such as CHOP, to induce clinical remission before transplant. Disease relapse was documented following the RECIST criteria (v1.0) of progressive disease. Twenty-four hours before TBI, all recipient dogs underwent gastrointestinal sterilization using neomycin sulphate (6 mg/kg PO q8h) and polymyxin B (8,333 IU/kg PO q8hrs) as previously described.7, 8 In addition, all dogs received immunosuppressive doses of cyclosporine (5 mg/kg PO q8hrs) which was continued until ~30d post-alloHCT, as previously described (Supplemental Figure 1).4, 9, 10
2.2 DLA genotyping and donor preparation
DNA Isolation. DLA genotyping was performed at the Fred Hutchinson Cancer Research Center Clonal Tracking and Canine Resource Development Laboratory. Genomic DNA was purified from 200 μl donor/recipient blood using a BioSprint 96 Blood kit (Qiagen, Valencia CA, USA) according to the manufacturer's instructions. DNA was eluted in 200 μl 10 mM Tris, pH 7.9 and quantified (NanoDrop spectrophotometer, Fisher Scientific, Pittsburgh, PA, USA). Final concentrations ranging from 20 to 100 ng/μl were used in PCR reactions.
DNA Amplification. Candidate donor/recipient pairs were matched at the hypervariable regions of the class I MHC gene, DLA-88, and the class II genes, DRB1, DQA1, and DQB1 in the canine major histocompatibility complex (MHC).11 Amplification of polymorphic regions of DLA-88 was performed essentially as described,11-13 with minor modifications of primer length and PCR conditions. Typically, primers 2924 and 2928 (200 nM each), or alternative primers, when the 2924/2928 primers did not yield a product (Supplemental Table 1), were used to amplify a 1.1 kb region, containing exons 2 and 3. Cycling conditions were: Pre-melt at 95°C for 5 min, hold 72°C for 15 sec, followed by 35 cycles of 95°C for 15 sec., 62°C for 30 sec., and 72°C for 90 sec., and a final extension at 72°C for 2 min. Amplification of the polymorphic exon 2 regions of DRB1, DQA1, and DQB1 was performed essentially as described for DRB113, 14 with gene-specific primers (Supplemental Table 1). As with DLA-88, the secondary primer sets were used when the initial PCR attempt failed. The primer positions for all four MHC genes are shown in Supplemental Figure 2. Cycling conditions for all three genes were: Pre-melt at 95°C for 5 min, followed by 35 cycles of 95°C for 15 s, 60°C for 30 s, and 72°C for 30 s, and a final extension at 72°C for 1 min. All target regions were amplified using a Gene Amp 9700 thermocycler (Applied Biosystems/ ThermoFisher Scientific Grand Island, NY). Thirty μl PCR reactions contained 20–100 ng genomic DNA, primers at 200 nM each, and 1X MyFi high-fidelity polymerase mix (Bioline USA Inc., Tauton, MA, USA). PCR products were separated by electrophoresis on 1.5% agarose gels, the predicted DNA bands excised and purified on QIAquick gel extraction columns (Qiagen, Valencia CA, USA), and the DNAs eluted in 50 μl10 mM Tris, pH 7.9.
Sequence Analysis and Allele Calls. Gel-purified PCR products were sequenced in both directions using amplification primers on a GeneAmp 9700 thermocycler using BigDye Terminator Cycle Sequencing Reagent (Applied Biosystems: Waltham, Mass., USA) using 2–4 μl of gel-purified product. Products were separated and analysed by capillary electrophoresis (ABI 3730xl DNA Analyzer, Applied Biosystems). Heterozygous base positions were recorded only when observed in both directions. In cases where the two alleles were not clearly identifiable by direct sequencing, the PCR products were cloned using a TOPO TA PCR cloning kit (Invitrogen: Carlsbad, CA, USA), and at least eight clones were sequenced for confirmation of specific alleles. Sequences were compared to the Fred Hutchinson data base of DLA alleles accumulated from the Immuno Polymorphism Database-MHC (IPD-MHC) (http://www.ebi.ac.uk/ipd/mhc/dla) and GenBank (http://www.ncbi.nlm.nih.gov/genbank) using the NCBI BLAST program (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
All DLA-matched donor dogs underwent routine general health screening which included physical examination, CBC, serum chemistries, and vector-borne disease screening. All dogs received a 2-week course of doxycycline before mobilization.
2.3 Allogeneic HCT
2.3.1 Donor dog mobilization and peripheral blood mononuclear cell collection
Donor dogs were mobilized using 5 μg/kg filgrastim (Neupogen, Amgen, Thousand Oaks, CA) SC q12h for 5 days, as previously described.7, 8, 15 Six of fifteen dogs also received 400 μg/kg of the CXCR-4 inhibitor plerixafor (Mozibil, Sanofi US, Bridgewater, NJ), in addition to filgrastim, ~10 h before apheresis,16, 17 based on clinician preference. Peripheral blood mononuclear cell (PBMNC) collections were performed using either a CobeSpectra or Spectra Optia Apheresis System, as the previously described.7, 8, 15 Two dogs required more than one procedure to collect an adequate number of CD34+ cells to ensure complete hematologic reconstitution. After the collection was complete, the donor dogs recovered uneventfully, had their jugular and peripheral catheters removed, and released from the hospital the same day.
2.4 Apheresis product analysis
Proportions of CD34+ cells of the final harvest products were determined by flow cytometry as previously described.7, 8, 15 If an adequate number of CD34+ cells/kg were harvested, 2–3 donor-lymphocyte infusions (DLI),18 at a dose of 1–2 × 107 CD3+ lymphocytes/kg, then were aliquoted and frozen in a 1:1 dilution of freezing medium as the previously described,15 for the purpose of inducing GvL in the setting of relapsed disease.19 The remaining apheresis product was refrigerated at 4°C without further manipulation until infusion one or two days later, immediately after the completion of total body irradiation (TBI).
2.5 Total body irradiation
Recipient dogs received TBI consisting of two 4 Gy fractions at a dose rate of 7.5–10 cGy/min administered with 6 MV photons from a Varian Clinac 1800, Varian Trilogy, or similar machines, as the previously described,20 although radiation setup protocols varied between facilities. TBI was considered day 0.
2.6 Post-alloHCT care
Immediately after TBI, the apheresis product was warmed to room temperature and administered intravenously over a period of 30 min to 1 h. Post-alloHCT care was essentially as the previously described.7, 8, 15 Toxicities are described using VCOG-CTCAE v2 guidelines. Post-transplant immunosuppression consisted of oral cyclosporine (CSP) at a dose of 5–10 mg/kg from day −1 to ~day +30 post-transplant. Weekly serum cyclosporine trough levels were drawn ~12 h post-dosing and doses were adjusted accordingly to keep the trough level in the therapeutic range of 400–600 ug/dl. To augment platelet recoveries, most dogs received either fresh whole blood, fresh leukoreduced platelets, platelet-rich plasma, DLI, or a lyophilized platelet product (StablePlate RX™, BodeVet, Rockville, MD), depending on the clinician's preference. Follow-up care included weekly recheck visits at the referring veterinarian for physical examination, CBC until platelets were > 100 000/μl, and chemistry panels to monitor for elevations in liver values. Thereafter, follow-up care was performed monthly for 12 months. In the event of persistent clinical signs consistent with progressive cutaneous acute GvHD (aGvHD) dogs were restarted on cyclosporine until a complete response was seen and then were slowly weaned off for 1–3 months.
2.7 Post-HCT donor chimerism analysis
Chimeric ratios were determined by Variable Number Tandem Repeats (VNTR) analysis by the Clonal Analysis Lab in the Core Center of Excellence in Molecular Haematology at the Fred Hutchinson Cancer Research Center. A fluorescent VNTR, as described for human VNTR,21, 22 was utilized.23, 24 Chimeric ratio was determined by the ratio of the areas of unique donor and recipient peaks and normalized to a standard curve produced from mixtures of donor and recipient DNA.
2.8 Statistical analysis
Long-term follow up of the treated dogs was achieved via regular communication with the owners, referring veterinarians, or both. Median disease-free interval (DFI), defined as the time from alloHCT (day 0) until the time of relapse, and median overall survival (OS), defined as the time from alloHCT(day 0) until death, were calculated using the Kaplan–Meier method. For comparison to historical autoHCT data, we combined cases from two previous reports our group published in 2012 (24 dogs) and 2022 (10 dogs).7, 8 There was no statistically significant difference between these three groups of dogs when comparing mean age, mean weight, or mean days from diagnosis to transplant. In addition, when combined (49 dogs total), 94% of dogs were diagnosed with Stage III-Va lymphoma. Analysis included intent-to-treat (all dogs) and dogs treated in the first remission from each study. For the later, four dogs who died in the hospital were censored from DFI analysis and two dogs who died from unrelated causes were censored from the OS analysis. DFI and OS were compared between the three groups using the log-rank (Mantel–Cox) test, with a trial end-point of 4.1 yrs, since all dogs from all 3 studies who lived >2 yrs post-transplant have lived >4 yrs. All statistics were calculated using the GraphPad Prism 9 (version 9.0.0). A P-value ≤.05 was considered significant.
3 RESULTS
3.1 Donor dogs
3.1.1 DLA-genotyping
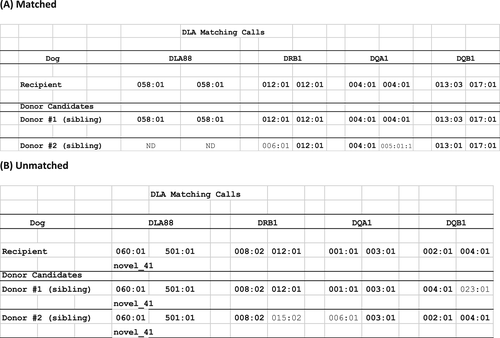
Table 1 describes the characteristics of the DLA-matched donor dogs. The mean number of dogs screened was 5 (range: 1–28). An example of genotyping data is shown in Figure 1. In Panel A, the recipient dog was fully matched to donor dog #1 at 8/8 DLA alleles (identical match). DLA88 genotyping was not performed on donor #2, since both DRB1 and DQA1 loci were not matched. In Panel B, donor dog #1 was matched to the recipient at 7/8 DLA alleles (haploidentical match), while donor #2 was not matched.
| Signalment | |
| Number screened | Mean = 5 (range: 1–28) |
| Relationship to recipient | 6FS, 6MS, 2B, and 1MDL |
| Spay/neuter | 6SF, 2IF, and 7NM, |
| Age (months) | Mean, 57 (24–108) |
| Sex matching | 10SG, 5OG |
| Mobilization | |
| Filgrastim only | 9 |
| Filgrastim + plerixafor | 6 |
| Pre-apheresis WBC count | Mean = 42.0 × 103/μl (range: 22.5–89.7 × 103/μl) |
| Pre-apheresis monocyte count | Mean = 2.0 × 103/μl (range: 1.06–4.28 × 103/μl) |
| Apheresis | |
| Number of procedures | 13 dogs (1), 1 dog (2), and 1 dog (4) |
| Time (m) | Mean = 253 (range: 180–401) |
| Harvest volume (ml) | Mean = 220 (range: 41–401) |
| TBV processed | Mean = 2.7 (range: 2–3.9) |
| WBC count | Mean = 71.0 × 103/μl (range: 44.3 –102.3 × 103/μl) |
| CD34+ % | Mean = 1.9 (range: 0.3–5) |
| CD34+ cells/kg infused | Mean = 8.0 × 106 (range: 2.08 × 106–2.9 × 107) |
| Adverse events | 0 |
- Abbreviations: FS, female sibling; MS, male sibling; B, bitch; MDL, male from a different litter of same mating pair; SF, spayed female; IF, intact female; NM, neutered male; SG, same gender; OG, opposite gender; TBV, total blood volume.
3.2 Donor dog mobilization and apheresis cell yield
Thirteen of fifteen donor dogs responded appropriately to mobilization with either filgrastim alone or filgrastim plus plerixafor and completed peripheral blood mononuclear cell apheresis uneventfully (Table 1). Three dogs developed Grade 2 neutrophila, while the remaining 12 dogs neutrophil counts did not exceed 50 k/μl. The apheresis in two dogs, mobilized with filgrastim only, yielded an inadequate number of CD34+ cells/kg, so additional collections, using a mobilization protocol including plerixafor, were successfully used to harvest an adequate number of CD34+ cells/kg. Two dogs weighed <15 kg necessitating machine priming to ensure hemodynamic stability during the initial stages of the procedures.25, 26 All dogs tolerated the mobilization protocol and apheresis procedure well, with no AE noted. The mean time of the procedures and mean volume of the harvests were 253 m (range: 180–401 m) and 220 ml (range: 41–401 ml), respectively. The mean pre-apheresis WBC count was 42.0 × 103/μl, while the mean pre-apheresis mononuclear cell count was 2.0 × 103/μl.
For human alloHCT, infusing ≥5x106 CD34+ cells/kg after myeloablation is generally recommended to ensure timely complete hematologic engraftment.27 The mean CD34+ percentage of the apheresis products was 1.9% (range: 0.3%–5%) (Table 1). The mean infused donor dog CD34+ cell dose was 8.0 × 106 cells/kg (range, 2.08 × 106–2.9 × 107 cells/kg). Overall, >5 × 106 CD34+ cells/kg were harvested from 11/15 donor dogs. There was no statistical difference between the mean apheresis product WBC counts (p = .6647) or CD34% (p = .0816) of the apheresis products between donor dogs mobilized with filgrastim alone or mobilized with filgrastim and plerixafor (Supplemental Table 2).
4 RECIPIENT DOGS
4.1 Recipient dog characteristics
In Table 2, the characteristics of the 15 recipient dogs with BCL, including disease stage and relapse status, are shown. Five dogs relapsed before the procedure: three dogs once, one dog twice, and one dog 3 times. Four of the relapsed dogs were not in remission when transplanted. The mean time from diagnosis to alloHCT was 118 days (range, 29 days-379 days). No AE in any recipient dog relating to the infusion of the allogeneic harvests were seen.
| Signalment | |
| Number of dogs | 15 |
| Breeds | 12 (4 LR) |
| Age (months) | Mean = 55 (range: 24–96) |
| Spay/neuter | 6SF, 2IF, 6NM, 1IM |
| Weight (kg) | Mean = 30.4 (range: 7.7–69.1) |
| B-cell lymphoma | 15 LN FNA/biopsy |
| Diagnosis | |
| Stage 1 | 2 |
| Stage III | 6 |
| Stage IV | 3 |
| Stage V | 4 |
| Substage a | 11 |
| Substage b | 4 |
| Relapse before alloHCT | 5 |
| Remission before alloHCT | 11 |
| Days from dx to alloHCT | Mean = 118d (range: 29–379) |
| PARR + tumours | 12 |
- Abbreviations: HCT, haematopoietic cell transplantation; LR, Labrador retriever; SF, spayed female; IF, intact female; NM, neutered male; IM, intact male; LN FNA, lymph node fine needle aspirate; alloHCT, identical allogeneic haematopoietic stem cell transplant; and PARR, PCR for antigen receptor rearrangements.
4.2 Donor cell engraftment
All 12 dogs who had VNTR chimerism analysis post-alloBMT achieved >95% donor WBC cells within 2 weeks of donor cell infusion (data not shown). Chimera testing was not performed on the two dogs who died in the hospital before the analysis could be run and on one dog whose owner declined testing due to financial constraints (this dog engrafted appropriately).
4.3 Acute TBI Toxicity
All dogs experienced GI AE, including Grades 1–3 vomiting, diarrhoea, inappetence, anorexia, or some combination of these, as previously described.7, 15 However, no dogs in this cohort developed any Grade 4 GI AE, while eight dogs in the cited studies developed Grade 4 anorexia and one dog developed Grade 4 diarrhoea. In addition, no dogs in this cohort required nutritional supplementation, while 8 dogs from the cited studies received either partial or total parental nutrition.
All dogs developed Grade 4 neutropenia (0 neutrophils/μl) at a mean of 6 days post-TBI and achieved a neutrophil count >1000 × 103/μl in 9–18 days (mean, 11 days), as the previously reported.7, 15, 28 All dogs also developed Grades 3–4 thrombocytopenia, as the previously reported,7, 15, 28 although it was not possible to determine true platelet nadirs since most dogs (11/15) received various platelet products to augment platelet recovery. Three of fifteen dogs exhibited clinical signs related to thrombocytopenia. A summary of these data is found in Supplemental Table 3. The mean hospital stay of all dogs was 22 days.
4.4 Treatment-related mortality (TRM)
Two dogs (13%), both not in remission when treated, died in the hospital, presumably secondary to TBI toxicity. One dog, died 7 days post-alloHCT with clinical signs consistent with septic shock. A cosmetic necropsy revealed mild pleural effusion and mixed Gram-positive and Gram-negative bacteria and variable fungal yeast and pseudohyphae (possible Candida sp.) within both blood vessels and airway lumina. The second dog, appeared to be engrafting slowly and was administered two DLI doses days 12 and 13 post-TBI. She was found deceased 13 days post-alloHCT. Necropsy was declined by the owners.
4.5 GvHD
The target organs of aGvHD in dogs and people are the skin, gastrointestinal tract, and liver.29, 30 No dogs developed prolonged liver enzyme elevations after CSP was discontinued. Three out of thirteen dogs (23%) who left the hospital developed cutaneous signs consistent with acute Grade 2 aGvHD, characterized by periocular and/or muzzle alopecia and scaling. One dog developed cutaneous signs of aGvHD ~2wks after a DLI (given 8 weeks post-alloHCT), which eventually resolved within ~4 wks. One dog who was managed at home by a non-compliant owner, developed cutaneous signs ~3 wks after CSP cessation that waxed and waned for the rest of its life, depending on the dose of CSP that was given. One dog, whose platelet recovery was prolonged (26 k/μl 2 months post-alloHCT), developed cutaneous aGvHD ~2wks after receiving a fresh whole blood transfusion from the mobilized donor and CSP was discontinued. A biopsy of an upper lip lesion confirmed aGvHD (hyperplastic moderate lymphocytic interface dermatitis). All lesions resolved without treatment over 1.5 months.
4.6 Cyclosporine toxicity
One dog, after beginning CSP, had markedly elevated CSP serum levels (1600 ng/ml) that persisted in spite of the drug being discontinued. Diabetic ketoacidosis was confirmed ~14 days after CSP was started and insulin therapy was initiated.31, 32 This dog remained a lifelong diabetic.
4.7 Factor V inhibition
As previously reported, one dog developed acquired circulating coagulation Factor V inhibitors that required intensive platelet support.10
5 ALLOHCT OUTCOME
5.1 All cause and disease specific mortality
The all-cause mortality was 40%. Two dogs died in the hospital and three dogs were euthanized due to disease progression. One dog died of presumptive Grade 5 gastric-dilatation volvulus.
The disease specific mortality was 27%, with 3/15 dogs developing progressive disease post-transplant.
5.2 Remission duration
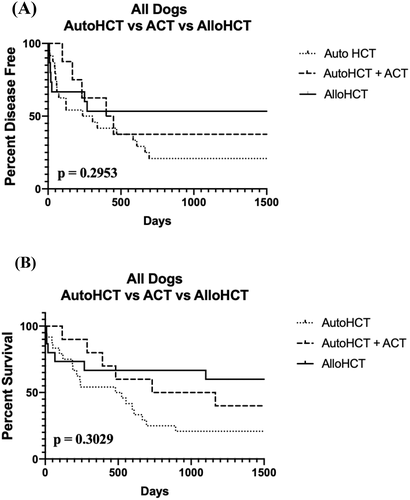
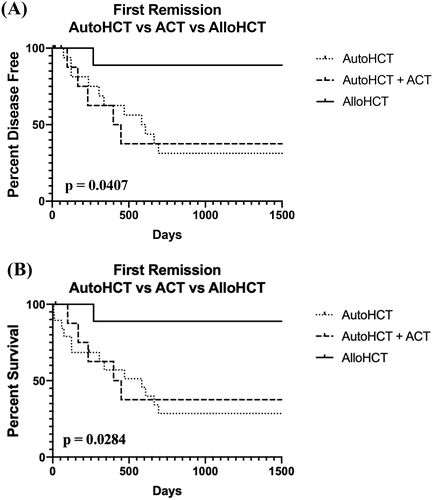
The median DFI of all dogs was 1095 days (range: 9–2920 days). When compared to two historical control groups of 34 dogs with BCL treated with autoHCT or autoHCT + SCT,7, 8 there was a not a statistically significant difference in median DFI or OS between the three groups (p = .2953) (Figure 2A). The median DFI of the 13 dogs discharged from the hospital was 1155 days (range: 15–2902 days). The median DFI of the 10 dogs transplanted in the first remission was 1235 days (range: 19–2920 days). However, one dog died of presumptive Grade 5 gastric dilatation-volvulus 19 days post-alloHCT. With this dog censored, the median DFI was 1285 days (range: 268–2290 days). When compared to 2 historical control groups of 27 dogs with BCL in the first remission treated with autoHCT or autoHCT + SCT,7, 8 there was a statistically significant difference in median DFI between the three groups (p = .0407) (Figure 3A). The median DFI of three dogs who relapsed with disease and did not die in the hospital was 25 days (range, 15–250 days). The dog with the longest median DFI in this group was in remission when transplanted.
5.3 Overall survival
The median OS of all dogs was 1115 days (range: 9–2920 days). When compared to 2 historical control groups of 34 dogs with BCL with autoHCT or autoHCT + SCT,7, 8 there was not a statistically significant difference in OS (p = .3029) (Figure 2B). The median OS of the 13 dogs who were discharged from the hospital was 1185 days (range: 19–3285 days). The median OS of the 10 dogs who were transplanted in their first remission was 1235 days (range: 19–2920 days). As above, when censoring the dog who died of presumptive Grade 5 gastric-dilatation-volvulus, the median OS was 1285 days (range: 268–2920 days). Of the remaining nine dogs, only one dog died from lymphoma <2 yrs post-alloHCT (268 days). Of the remaining eight dogs, one was killed ~8 yrs after transplant, while another died ~4.5 yrs after transplant-neither due to recurrent BCL. The remaining six dogs are still alive and disease-free. When compared to 2 historical control groups of 27 dogs with BCL in the first remission treated with autoHCT or autoHCT + SCT,7, 8 there was a statistically significant difference in OS (p = .0284) (Figure 3B). The cure rate of alloHCT, as defined as living >4 yrs after the procedure and/or not dying from lymphoma, in these dogs was ~89%.
The median OS of the three dogs who relapsed and did not die in the hospital before alloHCT was 1100 days (range: 66–1980 days). One dog, with the shortest median DFI and the longest median OS, who was not in remission when transplanted, died ~5.3 yrs post-transplant for reasons unrelated to lymphoma.
6 DISCUSSION
This retrospective study describes the feasibility, safety, and efficacy of using an aggressive treatment protocol to treat 15 dogs with BCL using a multiagent chemotherapy protocol combined with identical alloHCT. We reasoned, based on human alloHCT literature, that alloHCT would decrease the ~65% relapse rate of dogs with BCL treated with autoHCT. In all cases, using a commercial laboratory, we identified a DLA-matched donor dog and safely isolated, using cell separator machines, an adequate number of donor-derived CD34+ cells to ensure complete post-TBI hematologic recovery. While two dogs died in the hospital, all surviving transplanted dogs developed >95% donor WBCs within 2 weeks. Similar to our experience in the autoHCT setting, dogs who had relapsed before transplant or were transplanted when out of remission had a significantly shorter DFI when compared to dogs that had not relapsed and were in clinical remission. Of the dogs treated in the first remission, ~89% were cured of their disease and have lived >4 yrs after transplant.
The most critical component of alloHCT is identifying an identical DLA-matched donor. DLA loci segregate according to Mendelian genetics,33 so all siblings, the bitch/sire, and any other dogs produced by the same mating pair, have a 25% chance of matching. More distantly related dogs, such as cousins, have a 12.5% chance of matching. Since no bone marrow donor registries exist for dogs as in humans (https://bethematch.org), DLA-genotyping must be performed on both the recipient and donor. In this study, 10 cases required DLA-genotyping of ≤3 related dogs to identify a matched donor. Importantly, we show the mobilization protocol is well tolerated with no AE seen during or after the cell collection process-an important point for donor dog owners. Because multiple collections were required in two cases, donor dog owners and clinicians must be aware that, although uncommon, multiple collections may be needed for the procedure to proceed.
In this study, filgrasim and plerixafor were used to mobilize 7/15 donor dogs, while 8/15 dogs received filgrastim alone. We did not find any statistically significant differences in pre-apheresis WBC counts, monocyte counts, or apheresis CD34+ cell yield between the protocols, in contrast to the previous report.17 In two cases, plerixafor was added to filgrastim for additional collections when an inadequate number of CD34+ cells were collected using filgrastim alone, and this combination resulted in the collection of an adequate number of CD34+ cells. For these reasons, due to the extremely high cost of plerixafor, we recommend adding plerixafor to the mobilization protocol only when needed.
In all transplanted dogs, complete donor chimerism was seen within 2 weeks of the procedure. Acute GvHD, a complex process manifested by damage and activation of host tissues secondary to TBI, elaboration of proinflammatory cytokines, and expansion of alloreactive donor-derived T-cells, is defined as a syndrome of dermatitis, hepatitis, and enteritis developing within ~100d of alloHCT. Prophylaxis against aGvHD includes steroids, methotrexate, and calcineurin inhibitors such as CSP and tacrolimus, among others. In this study, aGvHD, which manifested as inner ear, periocular, muzzle/lip scabbing/alopecic cutaneous lesions, was seen in only three dogs within ~2 wks after CSP was discontinued. In two dogs, the lesions eventually resolved over a period of 2–3 months, while one dog, with a long history of skin allergies before treatment, had waxing/waning lesions for the rest of its life despite being on varied doses of CSP. None of the lesions in any of these dogs were life-threatening. Additionally, no dogs developed clinical signs associated with chronic GvHD (cGvHD, ocular or oral sicca syndromes, dysphagia, wasting syndrome, and chronic infections) that typically develops in people within 2 yrs of alloHCT. This is in contrast to human data suggesting a 38% and 33% incidence of aGVHD and cGvHD in HLA-matched sibling alloHCT patients, respectively.34, 35 The reasons why both aGvHD and cGvHD in these dogs was less pronounced than seen in people is unknown.
Finally, although the gastrointestinal side effects of TBI in alloHCT tend to be milder than with autoHCT, the post-procedure care is more complicated due to our observation that the dogs have delayed platelet recoveries (>100 k/μl) requiring, in some cases, intensive platelet support, continued monitoring of CSP levels after discharge, and managing any aGvHD symptoms once CSP is discontinued. Additionally, in the setting of post-alloHCT relapse, the use of DLIs (harvest cryopreserved CD3+ lymphocytes, in vitro expanded donor T-cells, mobilized or unmobilized donor peripheral blood, or the second infusion of a mobilized apheresis product) to incite a renewed GvL response can be considered.
In summary, our results suggest that DLA-genotyping at a commercial laboratory can identify suitable DLA-matched donor dogs in a timely manner to treat dogs with BCL using identical alloHCT. No AE were seen regarding donor dog mobilization and stem cell collection procedures and all recipient dogs tested developed >95% donor cells within 2 weeks of transplant. Importantly, few recipient dogs experienced signs associated with severe aGvHD or cGvHD. We suggest that alloHCT can reduce the relapse rate seen in the autoHCT setting by ~50%, with ~89% of dogs in the first remission cured of their disease.
ACKNOWLEDGEMENTS
The authors wish to thank the dedicated hospital staff for their excellent care of these dogs.
FUNDING
This work was not supported by any grant.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
Open Research
DATA AVAILABILITY STATEMENT
Data was obtained from medical records at the respective clinical practices.