Establishment of cell line and in vivo mouse model of canine Langerhans cell histiocytosis
Abstract
A cell line named FB-LCH01, derived from a dog diagnosed with Langerhans cell histiocytosis (LCH), was established and characterized. FB-LCH01 had C-shaped nucleoli, characterized by modal chromosome aberrations. The original tumour cells as well as established FB-LCH01 cells were immunopositive for human leukocyte antigen-DR, Iba-1 and E-cadherin, and immunonegative for CD163 and CD204, suggesting Langerhans cell origin. Furthermore, the characteristics of FB-LCH01 were compared with those of two canine histiocytic sarcoma cell lines (PWC-HS01 and FCR-HS02) established previously. Expression of E-cadherin was detected only in FB-LCH01, but not in PWC-HS01 and FCR-HS02. All (n = 9) the severe combined immunodeficiency mice inoculated with the FB-LCH01 cells developed subcutaneous tumour masses after 3 weeks. Eight of nine mice also developed metastatic lesions in the lymph nodes (8/8; 100%), lung (5/8; 62.5%), stomach (5/8; 62.5%), heart (4/8; 50%), pancreas (4/8; 50%), kidney (3/8; 37.5%), skin (3/8; 37.5%) and bone marrow (1/8; 12.5%). Tumour cells were pleomorphic and round- to polygonal-shaped with prominent anisocytosis and anisokaryosis. The xenotransplanted tumour cells maintained the immunohistochemical features of the original tumour with persistent E-cadherin expression at injection site and some visceral organs. In conclusion, the established cell line as well as the mice xenotransplant model in this study reflect the nature of canine LCH and may serve as promising models for investigating the patho-tumorigenesis and therapy of the disease.
1 INTRODUCTION
Langerhans cell histiocytosis (LCH) is an uncommon histiocytic proliferative disorder characterized by Langerhans cell (LC) proliferation.1-7 In a recent classification, canine cutaneous LCH and canine cutaneous histiocytoma (CCH) have been classified within a single disease spectrum with diverse biological behaviours.4 Canine LCH shares histological and immunohistochemical characteristics with CCH, however the clinical features of LCH are different from those of CCH because LCH develops multiple lesions. In contrast to histiocytoma, which spontaneously regresses, cutaneous LCH may progress and metastasize to lymph nodes, lung and other internal organs.2, 4, 6 The manifestation and behaviour of canine cutaneous LCH resemble those of human cutaneous LCH.3, 4, 7 Human LCs are characterized by positive immunostaining for CD1a, S100 and CD207 (Langerin), and by the presence of cytoplasmic Birbeck's granules on electron microscopy.3, 4, 7, 8 However, canine LCs lack S100 expression and Birbeck's granules.4, 5, 9 The immunohistochemical feature of CCH and canine cutaneous LCH corresponds to that of epidermal LCs: positive for CD1a, major histocompatibility complex class II, CD11c and E-cadherin.2, 4, 5, 10-12 Immunohistochemistry for CD1a and CD11c is available only with frozen sections.2, 4, 5, 13 Canine LCH most commonly affects Shar Pei dogs (about 20% of total cases) without sex predilection, though other breeds may also develop this disease.4 To date, a rare case of canine LCH in a Dachshund exhibited multiple cutaneous masses with eventual metastasis.6
Despite the accumulation of knowledge from recent studies, clinical behaviours and the origin of canine LCH are still unclear maybe because of the broad spectrum of canine histiocytic diseases (ie, resemblance to CCH) and limitation of cell markers. Moreover, there have been no studies on the treatment for canine LCH. Establishment of a cell line derived from a canine cutaneous LCH lesion may provide information regarding the cell origin, and pathogenesis of the disease, which facilitates further in vitro studies for therapeutic intervention. The aim of this study is to establish a cell line, derived from a canine LCH case, and to characterize its phenotype and tumorigenicity. Furthermore, the nature of the cell line was compared with those of the other two canine histiocytic sarcoma (HS) cell lines that were established previously.13
2 MATERIALS AND METHODS
2.1 Animal
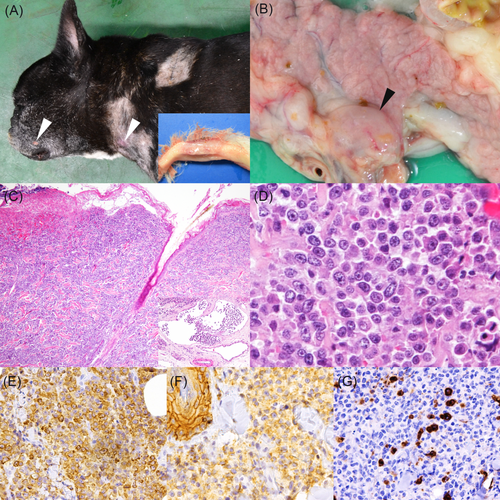
A 5 years and 4 months old female French Bulldog presented with a small mass located between the third and fourth digits of the right foreleg. The mass was surgically removed and diagnosed as CCH by histopathological examination. Two months after the surgery, recurrence was noticed on the same site, and additional multiple masses developed in other regions of the skin: top of the head, both pinnae, right shoulder, right lumbar, right inguinal, both sides of thigh and ventral. The masses presented erythema and ulceration. Also, systemic lymphadenopathy was noticed by palpation and ultrasound examination. Skin masses in the right shoulder, thigh, right inguinal and right lumbar regions were biopsied, and these lesions were diagnosed with LCH based on the results of histopathological examination. The dog was treated with chemotherapeutic agents including lomustine, vinblastine and nimustine, however, the dog died 88 days after the first biopsy was taken, and necropsy was subsequently performed. Gross examination revealed multiple skin masses in the right mandible, both sides of neck and both sides of inguinal region (Figure 1A). In addition, enlargement of bilateral mandibular and pancreaticoduodenal lymph nodes (Figure 1B), and masses in the bilateral ureter, mediastinum and aorta were observed. All visceral organs including the lesions were fixed in 10% neutral-buffered formalin for pathological examination. Also, fresh tissues were collected from the inguinal mass and used for cell line establishment.
2.2 Cell cultivation
Tumour cells were dissociated from the fresh tumour sample, washed and centrifuged three times with sterile phosphate-buffered saline (PBS; pH 7.4) containing 1% penicillin-streptomycin. The tumour cells were suspended in Dulbecco's modified eagle medium (DMEM; Gibco, Fisher Scientific, Paisley, Scotland, UK) containing 0.25% crude trypsin (BD Difco, Sparks, Maryland) and incubated at 4°C for 16 to 18 hours. Thereafter, the cells were centrifuged at 1000 rpm, at 4°C for 5 minutes and the pelleted cells were in new DMEM supplemented with 10% heat-inactivated fetal bovine serum (FBS; GE Health Life Sciences, South Logan, Utah) and 1% penicillin-streptomycin (Wako Pure Chemical, Osaka, Japan), and then filtered through a cell strainer (70 μm; Falcon, Tokyo, Japan). The cell suspension was seeded into 100-mm petri dishes (Thermo Scientific, Tokyo, Japan) and maintained in a humidified atmosphere of 5% carbon dioxide and 95% air at 37°C. Cell culture was observed daily by a phase-contrast microscopy. Tumour cells were cloned using a serial dilution method. Once the cultured cells reached a 90% confluence, they were removed and seeded into 60-mm petri dishes with 10% FBS in DMEM. Medium was replaced every 3 to 4 days and cell passages were performed weekly. The cells were stocked in DMEM containing 10% FBS and 10% dimethylsulphoxide (DMSO; Sigma-Aldrich, Saint Louis, Missouri). Cloned cells were characterized as described later, and cells at the passage 30th were used for all in vitro experiments and each study was repeated at least three times to verify the results.
PWC-HS01 and FCR-HS02 cell lines were established from the tumour tissues of canine HS cases by Thongtharb et al.13 These HS cell lines were maintained in DMEM containing 10% FBS in a humidified atmosphere of 5% CO2 and 95% air at 37°C. HS cell lines were observed daily to check cell viability and growth before using for E-cadherin expression in Immunocytochemistry and Western blot analysis.
2.3 Growth curve
The cells were seeded at 1 × 104 cells in a well of a 24 well multiplate and maintained in the growth medium at 37°C for 14 days. Cell viability was counted every 24 hours by trypan blue staining with a haemocytometer. After the growth curve was obtained, the population doubling time was determined from the exponential phase of the curve.
2.4 Morphologic and immunocytochemical characterizations
Cells (1 × 105 cells/mL) were placed into 2-well chamber slides (Thermo Scientific) and maintained in a humidified atmosphere of 5% carbon dioxide and 95% air at 37°C. At about 80% confluence, the cells were fixed in methanol and stained with a Giemsa solution (Merck Millipore, Tokyo, Japan).
Immunocytochemistry was performed using monoclonal and polyclonal antibodies listed in Supplemental table. All cultured cells (FB-LCH01, PWC-HS01 and FCR-HS02) were cultured on 8-well chamber slides (Thermo Scientific) and fixed with 4% paraformaldehyde. Cell membrane permeabilization was then performed by treating the cells with 0.25% Triton X-100 prior to a blocking step. After a 40 minutes-incubation in PBS with 1% bovine serum albumin and Tween 20 (PBST) in order to block nonspecific reactions, primary antibodies diluted in PBST were applied at 4°C overnight. The slides were then incubated with fluorescein-labelled horse anti-mouse immunoglobulin G (IgG) (Vector Laboratory, Tokyo, Japan), or fluorescein-labelled goat anti-rabbit IgG (Vector Laboratory) at room temperature for 1 hour and counterstained with DAPI (Vectashield; Vector Laboratory). All slides were observed under the Zeiss LSM 700 laser scanning confocal microscope (Carl Zeiss Meditec, Tokyo, Japan).
2.5 Chromosome analysis
The tumour cells at the 35th passage were treated with 0.02 μg/mL of colcemid (KaryoMax; Invitrogen, Life technologies, Grand Island, New York) in PBS at 37°C for 2 hours in a humidified atmosphere of 5% CO2 and 95% air. They were then trypsinized, washed and treated with a prewarmed hypotonic solution of 0.075 M potassium chloride (Wako). After fixation with a methanol/acetic acid (1:1) solution, fixed cells were applied to glass slides and subsequently stained with a Giemsa solution. The modal chromosome numbers were counted with an oil-immersion objective (1000×) lens using a light microscope.
2.6 DNA distribution analysis by flow cytometry
Canine peripheral blood mononuclear cells (PBMCs) were isolated from peripheral bloods of three healthy beagle dogs using the ordinary gravity sedimentation method using Ficoll-Paque PLUS (GE Healthcare, Little Chalfont, UK). The use of experimental animals in this study was approved by the Institutional Animal Care and Use Committee of the Graduate School of Agriculture and Life Sciences, The University of Tokyo (Approval No. P18-129). Isolated PBMCs and FB-LCH01 cells were washed twice with PBS, fixed overnight in ethanol (70%, v/v) at −20°C, and washed twice with PBS. Thereafter, the cells were stained with 50 μg/mL of propidium iodide (Sigma-Aldrich, St. Louis, Missouri) and 200 units/mL of DNase-free RNase in PBS (pH 7.4) (Sigma-Aldrich) at 37°C for 5 minutes. DNA content was determined using a FACSVerse flow cytometer (BD Biosciences, Franklin Lakes, New Jersey). All procedures were repeated two times in separate experiments.
2.7 Western blot analysis
Protein extractions from FB-LCH01 and two previously established HS cell lines (PWC-HS01 and FCR-HS02)13 were performed by using radioimmunoprecipitation assay buffer (50 mM Tris-HCl, 150 mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulphate, 5 mM EDTA, 2 mM Na3VO4, 1 mM phenylmethylsulphonyl fluoride, 10 mM sodium fluoride), with a proteinase inhibitor (complete mini EDTA-free; Roche Diagnostic, Mannheim, Germany). Total cell lysates were agitated at 4°C for 30 minutes and centrifuged at 12 000 rpm, at 4°C for 20 minutes. Protein samples diluted (1:1) with a loading buffer (2× Laemmli sample buffer containing with β-mercaptoethanol) were put onto a 10% polyacrylamide gel (e-PAGEL; ATTO, Tokyo, Japan) and electrophoresed at 600 V, 20 mA for 90 minutes. The protein bands were transferred to a polyvinylidene fluoride membrane (Immobilon-P transfer membrane; Millipore, Billerica, Massachusetts). After blocking nonspecific binding, following primary antibodies were applied: anti-E-cadherin (1:500; BD Transduction Laboratories, San Jose, California), anti-CD204 (1:500; TransGenic, Kobe, Japan) and anti-β-actin (1:2000; Cell Signalling Technology, Tokyo, Japan). The enhanced chemiluminescence detection system (GE Healthcare) and chemiDoc XRS+ system (Bio-Rad Laboratories, Tokyo, Japan) were used for visualization.
2.8 Tumorigenicity assay
Nine female immune-deficient C.B-17/Icr-scid/scidJcl severe combined immunodeficiency (SCID) mice at 5 weeks of age were subcutaneously injected with 200 μL of the cultured FB-LCH01 cells at the lower back. After injection, the mice were inspected weekly for tumour formation. When tumours were observed, the volume was calculated every day by using the following formula: Tumour volume (mm3) = (Length × Width2)/2.14 Animals were sacrificed with isoflurane at 2 months after inoculation or when mice skin ulceration was detected or when tumour volume exceeded 500 mm3. Tumour masses and organs were collected at necropsy and fixed in 10% neutral-buffered formalin. All experiments using SCID mice were approved by the Institutional Animal Care and Use Committee of the Graduate School of Agriculture and Life Sciences, The University of Tokyo (Approval No. P17-017).
2.9 Histopathology and immunohistochemistry
Formalin-fixed tissues from the dog and mice were embedded in paraffin wax. Glass slides with 4 μm-thick tissue sections were prepared and stained with haematoxylin and eosin. For immunohistochemistry, deparaffinized sections were immersed in 10% hydrogen peroxide (H2O2) in methanol at room temperature for 5 minutes to inactivate endogenous peroxidase. After three washes in Tris-buffered saline, all tissue sections were incubated with 8% skim milk at 37°C for 30 minutes in order to block non-specific reactions. Subsequently, sections were incubated with the primary antibodies at 4°C overnight. Primary antibodies used and their dilution and antigen retrieval methods were summarized in Supplemental table. The EnVision system (Dako, Tokyo, Japan) of rabbit/mouse reagents conjugated with horseradish peroxidase was applied at 37°C for 40 minutes. The slides were then reacted with 3-3′-diaminobenzidine, 0.03% H2O2 in a Tris-hydrochloric acid buffer before a counterstain with Mayer's haematoxylin (Muto Pure Chemicals, Tokyo, Japan). Normal canine and mouse tissues were used as positive controls, and negative controls were performed by applying antibody diluent, irrelevant antibody and buffer instead of the primary antibodies.15
3 RESULTS
3.1 Histological and immunohistochemical features of the original tumour
Histologically, tumour cells invaded from the superficial dermis to the subcutis and often to the skeletal muscle (Figure 1C). The tumour cells were present in dermal lymphatic vessels (Figure 1C, inset). The proliferation of tumour cells was also detected in the spleen, pancreas, kidney, urinary bladder, bilateral mandibular and pancreaticoduodenal lymph nodes. Tumour cells were pleomorphic and round- to polygonal-shaped with a variable amount of eosinophilic cytoplasm and discrete cell border. Nuclei were atypical with severe anisokaryosis (Figure 1D). Mitotic figures were frequently observed (>60 mitoses/10 high power fields, 400×). In addition, tumour cells were blended with a variable infiltrate of macrophages, lymphocytes, neutrophils and eosinophils. Focal necrosis was randomly observed.
Immunohistochemically, the tumour cells of the skin masses were positive for human leukocyte antigen-DR (HLA-DR), vimentin, Iba-1 and E-cadherin (Figures 1E,F). However, they were negative for CD163, CD20, CD3, cytokeratin, S100 and CD204 (Figure 1G). In the lymph nodes and other visceral organs, E-cadherin-immunopositivity of the tumour cells was weaker compared to that in the skin mass, whereas immunopositivity of other markers were comparable. Based on the histopathological and immunohistochemical findings, the tumour was diagnosed as canine cutaneous LCH.
3.2 Morphological, chromosome features and DNA content of the established LCH cell line
The FB-LCH01 cells were variable sized and round- to polygonal-shaped. The tumour cells displayed a broad and weakly basophilic cytoplasm, and round to oval nuclei. In addition, C-shaped, coffee bean-shaped or folded nuclei were present. The nuclei had prominent single or several nucleoli. The cells also showed mild anisocytosis and anisokaryosis (Figure 2A). The doubling time at the logarithmic phase of the FB-LCH01 cell line was approximately 35.4 hours (Figure 2B, Table 1). By karyotyping, the number of chromosomes of the FB-LCH01 cell was 43-67 (average 59, mean 58.6) (Table 1). By flow cytometry, the G0/G1 population of FB-LCH01 cell had a DNA content that was 1.1 times of canine PBMC (Supplemental figure 1).

| Features | Cell line | ||
|---|---|---|---|
| FB-LCH01 | PWC-HS0113 | FCR-HS0213 | |
| Biological | |||
| Population doubling time (h) | 35.4 | 64.8 | 56.5 |
| Chromosomal number | 43-67 | 63-88 | 42-49 |
| Metastatic potentiala | + | −a | −a |
| Immunocytochemical | |||
| HLA-DR | + | + | + |
| CD1a | + | + | + |
| Iba-1 | + | + | + |
| E-cadherin | + | − | − |
| CD204 | − | + | − |
| CD163 | − | + | + |
| CD11b | − | + | +w |
| Myeloid/Histiocyte antigen | − | − | − |
| Cytokeratin | − | − | − |
| Vimentin | + | + | + |
| S100 | + | + | + |
- Abbreviations: −, negative; +, positive; CD, cluster of differentiation; HLA-DR, human leukocyte antigen-DR; HS, histiocytic sarcoma; Iba-1, ionized calcium-binding adapter molecule-1; LCH, Langerhans cell histiocytosis; +w, weakly positive.
- a Thongtharb et al13 have demonstrated that these cell lines have metastatic ability when intravenously injected into SCID mice, but such finding was not detected using the subcutaneous injection.
3.3 Immunocytochemical features of the established LCH cell line
The results of immunocytochemical characterization of FB-LCH01 and E-cadherin expression of HS cell lines (PWC-HS01 and FCR-HS02) are summarized in Table 1. In the LCH cell line and both HS cell lines,13 cultured cells were strongly positive for HLA-DR, CD1a, vimentin, S100 and Iba-1, while they were negative for cytokeratin and Myeloid/Histiocyte antigen. Moreover, FB-LCH-1 cells were positive for CD11c. FB-LCH01 cells were positive for E-cadherin, but both HS cells were negative (Figure 2C). Whereas HS cells were positive for CD163 and CD11b,13 but FB-LCH01 cells were negative. In addition, PWC-HS01 cells were positive for CD204, but FB-LCH01 and FCR-HS02 cells were negative (Table 1).13
3.4 Western blot analysis
A consistent band of E-cadherin was detected at approximately 120 kDa only in FB-LCH01 cells but not in PWC-HS01 and FCR-HS02 cells. Whereas, a band of CD204 (72 kDa) was detected only in PWC-HS01 cells but not in FB-LCH01 and FCR-HS02 cells (Figure 2D).
3.5 Xenotransplant mouse model
All the SCID mice injected with FB-LCH01 cells developed palpable masses in the subcutaneous tissue of the injection site after 3 weeks. The masses were solid and dome-shaped (Figure 3A). Alopecia and skin ulcers in the mass region were observed in 6 of 9 mice.
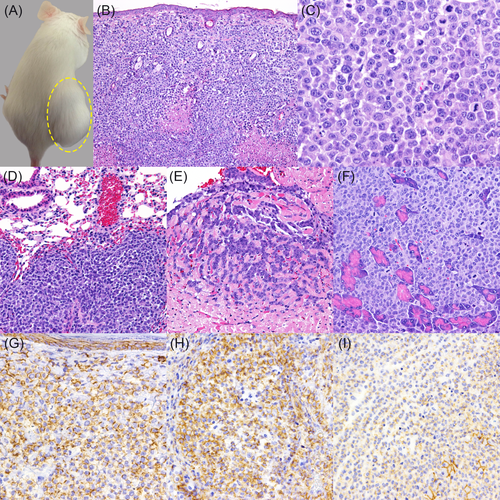
Tumour cells were located in the subcutaneous tissue and exhibited dermal tropism. In metastatic lesions of the visceral organs, tumour cells were arranged in a solid pattern. Extensive necrotic foci were observed in the centre of the tumour masses (Figure 3B). The tumour tissues were composed of round to polygonal neoplastic cells with an abundant pale eosinophilic cytoplasm. The shape of nuclei was round, or C-shaped to polymorphic with prominent single or several nucleoli. Mitoses and nuclear atypia were frequently observed (>100 mitoses/10 high power fields, 400×) (Figure 3C).
Distant metastases were detected in 8 of 9 mice. Metastases to the lymph node were found in 8 of 9, those to the lung or stomach in 5 of 9 (Figure 3D), to cardiac muscle or pancreas in 4 of 9 (Figures 3E,F), to the skin or kidney in 3 of 9 mice and to the bone marrow in one of 9 mouse. Metastatic lesions in all the SCID mice presented the similar histological features.
Transplanted tumour cells in the subcutaneous tissues at the injection site were strongly positive for Iba-1, HLA-DR, E-cadherin and vimentin. However, the tumour cells were negative for cytokeratin, CD3, CD20, CD163, CD204 and S-100. In metastatic tissues, tumour cells expressed the same markers with the tumour cells at the injection site except for E-cadherin. Tumour cells in the lymph nodes, lung, heart and stomach were strongly positive for E-cadherin, while the cells in the kidney, pancreas and bone marrow were weakly positive or a limited subpopulation was positive (Figures 3G,H,I). The immunohistochemical features of the original canine tumour and xenotransplanted and distant metastatic tumours of the FB-LCH01 cell line were summarized in Table 2.
| Sample | IHC markers | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| E-cadherin | HLA-DR | Iba-1 | CD204 | CD163 | CD20 | CD3 | Cytokeratin | Vimentin | S100 | ||
| Original tumour | FB-LCH01 | + | + | + | − | − | − | − | − | + | − |
| PWC-HS0113 | ND | + | + | + | + | − | − | − | + | + | |
| FCR-HS0213 | − | + | + | + | + | − | − | − | + | + | |
| Xenotransplanted tumour | FB-LCH01 | + | + | + | − | − | − | − | − | + | − |
| PWC-HS0113 | − | + | + | + | + | − | − | − | + | - to + | |
| FCR-HS0213 | − | + | + | +w | + | − | − | − | + | − | |
| Distant metastatic tumoura | FB-LCH01 | +w to + | + | + | − | − | − | − | − | + | − |
| PWC-HS0113 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| FCR-HS0213 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
- Abbreviations: −, negative; +, positive; CD, cluster of differentiation; HLA-DR, human leukocyte antigen-DR; Iba-1, ionized calcium-binding adapter molecule-1; IHC, immunohistochemistry; NA, not applicable; ND, no data; w, weak.
- a In SCID mice tumorigenicity assay.
4 DISCUSSION
Canine cutaneous LCH is an aggressive type of cutaneous histiocytoma, which exhibits multiple skin lesions and may progress to develop lesions in the lymph nodes and internal organs.2, 4-6 In the present study, a new cell line (FB-LCH01) was established from the tumour lesion of cutaneous LCH in a dog. Morphological and immunohistochemical phenotype of the cell line was comparative to that of cutaneous LC. Moreover, the phenotype of FB-LCH01 was distinct from that of previously established two HS cell lines13 that share both macrophage and dendritic cell phenotypes (Table 1).
In consistent with prior case reports on cutaneous LCH, multiple cutaneous masses developed also in the present case.4-9 The lymph nodes, ureter, mediastinum and posterior aorta were involved in the present case, indicating that the dog was at advanced stage of the disease. In the WHO classification of human haematopoietic tumours, LC tumours are classified into LCH and LC sarcoma (LCS), based on cytological atypia and clinical aggressiveness.8 In human, LCH occurs commonly in children, whereas almost all cases of LCS occur in adults.7, 8 The present canine case was 5 years and 4 months old, older than the prior canine LCH case.6 It is considered that, LCS can develop from LCH or separately (de novo).7, 8 In the present case, skin lesions were centred in the epidermis and superficial dermis, which implicates the origin and affinity of the tumour cells. As described in human LCS, significant cellular pleomorphism with anisokaryosis, high mitotic index and focal necrosis were also observed in the present canine case.7, 8 Therefore, progressed canine LCH cases may be diagnosed as LCS, as in human. However, the lack of E-cadherin expression in metastatic lesions makes it difficult to determine the LC phenotype of the tumour cells, because other LC markers such as CD1a and CD11c molecules are currently not available on formalin-fixed sections from the dog.2, 4, 5, 12
In cell culture, FB-LCH01 exhibited a high proliferative rate (doubling time 35.36 hours) than other previously published canine HS.13 Chromosomal aberrations may contribute to carcinogenesis and increased oncogenic copies.16 Comparing with the number of chromosome of the domestic dog (2n = 78), FB-LCH01 demonstrated a decrease of chromosome number, although the DNA content was similar to that of normal canine cell. In human, the loss of chromosome number has been associated with mutations in tumour suppressor genes.17 Decrease chromosome number in canine LCH may also imply mutations of tumour suppressor genes.
All cultured cells (FB-LCH01, PWC-HS01 and FCR-HS02) were positive for histiocytic markers (HLA-DR, CD1a, Iba-1), S100 and vimentin (Table 1).13, 18 FB-LCH01 cells also were positive for CD11c which was known as a dendritic marker.4 However, FB-LCH01 cells were negative for macrophage markers such as Myeloid/Histiocyte antigen, CD11b, CD163 and CD204.2, 4, 19 Importantly, the cell membrane of FB-LCH01 cells expressed E-cadherin, which is in consistent with normal canine cutaneous LCs and the original tumour of the present case.2, 4, 10 In contrast to FB-LCH01 cells, both HS cells (PWC-HS01 and FCR-HS02) were immunopositive for CD11b and CD163,13 but were immunonegative for E-cadherin. Western blot analysis confirmed the differential expression of E-cadherin and CD204 in LCH and HS cell lines. Expression of E-cadherin, which was found only in FB-LCH01 cells, may support LC origin of this established cell line (Figure 2D).4, 10, 11
In the xenotransplanted experiment, all of the immune-deficient SCID mice developed masses in the skin (dermis and subcutaneous) of the injection site. More than 90% (8/9) of FB-LCH01-transplanted mice showed metastases to other areas of the skin, lymph nodes and thoracic and abdominal visceral organs. In order to determine the metastatic ability, tumour cells are commonly injected into circulation via the lateral tail vein or directly into the left ventricle of the heart.13, 20 Besides that, orthotopic transplantation of tumour cells is used as a spontaneous metastasis model, which virtually resembles the original tumour.20 In the present study, the SCID mice were subcutaneously injected with FB-LCH01 cells at the primary location of the tumour. Subsequently, tumour cells were provided with a micro-environment analogous to that of the original tumour. As a result, FB-LCH01 cells demonstrated a prominent metastatic potential which was consistent with the biology of the original tumour. These findings support that multiple cutaneous lesions in canine LCH are likely to be metastatic lesions from a primary lesion rather than multicentric tumour development. Besides the cutaneous lesions, lymph nodes were the most frequent location of metastasis (100%, 8/8), followed by the lung, stomach, cardiac muscle, pancreas, kidney, subcutis and bone marrow. These results suggest that tumour cells of the primary site are most likely to metastasize to the lymph nodes, and then spread to other distant organs.4, 5 Furthermore, the most frequently affected visceral organ was the lung, which also represents the biological and behaviours of LCH.1, 4
E-cadherin is a cell to cell adhesion molecule, which is expressed in epithelial cells as well as cutaneous LCs.1, 3, 4, 10 Downregulation of E-cadherin expression is associated with the migration of LCs from the skin to the draining lymph nodes.3, 11 In the present study, E-cadherin expression of the tumour cells was decreased in metastatic sites in the original canine case and also in the xenotransplantation mouse model. These findings indicate that downregulation of E-cadherin is associated with detachment of the tumour cells from the primary site, and migration to the lymph nodes and/or distant organs.3 Moreover, E-cadherin expression of tumour cells may be recovered after a subpopulation of tumour cells spread to new residential distant organs. However, further prospective studies supporting our hypothesis are needed.
The present study represents the biological behaviour, morphology and immunophenotype of FB-LCH01, a newly established canine LCH cell line. Furthermore, after the inoculation in immune-deficient SCID mice, FB-LCH01 cells showed similar clinical behaviour, and histological and immunohistochemical characteristics to those of the original LCH. Therefore, the established cell line as well as the mice xenotransplant model can be used as useful tools for investigating the pathogenesis and therapy of the disease.
ACKNOWLEDGEMENTS
The authors thank Ms. S. Kato for her technical assistance.
CONFLICT OF INTEREST
The authors declare no potential conflicts of interest.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.