Canine oral primary melanoma cells exhibit shift to mesenchymal phenotype and phagocytic behaviour
Funding information: University of Veterinary Medicine, Vienna
Abstract
Canine oral malignant melanoma (COMM) is a potentially lethal cancer disease. We established primary cell lines from mostly amelanotic primary COMM and metastases and assessed lesions and derived cells for Melan A, PNL2 and CD146 expression. Then, migration and invasion of CD146-enriched vs -depleted COMM cells were analysed. Epithelial-to-mesenchymal transition (EMT) was addressed by Vimentin-staining and MMP2/MMP9 zymography. Phagocytic behaviour was analysed by histopathological examination and phagocytosis assay. While Melan A- and PNL2-staining yielded inconsistent data, 100% of COMM sections and primary cells showed CD146 expression, suggesting that this protein may serve as a prognostic marker. An overall correlation between CD146-expression and migration/invasion was not observed. All primary cell lines consistently expressed Vimentin and secreted biologically active MMP2, indicating that they had undergone EMT. Importantly, COMM sections exhibited cell-in-cell structures, and all primary cell lines exhibited phagocytic activity, supporting the concept that cell cannibalism may have a role in COMM progression.
1 INTRODUCTION
Canine melanoma is a relatively common tumour disease that arises from melanocytes and preferentially develops at highly pigmented cutaneous and mucosal sites on the dog's body. Primary melanomas commonly affect haired skin where they present as dark nodular, flat or wrinkled masses, but can also occur in the oral cavity, nail bed, foot pad, ocular region, gastrointestinal tract or mucocutaneous junctions. Interestingly, haired-skin melanomas only rarely metastasize and usually have a good prognosis following excisional therapy. In contrast, (inter) digital and oral tumours show malignant behaviour and, similar to human malignant melanoma (MM), have a high metastatic potential and often resist to irradiation or chemotherapy.1 In general, canine and human MM display striking similarities at clinical, histopathological and molecular level.2 For example, canine and human MMs typically show activation of the activated protein kinase B mechanistic Target of Rapamycin pathway, tyrosine Kinase kit aberrations, cyclooxygenase-2 and chondroitin sulfate proteogycan 4 overexpression and deregulation of the Wnt/β-catenin pathway.3, 4 A high degree of resemblance on cellular and subcellular level is particularly observed between canine oral MM (COMM) and human mucosal MM.5 Most notably, mucosal lesions lack BRAF mutations in both species. These mutations are a typical feature of human cutaneous MMs known to enhance mitogen-activated protein kinase activity.6, 7 Canine MM thus possibly represents the best natural animal model for human MM.
MM progression and metastasis is a multi-step process that is initiated by genetic alterations, leading to modulation of cell-cell interactions, so that tumour cells can detach from the primary lesion, migrate through the extracellular matrix (ECM) and enter the microvasculature, leading to their dissemination via the blood stream.8, 9 This cascade of events closely resembles an embryologic cellular program termed epithelial-mesenchymal transition (EMT). This program is activated in embryonic development and wound healing and orchestrates the conversion of various types of epithelial cells into mesenchymal cells.9-12 EMT can be grossly characterized by (a) loss of typical epithelial characteristics such as apical-basolateral polarization, basement membrane integrity and cell-cell adhesion and (b) gain of mesenchymal features via suppression of E-cadherin expression allowing EMT transcription factors to induce increased expression of mesenchymal proteins. The later include matrix metalloproteinases (MMPs) and Vimentin that promote ECM degradation, cell motility and invasion.9, 13, 14 In human MM, EMT-mediated changes prevent senescence and apoptosis and enhance the migratory capacity and invasiveness of tumour cells, thus promoting the transition from MM in situ to metastatic MM.13 Moreover, tumour cells exhibiting a mesenchymal morphology have been shown to resist to chemotherapy and radiation, indicating that targeting MM-associated EMT-mediated processes might represent an expedient therapeutic approach.13
Recently, the cell adhesion protein CD146 (MCAM; MUC18) has been identified as having a multifaceted role in human MM progression and metastasis. CD146 is expressed on the surface of and within MM cells and metastases, while benign nevi usually lack this protein. in vitro and in vivo analyses have revealed CD146 acting as promoter of protumoural inflammatory processes by facilitating proinflammatory leucocyte extravasation. CD146 also contributes to MM cell survival via inhibition of proapoptotic pathways. Furthermore this protein drives metastasis by (a) inducing heterotypic adhesion and diapedesis, (b) contributing to the degradation of the ECM, (c) supporting MM cell motility and (d) promoting tumour vascularization. Hence, CD146 has been proposed as additional driver of EMT and valuable prognostic marker.15
Engulfment and digestion of live and viable cells by cancer cells in an act of cannibalism probably represents an ancient process. Pathologists have long observed cell-in-cell (CIC) structures in various human cancer tissues, including melanoma. However, the biological significance of cancer cell cannibalism and the mechanism underlying this entotic process remain largely unknown.16 To our knowledge, this phenomenon has not been described for canine MM thus far.
Herein, we report on the establishment of primary cell lines from primary COMM and lymph node metastases and on their characterization in regard to expression of the mesenchymal proteins Vimentin, MMP2 and MMP9 and of CD146 (MCAM) as potential driver of cell motility and invasiveness. In addition, we provide evidence on the phagocytic activity of primary COMM cells and metastases and discuss the possible pathobiological impact of this interesting finding.
2 MATERIALS AND METHODS
2.1 Canine tumour patients
Tumour specimens were obtained from COMM patients presenting at the University Hospital for Small Animals, University of Veterinary Medicine, Vienna, Austria (animals 1, 3 and 4), or a private Clinic for Small Animals (dog 2) with the owner's written consent and in accordance with institutional guidelines allowing tissue excised for diagnostic or therapeutic reasons to be used for scientific purposes. All lesions underwent histopathological examination, leading to the diagnosis of COMM in all cases. Tumour material consisted of1 a fine needle aspiration biopsy (FNAB) from a primary predominantly amelanotic palatal COMM and biopsies from metastases affecting the right (1.1; Ø 4 cm) and the left mandibular lymph node (1.2; Ø 2 cm) of a castrated female 11-year-old mongrel2; a biopsy from a low pigmented mandibular COMM diagnosed in a castrated male 15-year-old mongrel3; a biopsy from an amelanotic mandibular COMM detected in a female spayed 11-year-old Papillon; and4 a biopsy from a pigmented COMM affecting the right oral commissure of a male 9-year-old Golden Retriever.
2.2 Establishment of primary cell lines
Following excisional biopsy (or FNAB in one case), the tumour material was immediately supplied with Dulbecco's Modified Eagle Medium with high glucose (4.5 g/L) and GlutaMAX supplemented with 10% heat-inactivated (30 minutes, 56°C) foetal bovine serum (FBS-HI), 1% antibiotic-antimycotic mixture, 50 μg/mL Fungizone (all from GIBCO-Life Technologies, Carlsbad, California) and 250 μg/mL Gentamycin (Vana GmbH, Vienna, Austria) and transferred to the laboratories of the University's Research Group Oncology. Then samples were washed twice with Dulbecco's phosphate-buffered saline (PBS) without magnesium and calcium (GIBCO-Life Technologies) and dissected from surrounding tissue using a scalpel. Subsequently, biopsies were cut into small pieces and, including the FNAB sample, digested in 2.5% Trypsin (GIBCO-Life Technologies) solution diluted 1:10 in Hanks balanced salt solution (HBSS) at 4°C overnight, followed by incubation at 37°C for 30 minutes. In case of incomplete digestion, tissue was further incubated with 100 U/mL Collagenase I (GIBCO-Life Technologies), diluted in HBSS at 37°C for 30 to 60 minutes on an incubator shaker. Thus digested samples were filtered and scraped through a 100 μm Cell Strainer (BD Falcon, Heidelberg, Germany), collected in FBS and rinsed with HBSS. Then, cells were collected by centrifugation (Sigma 1-15 K; SIGMA GmbH, Osterode, Germany) at 600g for 5 minutes, and the supernatants were discarded. Cells were washed repeatedly with HBSS until supernatants were clear, seeded at a density of 10 000 viable cells/cm2, and incubated at 37°C with 5% CO2. Cells were split according to their density using 0.05% Trypsin-EDTA (GIBCO-Life Technologies). According to their respective source, obtained primary cell lines were designated as cRGO1, cRGO1.1, cRGO1.2, cRGO2, cRGO3 and cRGO4. All primary cell cultures were screened for contamination by mycoplasma using the EZ-PCR Mycoplasma Test Kit (Biological Industries, Beit-Haemek, Israel) and Mycoplasma Detection Kit-Quick Test (Bimake, Houston, Texas), according to manufacturer's instruction. Cultures testing positive for the presence of mycoplasma were repeatedly treated for 3 weeks with MycoKill AB (GE Healthcare Life Sciences, Little Chalfont, UK), diluted 1:50 in standard medium and screened again for the absence of mycoplasma. To comparatively assess the morphology of cRGO1 cells explanted from a primary COMM vs cRGO1.1 and cRGO1.2 cells derived from metastases of the primary lesion, cells were seeded at an initial standard concentration of 250 000 cells per six-well and cultured for 72 hours. Then, cells were subjected to microscopy and photographs at 40× and 100× magnification were taken.
2.3 Control cell lines
The canine MM cell line Shadow was established and kindly provided by Dr. J.F. Modiano,17 the canine macrophage-like cell line DH82 derived from a histiocytic sarcoma was kindly provided by Dr. Cheryl London.18 The human fibrosarcoma cell line HT1080 was purchased from ATCC (Manassas, Virginia). Shadow and HT1080 cells were cultured in standard COMM medium as described above. DH82 cells were cultured in RPMI 1640 medium with L-Glutamine (Lonza BioWhittaker, Cologne, Germany) supplemented with 10% FBS-HI, 1% antibiotic-antimycotic mixture, 1% Non-Essential Amino Acids (GIBCO-Life Technologies), 1% Sodium Pyruvate (GIBCO-Life Technologies) and 1% (10 mM) HEPES buffer solution (GIBCO-Life Technologies). For cryoconservation, melanoma and HT1080 cells were frozen in standard medium plus 10% dimethyl sulfoxide (DMSO) (Sigma-Aldrich Co., St. Louis, Missouri) and 30% FBS. DH82 cells were cryoconserved in 90% FBS plus 10% DMSO.
2.4 Histopathological tumour diagnosis
Tumour material was fixed in 4% neutral buffered formalin for 24 hours, gradually dehydrated and embedded in paraffin. Then 2.5-μm tissue sections were prepared using rotation-microtome Microm HM355S (Thermo Fisher Scientific, Waltham, Massachusetts). Sections were dried over night at 37°C, then deparaffinized in xylene, rehydrated in ethanol at decreasing concentrations and subjected to haematoxylin and eosin (H&E) staining. Then sections were analysed by microscopy using a Leitz Dialux 20 microscope (Leica Microsystems GmbH, Wetzlar, Germany).
2.5 Immunohistochemical and immunofluorescence staining of COMM tissue
Sections derived from the left lymph node metastasis of dog 1 (1.2) and from the tumour of dog 3 were prepared as described above. Following deparaffinization, heat-induced antigen retrieval was performed in citrate buffer at pH 6 (Melan A, Vimentin, MMP2, MMP9) or Tris/EDTA buffer at pH 9 (PNL2; CD146) in a microwave for 30 minutes. Then, sections were blocked with 3% donkey serum or 1.5% goat serum (depending on the secondary antibody) at room temperature for 30 minutes. Following short draining, sections were incubated with primary antibodies confirmedly recognizing respective canine antigens according to information by manufacturer and/or the University's pathological Institute, that is, mouse anti-Melan A (1:500; Dako Agilent Pathology Solutions, Santa Clara, California), mouse anti-PNL2 (1:600; Santa Cruz Biotechnology Inc., Heidelberg, Germany), mouse anti-CD146 (1:100; Abcam, Cambridge, UK), mouse anti-Vimentin (1:10000; BioGenex, Fremont, California), rabbit anti-MMP2 (1:100; Abcam) or rabbit anti-MMP9 (1:100; Abnova GmbH, Heidelberg, Germany) at 4°C overnight. Negative controls included in the experiment consisted of “no-primary-antibody” controls. Then slides were washed and subjected to immunohistochemical (IHC) (Melan A, Vimentin) or immunofluorescence (IF) staining (PNL2, CD146, MMP2, MMP9). For IHC, horseradish peroxidase (HRP)-labelled secondary antibody (BrightVision Poly-HRP, Immunologic, Duiven, Netherlands) was applied for 30 minutes at room temperature, and the signal was developed with 3,3′-Diaminobenzidine (DAB; Sigma-Aldrich) for 10 minutes. Cell nuclei were counterstained with haematoxylin, dehydrated through a graded series of ethanol concentrations and xylene and covered with DPX Mountant (Sigma-Aldrich) 44581. For IF analysis, sections were incubated with fluorescent secondary antibody, that is, Alexa Fluor 488-conjugated goat anti-mouse or donkey anti-rabbit antibody (both 1:500; GIBCO-Life Technologies) at room temperature for 1 hour. After washing, cell nuclei were counterstained with diamidino-2-phenylindole (DAPI; Sigma-Aldrich) for 10 minutes at room temperature and slides were mounted with Aqua-Poly/Mount (Polysciences Europe GmbH, Hirschberg, Germany). Then all sections were analysed and imaged using a Zeiss Axio Imager Z2 (Carl Zeiss Microscopy GmbH, Jena, Germany).
2.6 Flow cytometric analysis of established primary COMM cell lines
Intracellular staining was performed using Cytofix/Cytoperm (BD Biosciences, San Diego, California) according to instructions of the manufacturer. In brief, detached cells were washed twice with PBS + 0.2% BSA (Sigma Life Science) and then resuspended in 250 μL BD fixation and permeabilization solution. After an incubation time of 20 minutes at room temperature, cells were washed with 1 mL BD Perm/Wash Buffer (BD Biosciences). Staining of cell surface antigens was carried out in PBS plus 5% FBS. Primary mouse anti-Melan A (Mart-1) clone M2-7C10 (20 μg/mL; Thermo Fisher Scientific), mouse anti-PNL2 clone F-7 (10 μg/mL; Santa Cruz Biotechnology Inc.), mouse anti-CD146 clone A32 (5 μg/mL; Upstate, Inc., Charlottesville, Virginia), mouse anti-Vimentin clone 2D1 (10 μg/mL; Abcam), mouse anti-CD68 clone MAC387 (10 μg/mL; BioTrend, Destin, Florida) or isotype control antibodies (mouse IgG1; mouse IgG2a; Abcam) were diluted in BD Perm/Wash buffer or PBS supplied with 5% FBS and incubated for 30 minutes at 4°C. After a double washing step, cells were incubated with Alexa Fluor 647-conjugated rabbit anti-mouse antibody (2 μg/mL; Abcam) for 30 minutes at 4°C in the dark. After a final washing step, cells were resuspended in 500 μL BD FACSFlow (BD Biosciences) and transferred into BD Falcon Tubes (BD Biosciences). The analysis was performed on a BD FACS Canto II flow cytometer, using BD FACS DIVA Software 6.0 for analysis (BD Biosciences).
2.7 Magnetic separation of CD146-positive and CD146-negative tumour cells
Enrichment of CD146-positive melanoma cells was carried out by magnetic bead separation according to instructions of manufacturer (Miltenyi Biotec, Bergisch-Gladbach, Germany). In brief, 107 cells per cell line were labelled with mouse anti-CD146 IgG1 antibody clone A32 (5 μg/mL PBS; Upstate) at room temperature for 30 minutes, followed by incubation of labelled cells with magnetic anti-mouse IgG1 MicroBeads for 15 minutes on ice. Then magnetic CD146-positive cells were separated from unlabelled CD146-negative cells (flow-through) by application of cell suspensions onto MS columns placed in the magnetic field of a separator (Multi-Stand; Miltenyi Biotec). Thereupon, labelled and unlabelled cells were counted and resuspended in serum-free medium at a cell density of 105/mL.
2.8 Cell migration and invasion assay
To assess the migratory capacity and invasiveness of CD146-positive vs CD-146 negative tumour cells, 24-well plates (BD Falcon) were supplied with 600 μL of standard COMM medium or serum-free medium (negative control) per well. Then uncoated (migration) or gelatine-coated (invasion) transwell-inserts (BD Falcon) were placed into the wells. Subsequently, 105 CD146+ or CD146− cells in serum-free medium (100 μL) per insert were added and incubated overnight at 37°C in a CO2 incubator. Before staining, the inside of each insert was swabbed with cotton swabs to gently remove non-migrated cells. After washing of inserts and wells with PBS, migrated cells were stained with 0.2% crystal violet (Sigma-Aldrich), and counted at 100× magnification using a Leica DMIL microscope (Leica Microsystems GmbH).
2.9 Gelatine zymography
Serum-free cell culture supernatants were collected after an incubation period of 72 hours with or without 50 ng/mL Phorbol-12-myristate-13-acetate (Sigma-Aldrich), snap frozen in liquid nitrogen and stored at −80°C until analysis. Gelatine zymography was carried out in 8% SDS polyacrylamide gels containing 0.1% gelatine (Sigma-Aldrich) under non-reducing conditions. Cell culture supernatants (40 μL per cell line) were mixed with 20 μL of 3 × SDS-Sample Buffer (New England BioLabs, Frankfurt, Germany), loaded into gels and separated by electrophoresis. Then, gels were washed in renaturation buffer (4×; 2.5% Triton X-100 in developing buffer) to remove SDS and renature proteinases. Subsequently, gels were incubated for 24 hours at 37°C in developing buffer (50 mM Tris, 200 mM NaCl, 5 mM CaCl2, 5 μM ZnCl2, 0.02% NaN3 at pH 7.5) and fixed in 30% ethanol plus 10% glacial acetic acid solution. Then, gels were stained with Page Blue (Thermo Fisher Scientific), destained in water and imaged on a LED light pad (Huion, Shifeng, Shenzhen, China) using a Sony C3 digital camera (Sony Corporation, Berlin, Germany).
2.10 Phagocytosis assay
Phagocytic activity was assessed using 500-nm FluoSpheres Carboxylate-Modified Latex Beads-488 (Life Technologies). In brief, tumour cells, canine macrophage-like DH82 cells as positive control, and primary equine ocular squamous cell carcinoma (SCC) cells19 as negative control19 were seeded 24 h before analysis at a density of 500 000 cells per 6-well (Thermo Scientific). Then, cell culture medium was substituted by serum-reduced medium (2% FBS-HI), fluorescence-labelled beads (1%) were added and cells incubated for 1 hour at 37°C with 5% CO2. Then cells were detached with 0.5 mL TrypLE Express (Thermo Fisher Scientific) and subjected to flow cytometric analysis as described above.
2.11 Statistics
Gaussian distribution was assessed by Shapiro-Wilk normality test,20 and significance of observed differences by Mann-Whitney U test.21 Where applicable, results were presented as arithmetic mean ± SD.
3 RESULTS
3.1 Histopathological examination revealed COMM in all cases
All lesions underwent routine histopathological analyses to establish the final diagnosis of oral melanoma. The primary tumour affecting the palate of dog 1 was diagnosed as predominately amelanotic, poorly differentiated/highly pleomorphic and highly MM with bilateral local lymph node metastases. Dog 2 presented with a mass affecting the left caudal mandible corresponding to a poorly pigmented, highly pleomorphic MM. Dog 3 exhibited a large mass affecting the right caudal mandible. The tumour was composed of dense heterogeneous cells of mesenchymal appearance containing one to two enlarged nuclei and was classified as malignant amelanotic melanoma. Dog 4 was diagnosed with pigmented, pleomorphic MM at the right oral commissure showing no signs of lymph node metastases.
3.2 Primary cell lines were successfully established from COMM and metastases
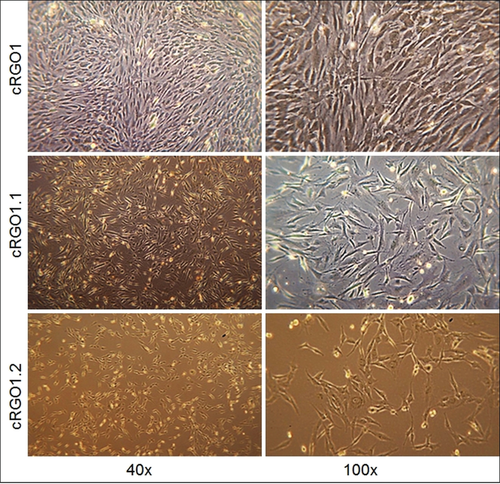
Tumour cells adhered within 24 hours, and reached confluency within 4 to 12 days. Multiple passaging (up to 48×) had no apparent effect on cell proliferation, as illustrated by average population doubling times of one doubling per day irrespective of the passage number. Primary cell lines derived from primary COMM displayed polygonal and spindle-shaped cellular growth characteristics as exemplarily shown for cRGO1. In comparison, metastases-derived cell lines cRGO1.1 and cRGO1.2 displayed a more fibroblastic, bipolar and elongated morphology (Figure 1).
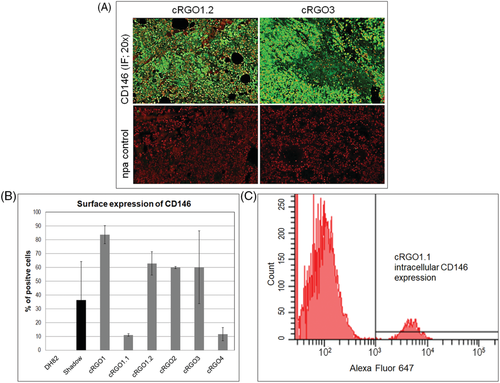
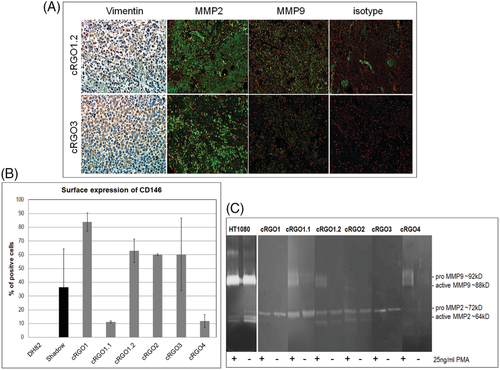
3.3 Tumour cells consistently expressed cell adhesion protein CD146
A primary tumour3 and a metastasis (1.2) tissue section, as well as all primary cell lines were assessed for expression of the melanoma markers Melan A (Mart-1), PNL2, and CD146, as well as macrophage marker CD68 using IHC or IF for tissue, and flow cytometry for cell analysis, with canine melanoma cell lines Shadow, or DH82 (for CD68) serving as positive controls. As negative control, specific antibodies were omitted (IHC/IF) or substituted by corresponding IgG isotype antibodies in the different assays. Melan A was exclusively detected from tissue sections, with metastasis 1.2 showing higher Melan A expression levels than primary tumour 3 (not shown). PNL2 was detected from the section of metastasis 1.2, and for 12% of cRGO2 cells. None of the melanoma cell lines tested positive for macrophage marker CD68, whilst control cell line DH82 yielded a positive result (Supporting information Figure S1). Importantly, both tumour sections analysed stained clearly positive for CD146 (Figure 2A). In analogy, 11% to 84% of cells per tumour cell line expressed this cell adhesion protein, with cRGO1 derived from a primary oral tumour revealing the highest CD146 expression level. In contrast, only 11% of cRGO1.1 cells derived from metastasis 1.1 of tumour 1 expressed CD146 on their surface (Figure 2B). On the other hand, cRGO1.1 was the only cell line expressing intracellular CD146, albeit at a low level (6.55% of cells; Figure 2C).
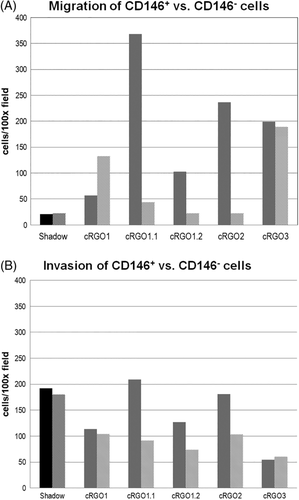
3.4 CD146 expression may influence the migratory potential of canine melanoma cells
To assess a possible influence of CD146 expression on the migratory potential and invasiveness of melanoma cell lines, CD146-enriched vs -depleted subpopulations of the cell lines except cRGO4 were subjected to migration and invasion assays. While CD146 expression had no impact on the migratory potential of cell lines Shadow, cRGO1 and cRGO3, this feature was significantly associated with migration of the two metastasis-derived cell lines cRGO1.1 and 1.2, and cRGO2 derived from a primary COMM. Metastasis cell line cRGO1.1 and COMM cell line cRGO2 also exhibited the highest migratory capacity (Figure 3A). The invasion assay yielded analogous findings, with CD146+ cRGO1.2 and cRGO2 displaying the highest levels of invasiveness. Interestingly, a high invasion capacity was also noted for Shadow, irrespective of CD146 expression (Figure 3B).
3.5 Tumour cells exhibited mesenchymal protein expression patterns
In a next step, sections of tumour 3 and metastasis 1.2 as well as all tumour cell lines were analysed for expression of mesenchymal proteins Vimentin, MMP2 and MMP9. Both sections tested clearly positive for Vimentin and MMP2, but showed poor MMP9 expression (Figure 4A). Flow cytometric analysis of primary tumour cell lines revealed medium to high Vimentin expression, with positive cells per primary cell line ranging between 36% and 98% (Figure 4B). MMP2 and MMP9 expression and secretion by tumour cells was assessed by zymography of respective cell culture supernatants. As depicted in Figure 4C, supernatants of phorbol-12-myristat-13-acetat (PMA)-stimulated and unstimulated cells tested positive for proteolytically active MMP2. In contrast, only PMA-stimulated primary cell lines cRGO1.1 and cRGO1.2 as well as cRGO4 secreted detectable amounts of proteolytically active MMP9 (Figure 4C).
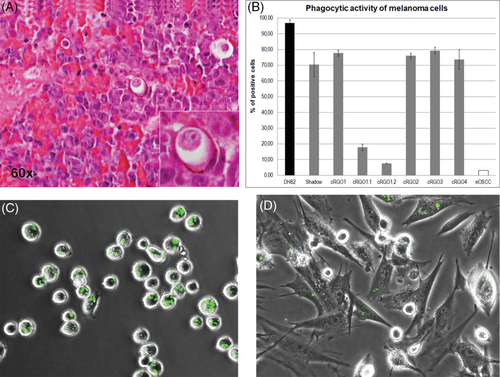
3.6 All primary canine melanoma cell lines showed phagocytic activity
The observation that tumour cells ingested neighbouring cells as revealed by H&E staining of COMM metastasis tissue 1.2 (Figure 5A) finally prompted us to assess established primary tumour cell lines for phagocytic behaviour. To this aim, cells were incubated with green fluorescent latex beads and then analysed by flow cytometry. Phagocytic activity was observed for all tumour cell lines. About 73% to 79.3% of tumour cells derived from primary oral lesions tested positive for bead ingestion (Figure 5B) as exemplarily shown for cRGO2 (Figure 5C). Significantly lower phagocytic activity (P = 0.05) was exhibited by cell lines cRGO1.1 and cRGO1.2 derived from mandibular metastases (17.7% and 7.5%; Figure 5B). Only 3.1% of primary equine ocular SCC cells used as negative control ingested green fluorescent latex beads (Figure 5B), thus testifying for the accuracy of the experiment and the validity of obtained data.
4 DISCUSSION
Primary cell lines explanted from tumour material are a valuable tool allowing in vitro study of various biological features and processes underlying carcinogenesis. We have successfully established primary cell lines from four distinct primary COMM, and two mandibular lymph node metastases of one of the primary lesions. Expression analysis of tumour sections and cell lines for classical melanoma markers Melan A and PNL2 yielded inconsistent results, with all (Melan A) and all but one cell line (PNL2; cRGO2) testing negative. This finding is in accordance with previous data showing that Melan A and PNL2 are not consistently expressed by COMM and primary cell lines derived thereof. Melan A seems to be predominantly expressed by benign melanotic melanomas of epithelioid type, whilst mesenchymal, aggressive amelanotic COMM often lack this protein.22-26 In accordance with this observation in dogs, feline amelanotic melanomas show likewise poor Melan A expression.27 Similarly, PNL2 expression is frequently observed for polygonal, epithelioid melanoma cells, while spindle-shaped melanoma cells of mesenchymal type often lack expression of this marker.23, 26 In concert with previous observations, our findings indicate that Melan A and PNL2 are not ideal markers for reliable identification of canine amelanotic melanomas that are predominantly constituted of spindle-shaped tumour cells. On the other hand, tumour sections and all primary cell lines clearly tested positive for CD146 expression. CRGO1 derived from a primary, metastasizing COMM and cRGO1.2 explanted from the left mandibular metastasis of this tumour revealed the highest CD146 expression scores, with 84% and 63% of cells testing positive for surface expression of the protein. In contrast, cRGO1.1 derived from the right lymph node metastasis exhibited the lowest CD146 surface expression score (11%), but revealed intracellular CD146 expression. This observation is suggestive for the right lymph node metastasis representing a different developmental state in comparison to the primary oral lesion and the left lymph node metastasis. When assessing surface CD146+ vs CD146− cell subpopulations in regard to their respective migratory and invasion potential, positive correlation of CD146 expression with cell migration and invasion was observed for the metastasis cell lines and cRGO2. However, Shadow, cRGO1 and cRGO3 did not exhibit this feature. Based on previous reports on the multifaceted role of CD146 as promoter of human melanoma progression,15, 28-30 our findings may reflect the advanced/metastatic state of cRGO1.1, cRGO1.2 and cRGO2. However, CD146-overexpressing cRGO1 revealed CD146-independent migratory and invasive behaviour, indicating that CD146 expression alone might not suffice to drive tumour cell dissemination.15 Together with constant cell doubling times as observed over 48 passages by May 2018, consistent expression of canine CD146 in tumours and by all primary cell lines confirmed their identity and suggested CD146 as additional marker for COMM, irrespective of the degree of pigmentation. This concept is strengthened by a recent report on the frequent expression (23/33 tumours) of CD146 in COMM. On the other hand, most ocular melanomas (5/7) lacked CD146 in this study, disqualifying CD146 as potential marker for canine ocular melanocytic neoplasms.31
In order for melanoma cells to dissociate from their cellular neighbourhood and invade the ECM, cells have to lose cell-cell adhesion and acquire motility, that is, shift from an epithelial to a mesenchymal state according to the highly conserved developmental EMT program.9, 14 EMT is initiated by down-regulation of E-cadherin, the major gatekeeper of the epithelial state. In a previous work, we hence assessed established primary cell lines for E-cadherin expression using anti-human or anti-mouse antibodies expected to cross-react with canine E-cadherin. Unfortunately, no antigen-specific antibody could be identified, thus leaving open the question whether or not the primary canine melanoma cell lines expressed this protein (personal communication). In another attempt to characterize melanoma sections and established melanoma cells in regard to their EMT state, we tested them for expression of EMT proteins Vimentin, MMP2 and MMP9. Vimentin is a type III intermediate filament protein and major cytoskeletal component of mesenchymal cells. Therefore, it is broadly used as marker of cells undergoing developmental and tumour progression-associated EMT.9, 32 Importantly, analysed COMM sections and all primary tumour cell lines consistently expressed medium to high levels of Vimentin. This finding is in total agreement with an early report on the consistent detection of Vimentin expression in six cell lines explanted from COMM (including Shadow), a skin melanoma-derived cell line and 28/29 tumour sections prepared from 11 COMM, 14 cutaneous and 4 digital canine melanomas.24 Vimentin is generally expressed by mesenchymal and neuroectodermal cells in normal tissue, and neoplastic cells that had undergone EMT in a high proportion of canine cancers.33-36 Along with Vimentin, tumour sections and primary cell lines consistently expressed biologically active MMP2 as revealed by IF and zymography. MMP9 expression was weaker or undetectable with only metastasis cells and melanotic cRGO4 secreting active MMP9 on PMA-stimulation. Weak MMP9 expression but MMP2 upregulation in COMM tissue and derived cell lines is in accordance with a previous report on insignificant MMP9 but high MMP2 transcription levels in progressive COMM and canine oral squamous cell carcinoma in comparison with low-grade tumour equivalents, suggesting that MMP2 may serve as prognostic marker for these canine oral tumour types.37
Histopathological examination of COMM sections repeatedly unveiled melanoma cells engulfing other cells as exemplarily shown in Figure 5A. This entotic process is also known as cellular cannibalism and thought to represent a primeval form of cell killing and feeding that remained conserved across evolution. Entotic CIC formation is a frequent, yet poorly studied event in cancer biology, and its significance still remains to be elucidated.16 Three biological factors triggering tumour cell cannibalism have been identified thus far, namely anchorage-independent growth, aberrant proliferation and starvation, that is, lack of available glucose. Accordingly, it has been proposed that entosis may confer tumour suppression via selective killing of dissociating, hyperproliferative or glucose-deprived cancer cells, notably at early tumour stages.38-40 On the other hand, entosis may promote tumour progression in several ways. It may provide tumour cells with nutrients and thus assure their survival under the starved conditions of the tumour stroma.40 Entosis may also contribute to immune evasion. In human MM, this concept is supported by the finding that metastatic but not primary MM cells engulf live CD8+ T cells.41 Tumour cells have also been shown to engulf natural killer cells under the presence of Ezrin.42 Finally, engulfment of cells by cancer cells directly promotes polyploidy by disrupting cell division, leading to bi- or multinucleated tumour cells. As a hallmark of tumour progression, the latter give rise to highly proliferative mutant clones that survive as the fittest at the expense of less aggressive or healthy cells.43 In this work, we provided evidence for CIC structures in COMM and also showed that primary cell lines established from COMM and metastasis thereof were able to engulf labelled latex beads. All primary tumour cell lines tested negative for CD68, thus ruling out the unlikely possibility of cultures being contaminated by macrophages. In contrast to the findings by Lugini et al,41 cells explanted from primary COMM showed significantly higher phagocytic activity than cells from lymph node metastases. This discrepancy may be because of COMM cells and cutaneous human MM cells differing in regard to their respective phagocytic behaviour. Alternatively or in addition, labelled latex beads may not represent an ideal surrogate for live cells. As a consequence, in-depth studies on COMM cell cannibalism involving GFP-transfected live cells are warranted.
Within the herein presented study, we have established several primary COMM and metastases cell lines that were mainly derived from amelanotic tumours. This fact may account for their poor reactivity with Melan A and PNL2 antibody. However, tumour sections and primary cell lines showed moderate to high CD146 expression levels, confirming that CD146 may serve as potent diagnostic and prognostic COMM marker. CD146 expression may also enhance the migratory potential of COMM; however, our in vitro data indicate that COMM cell migration and invasion is not dependent on the expression of this cell adhesion protein. Established primary cell lines had all undergone EMT as demonstrated by Vimentin expression and MMP zymography thus further confirming the histopathological diagnosis of progressive COMM. Intriguingly, all primary cell lines showed phagocytic activity, that was particularly pronounced when derived from primary lesions. Together with the histopathological observation of CIC phenomena in tumour sections, this novel finding points to tumour cell cannibalism in COMM. Although the pathobiological significance of this phenomenon is still poorly understood, there is general agreement on cell-in-cell structures representing a typical feature of high-grade human cancers including MM, and being associated with poor prognosis. On these grounds, further research on the phagocytic behaviour of COMM and its impact on canine tumour progression is warranted.
ACKNOWLEDGEMENTS
This study was funded in part by the the Austrian research association FFG. We wish to thank the teams of the VetCORE Facility and the Messerli Institute of the University of Veterinary Medicine, Vienna, for their kind support.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.