The effects of oncolytic reovirus in canine lymphoma cell lines
Abstract
Reovirus is a potent oncolytic virus in many human neoplasms that has reached phase II and III clinical trials. Our laboratory has previously reported the oncolytic effects of reovirus in canine mast cell tumour (MCT). In order to further explore the potential of reovirus in veterinary oncology, we tested the susceptibility of reovirus in 10 canine lymphoma cell lines. Reovirus-induced cell death, virus replication and infectivity were confirmed in four cell lines with variable levels of susceptibility. The level of Ras activation varied among the cell lines with no correlation with reovirus susceptibility. Reovirus-susceptible cell lines underwent apoptosis as proven by propidium iodide (PI) staining, Annexin V-FITC/PI assay, cleavage of PARP and inhibition of cell death by caspase inhibitor. A single intratumoral injection of reovirus suppressed the growth of canine lymphoma subcutaneous tumour in NOD/SCID mice. Unlike canine MCT, canine lymphoma is less susceptible to reovirus.
Introduction
Oncolytic viruses offer a new avenue for the treatment of cancer as these viruses are selective towards cancerous cells and spare bystander healthy cells from their cytotoxic effects. Adenoviruses, herpes simplex viruses, vaccinia virus and reovirus are among the oncolytic viruses that have been studied in depth so far. Due to the potential of these oncolytic viruses, various phase II and III clinical trials are being carried out for a range of neoplasms.1
Belonging to the genus Orthoreovirus within the family Reoviridae, reovirus is a small, non-enveloped dsRNA virus that can be commonly isolated from the human respiratory or gastrointestinal tract2 and the environment.3 Having the capacity to infect nearly every known mammalian species,4 reovirus neutralizing antibodies are detected in almost all adult humans5 and a high percentage of healthy dogs.6 As reovirus infection is usually asymptomatic, this virus poses no harm to its host.
Even though all three serotypes of reovirus possess oncolytic abilities,7 the serotype 3 Dearing strain is the most commonly explored reovirus in human neoplasms such as pancreatic, bladder, gastric, breast, neurological, lung, ovarian, colon, head and neck cancers.8-15 Transformation of cell lines that were naturally resistant to reovirus with v-erbB, sos or ras (all activators of Ras signaling pathway) allowed the cells to be highly susceptible to reovirus infection.16, 17 However, various recent studies have highlighted that Ras signaling pathway is not the sole pathway involved.18-21 Nonetheless, it has been proven that Ras activation enhances reovirus disassembly, infectivity and release of progeny virus.22
Lymphoma originates from lymphoreticular cells, and usually arises in lymphoid tissues, such as the spleen, lymph nodes and bone marrow. Canine lymphoma is the most common canine hematopoietic tumour, making up 83% of all canine hematopoietic malignancies and 7–24% of all canine neoplasms.23 As lymphoma is considered a systemic disease, systemic therapies, especially multi-agent chemotherapeutic approaches, are the best available therapeutic options, with response rates of up to 90%. Unfortunately, most animals will eventually succumb to relapse due to chemotherapy resistance.24 Alternative therapies using monoclonal antibodies and anti-tumour vaccines have been reported but effects were limited.23 Among the studies of oncolytic virotherapy in veterinary oncology, only the canine distemper virus (CDV) has been tested in canine lymphoid cells.25 Preclinical data from this study were encouraging, where CDV was reported to be a feasible therapeutic option for canine lymphoma. Besides that, adenovirus has been reported to be able to infect B-cell lymphoma,26, 27 which might make this virus a potential candidate for oncolytic virotherapy in canine cancers.
On the other hand, the effects of reovirus have been tested in various human lymphoid malignancies with variable results.28-30 Due to the similarities of canine lymphoma and the human variant of non-Hodgkin's lymphoma (NHL),31 we hypothesize that reovirus can serve as an alternative treatment of canine lymphoma. In the present study, the effects of reovirus in 10 established canine lymphoma cell lines were evaluated, followed by the assessment of the Ras activation status of the cell lines. In addition, investigation of the cell death pathway induced by reovirus in canine lymphoma was carried out. Lastly, the anti-tumour effects of reovirus were confirmed in canine lymphoma xenograft model.
Materials and methods
Cell cultures and reovirus
A total of 10 established canine lymphoma cell lines were tested for their susceptibility to reovirus in this study. CL-1,32 UL-1,33 CLGL-90,25 Nody-1, Ema and CLK34 are T-cell lymphoma cell lines while GL-1,35 17-7136 and CLBL-137 are B-ell lymphoma cell lines. CLC is a canine lymphoma cell line established in our laboratory but the cell type could not be determined.34 Mouse L929 fibroblastic cell line and human Burkitt's lymphoma cell line, Raji, were obtained from the Cell Resource Center for Biomedical Research (Institute of Development, Aging and Cancer, Tohoku University). All cell lines, except for 17-71 and CLGL-90, were maintained in R10 complete medium (RPMI1640 supplemented with 10% FBS, 100 U/mL penicillin, 100 µg/mL streptomycin and 55 µM 2-mercaptoethanol). 17-71 and CLGL-90 were maintained in Iscove's Modified Dulbecco's Medium (IMDM) supplemented with 10% FBS, 100 U/mL penicillin, 100 µg/mL streptomycin and 4.4 mM L-glutamine. All cells were grown at 37 °C in a humidified 5% CO2 incubator.
The Dearing strain of reovirus serotype 3 (Reolysin; clinical grade reovirus; GMP) was obtained from Oncolytics Biotech Inc.. Ultraviolet (UV)-inactivated virus was prepared by exposing live virus to short-wave UV radiation from a 15-watt germicidal lamp (Toshiba GL 15 UV lamp; predominant emission at 254 nm; Toshiba, Tokyo, Japan) for 90 min at a distance of 10 cm. Reovirus was confirmed to be UV-inactivated by 50% tissue culture infectious dose (TCID50) assay on L929 cells, as previously described38 with modifications.
Reovirus infection of cell lines
CL-1, 17-71, GL-1, Ema, Nody-1 and CLK were seeded at 2.5 × 104 cells; UL-1 and CLBL-1 were seeded at 1.25 × 104 cells; CLC and CLGL-90 were seeded at 3.125 × 103 cells before being mock-infected or infected with reovirus at a multiplicity of infection (MOI) of 70 plaque-forming units (PFUs) per cell. Cell infection was performed in triplicates in 48-well plates. Cell viability was quantified by trypan blue exclusion test at 72 h post-infection (hpi). Supernatant from each sample was collected and kept at −80 °C, pending titration of progeny virus using TCID50 assay. The titer of progeny virus was divided by the titer of input virus to obtain the fold increase of reovirus.
Cell lines susceptible to reovirus (CL-1, 17-71, CLC and GL-1) were selected for assessment of cell viability curves at 0, 24, 48 and 72hpi at MOI 70 of reovirus. In order to investigate the susceptibility towards various reovirus titers, the four reovirus-susceptible cell lines were also selected and incubated with UV-inactivated (equivalent of MOI 70), 2.8, 14 and 70 MOI of reovirus for 72 h.
Western blotting
All reovirus-susceptible canine lymphoma cell lines (CL-1, 17-71, CLC and GL-1) and two reovirus-resistant cell lines (Nody-1 and CLGL-90) were selected to assess the presence of reoviral proteins as confirmation of reovirus infectivity. These cells were seeded at 5.0 × 105 and mock-infected or infected with reovirus at MOI 70 for 48 h. Whole cell lysates were treated with RIPA lysis buffer [50 mM Tris HCl (pH 7.5), 150 mM NaCl, 1% NP40, 0.5% sodium deoxycholate] supplemented with complete, Mini EDTA-free protease inhibitor mixture (Roche Diagnostics K.K., Tokyo, Japan).
As for Poly(ADP-Ribose) Polymerase (PARP) cleavage detection, CL-1, 17-71, CLC and GL-1 were prepared in the same manner and harvested at 6 and 48hpi. Cell lysates were lysed with NP40 lysis buffer [1% NP40, 10 mM Tris HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA] also supplemented with complete, Mini EDTA-free protease inhibitor mixture.
Proteins were subjected to BCA protein assay (Thermo Fischer Scientific, Rockford, IL, USA) to determine the protein concentration before 30 µg of each protein sample was loaded into lanes in 9 and 12% SDS-PAGE gel for the detection of reoviral protein and PARP cleavage, respectively. Following electrophoresis, proteins were transferred to polyvinylidene fluoride (PVDF) membranes and blocked with 5% skimmed milk before probing with rabbit polyclonal anti-reovirus (dilution 1:100; produced by our lab) or rabbit polyclonal anti-PARP (dilution 1:1000; RB-1516-P0; NeoMarkers, Fremont, CA, USA) overnight at 4 °C on a rotary plate. After being washed three times with TBST, samples were probed with secondary labeling using goat anti-rabbit IgG HRP (dilution 1:4000; Zymed Laboratories, San Francisco, CA, USA) and incubated for 1 h at RT on a rotary plate. At the end of the incubation period, samples were washed again for three times with TBST. Immunoreactive bands were visualized by immersion in Western Lightning Chemiluminescence reagent (PerkinElmer, Shelton, CT, USA) using the Luminescent Image Analyzer LAS 3000 mini (FUJIFILM, Tokyo, Japan) and analysed using Science Lab 2005 (FUJIFILM). Membranes were stripped between antibody staining procedures with stripping buffer (100 mM 2-mercaptoethanol, 2% SDS, 62.5 mM Tris (pH 6.7)) for 30 min at 60 °C before being washed twice with TBST and blocking with 5% skimmed milk. To obtain an endogenous control, goat anti-actin (dilution 1:2000; sc-1615, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and rabbit anti-goat IgG HRP (dilution 1:4000; Bethyl Laboratories, Montgomery, TX, USA) were used. The immunoreactive bands of actin were also visualized using Western Lightning Chemiluminescence reagent (PerkinElmer).
GST pull-down assay for Ras status
Ras activation status of all the canine lymphoma cell lines was evaluated after GST pull-down according to Hwang et al.39 Western blotting was carried out as previously described using mouse anti-pan-Ras (Calbiochem) and goat anti-mouse IgG HRP (Zymed Laboratories) were used as primary and secondary antibodies respectively.
Apoptosis analysis using flow cytometry
CL-1, 17-71 and GL-1 were seeded at 1.0 × 105 while CLC was seeded at 1.25 × 104 before being infected with reovirus at MOI 70 and cells were harvested at 48 and 72hpi. For propidium iodide (PI) staining, cells were washed with cold PBS and fixed with cold ethanol before storage at −20 °C, pending analysis. Cells were incubated with 100 µl of 100 µg/ml Ribonuclease A (Nalacai Tesque, Kyoto, Japan) for 30 min and 4 µl of 1 mg/ml PI (Sigma–Aldrich Japan K.K., Tokyo, Japan) was added 5 min before acquisition. Flow cytometry was performed using CyFlow® Space (Partec GmbH, Münster, Germany) and results were analysed using FlowJo software (Tree Star, Inc., San Carlos, CA, USA). Cells in the SubG1 phase were gated to detect hypodiploid cells and the percentage of subG1 cells was considered as apoptotic cells.
Subsequently, Annexin V-FITC/PI assay was carried out to show the ratio of apoptotic and necrotic cells. The Annexin V-FITC/PI kit was purchased from Miltenyi Biotec (Auburn, CA, USA). Experiments were performed according to the manufacturer's protocol. Flow cytometric staining was analysed on a BD Accuri C6 Flow Cytometer (BD Biosciences, San Jose, CA, USA). Analyses were performed with FlowJo software.
Inhibition of reovirus killing by Z-VAD-FMK
CL-1, 17-71 and GL-1 were seeded at 2.5 × 104 cells while CLC was seeded at 3.125 × 103 cells and pre-treated with control DMSO, 10 µM or 100 µM of Z-VAD-FMK (caspase inhibitor I; Calbiochem) for 30 min at 37 °C before being infected with reovirus at MOI 70. Cell viability was quantified by trypan blue exlusion test at 48hpi.
Subcutaneous tumour xenograft model in NOD/SCID mice
Six-week-old NOD.CB17-Prkdc < scid>/J (NOD/SCID) mice were obtained from Charles River Japan Inc. (Shinagawa, Japan) and studies were conducted in a specific pathogen-free area in accordance with the Yamaguchi University Animal Care and Use guidelines. CL-1 (1.0 × 107 cells in 50 µl PBS) were implanted subcutaneously into the right flank of the mice under general anesthesia. When the desirable tumour size was achieved, 1.0 × 108 PFUs of live reovirus (experimental group) or UV-inactivated reovirus of equivalent titer (control group) in 20 µl PBS were injected intratumorally. Two-dimensional tumour measurements were performed with a caliper every other day until euthanasia due to excessive tumour burden. Tumour size was calculated by using the modified ellipsoid formula 1/2(Length × Width2) (mm3).
Statistical analysis
All data analyses were performed using Microsoft Excel and values were expressed as mean ± SD. Statistical differences were assessed using Student's t test. P < 0.05 was considered to be significantly different.
Results
Susceptibility of canine lymphoma cell lines to reovirus-induced cell death
In order to test the susceptibility of canine lymphoma towards reovirus infection, a total of 10 established canine lymphoma cell lines were screened for reovirus infection at a high MOI of 70 in order to clearly distinguish between the reovirus-susceptible and resistant cell lines. At 72hpi, trypan blue exclusion test revealed the direct reovirus-induced cell death in four out of 10 cell lines (Fig. 1A; P < 0.05). Next, to determine if reovirus-susceptible cell lines could sustain the infection, the amount of progeny virus produced was measured by TCID50 assay and the fold increase of reovirus was compared among the cell lines. The three cell lines that has the highest susceptibility to reovirus, CL-1, 17-71 and CLC, had a dramatic increment of reovirus titer (Fig. 1B). On the contrary, there was only a minimal increment of reovirus titer in GL-1, consistent with the low percentage of cell death. Morphological characteristics of cytopathic effects in the reovirus-susceptible cell lines such as granular appearance, cell clumping and loss of shape are shown in Fig. 1C. Among the cell lines that were susceptible to reovirus, one was T-cell lymphoma (CL-1), two were B-cell lymphoma (17-71 and GL-1) and the cell type of CLC was undetermined.

Ras activation status does not correlate with reovirus susceptibility
The baseline GTP-loading status of Ras was determined for the 10 canine lymphoma cell lines in order to investigate the involvement of Ras activation as the molecular determinant for reovirus susceptibility. Using Raji as the standard of Ras activation, GL-1, Ema, Nody-1, CLGL-90, CLBL-1, CLC and CLK had elevated Ras activities while Ras was not activated in CL-1, UL-1 and 17-71 (Fig. 2). Comparison of the susceptibility of the cell lines to reovirus with their activated Ras status revealed that even though CL-1 and 17-71 are highly susceptible to reovirus, they did not express higher GTP-bound Ras levels. This finding indicates that mechanisms other than the activation of Ras are involved in reovirus susceptibility in these cells.

Reovirus infectivity varies in canine lymphoma cell lines
Reovirus induced cell death gradually in a time-dependent manner from 24hpi to 72hpi in CL-1 and 17-71 but not in GL-1 and CLC (Fig. 3A). Minimal cell death was detected in CLC at 24hpi while the percentage of cell death in GL-1 remained the same from 48 to 72hpi. In Fig. 3B, the viability of the reovirus-susceptible cell lines was compared using reovirus at various MOI of 2.8, 14 and 70. Cell death was induced by reovirus in a MOI-dependent manner. Unlike the other cell lines, CL-1 had a higher susceptibility to reovirus at a low MOI, suggesting that there exists a difference in the sensitivity towards reovirus between the cell lines. In order to confirm that cell death was induced by reovirus, SDS-PAGE and Western blotting were carried out to detect the reovirus µ and σ proteins at 48hpi. Cells were not collected at 72hpi as the cells were adequately infected by reovirus at 48hpi to allow detection of reoviral proteins. The results confirmed that µ and σ proteins were detected only in the reovirus-susceptible cell lines but not in the two representatives of the reovirus-resistant cell lines, Nody-1 and CLGL-90 (Fig. 3C).

Reovirus induces canine lymphoma cell death via apoptosis
Previous reports have indicated that reovirus induces apoptosis in susceptible cell lines.40, 41 Therefore, in this study, apoptotic cell death due to reovirus was investigated using PI staining, Annexin V-FITC/ PI assay, detection of PARP cleavage and treatment of Z-VAD-FMK. As expected, the percentage of PI-stained cells increased from 48 to 72hpi in all the reovirus-susceptible cell lines (Fig. 4A). Subsequent experiment to elucidate the ratio between apoptotic and necrotic cells after reovirus infection was carried out using the Annexin V-FITC/ PI assay in 17-71, CLC and GL-1 (Fig. 4B). The results of CL-1 were not shown because of a high percentage of false positive stained by PI in the mock-infected CL-1. The percentage of reovirus-infected 17-71, CLC and GL-1 in late apoptosis (A + P+) was higher than those in early apoptosis (A + P−) in both 48 and 72hpi. The number of necrotic cells (A−P+) in the reovirus-infected cell lines was consistently lower than apoptotic cells (A + P− and A + P+) at both time points.
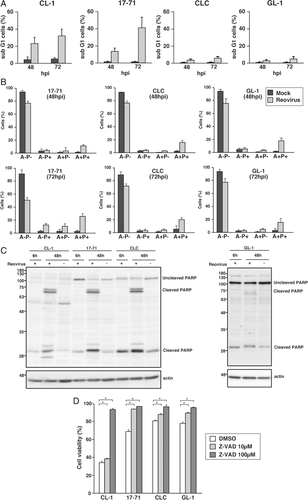
The cleavage of cellular substrate PARP is a hallmark of apoptosis. Signature cleavage product in CL-1, 17-71, CLC and GL-1 was not detectable at an earlier time point but became obvious at 48hpi (Fig. 4C). Cleaved PARP in GL-1 was the faintest among the cell lines, consistent with the level of susceptibility to reovirus. As PARP cleavage can be detected at 48hpi, samples at 72hpi was not carried out. On top of that, pre-treatment of canine lymphoma cell lines with Z-VAD-FMK before reovirus infection allowed the inhibition of reovirus-induced apoptosis in a dose-dependent manner (Fig. 4D; P < 0.05). Cell viability was quantified at 48hpi because co-culture with Z-VAD-FMK beyond 48 h seriously affected the viability of canine lymphoma cell lines. The combination of these four results strongly supports that the major cell death pathway induced by reovirus is apoptosis.
Reovirus suppresses growth of canine lymphoma mass in vivo
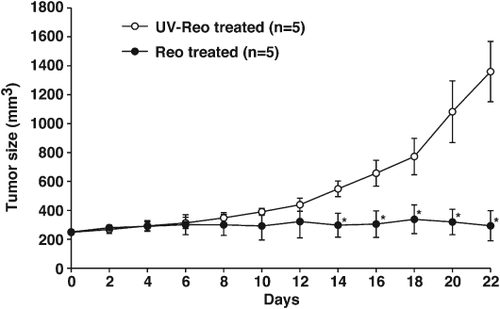
To assess the therapeutic potential of reovirus in canine lymphoma in vivo, CL-1 unilateral subcutaneous xenograft models were established in NOD/SCID mice and treated with a single intratumoral of reovirus injection. All mice treated with reovirus experienced significant supression of tumour growth by day 14 post-treatment as compared to those treated with UV-inactivated reovirus (Fig. 5A; P < 0.05).
Discussion
Research in oncolytic virotherapy has been impressive in the last decade in parallel with the advancement of technology. Many related studies have been carried out in human oncology, to the extent of phase III clinical trials. In comparison, this field in veterinary oncology is still lagging, with several oncolytic viruses being tested in preclinical settings such as adenovirus, CDV and vaccinia virus.42 However, none of these studies has proceeded to clinical trials as of yet. In hope to widen the availability of oncolytic viruses as therapeutic options in veterinary oncology, our laboratory is focusing on the feasibility of reovirus in the treatment of canine cancers.
We have previously demonstrated that reovirus induces prominent cell death in canine mast cell tumour (MCT) cell lines and primary MCT cells, followed by production of a high viral progeny titer. This phenomenon was dictated by apoptosis as the major cell death pathway.39 This subsequent study highlights the cytotoxic effects of reovirus in canine lymphoma cell lines and the cell death pathway involved. A panel of 10 established canine lymphoma cell lines was used to assess their susceptibility to reovirus. Out of these cell lines, six were T-cell lymphoma, three were B-cell lymphoma and one cell line with an undetermined cell type. Four of the cell lines were susceptible to the effects of reovirus as indicated by reovirus-induced cytotoxicity (Fig. 1A), viral progeny production (Fig. 1B) and reovirus infectivity (Fig. 3C). However, there was no distinguishable pattern as to which cell type had a higher susceptibility to reovirus (Table 1). Similar studies of the effects of reovirus in a variety of human NHL have also been performed. However, the susceptibility of these neoplasms towards reovirus ranges markedly, from sensitive to resistant.28-30 Similarly, the results of this study using canine lymphoma cell lines revealed noticeable differences in the susceptibility to reovirus. CL-1 and 17-71 were highly susceptible to reovirus (Figs. 3A and 4A) but CL-1 proved to be more sensitive at a lower MOI (Fig. 3B).
| Cell line | Cell type | Reovirus susceptibilitya | Viral progeny productionb | Ras activation |
|---|---|---|---|---|
| CL-1 | T cell | +++ | +++ | – |
| 17-71 | B cell | +++ | +++ | – |
| CLC | Undetermined | ++ | +++ | + |
| GL-1 | B cell | + | + | + |
| Ema | T cell | – | – | + |
| UL-1 | T cell | – | – | – |
| Nody-1 | T cell | – | – | + |
| CLGL-90 | T cell | – | – | + |
| CLBL-1 | B cell | – | – | + |
| CLK | T cell | – | – | + |
- a Reovirus susceptibility: – No significant difference between cell viability of mock and reovirus-infected cells; + <20% cell death; ++ 20–50% cell death; +++ >50% cell death.
- b Viral progeny production: – No significant increment as compared to input virus titer, + <5× increment of virus titer; ++ 5–10× increment of virus titer; +++ >10× increment of virus titer.
The major cell death pathway induced by reovirus was initially investigated by PI staining (Fig. 4A) and Annexin V-FITC/ PI assay (Fig. 4B). The detection of apoptotic cells via these two techniques proved to show differences which were more obvious in cell lines less susceptible to reovirus. On the other hand, similar percentage of cell death can be detected in the cell lines using trypan blue exclusion test (Fig. 1A) and Annexin V-FITC/ PI assay. The ratio of apoptotic to necrotic cells can be clearly seen in the Annexin V-FITC/ PI assay, where most of the reovirus-infected cells were confirmed to be undergoing apoptosis. However, the conventional Annexin V-FITC/ PI protocol was unable to be applied on CL-1 as there was a high percentage of cells that was falsely stained with PI. It has been reported that conventional Annexin V/ PI protocols can lead to as high as 40% false positives and it is associated with PI staining the RNA within the cytoplasmic compartment.43 Perhaps a modified Annexin V/ PI method that changes the cellular permeability during cell fixing to promote the entry of RNase A into cells after staining can prevent cells from falsely stained with PI.44
The reovirus-susceptible cell lines underwent apoptotic cell death in a caspase-dependent manner after reovirus infection (Fig. 4C, D), which is consistent with a previous report.22 Reovirus-induced apoptosis took place slower in canine lymphoma cell lines as compared to canine MCT, where the cleavage of PARP was detected as early as 6hpi in the MCT cell lines.39 Besides apoptosis, reovirus infection also leads to necrosis,45 necroptosis46 and autophagy47 in human cancer cells. As such, this emphasizes the need for additional studies to find out how reovirus utilises these cell death pathways in order to pinpoint the exact mechanism of reovirus oncolysis.
The initial studies on the mechanism of reovirus-induced cell death suggested that Ras transformation in cells promotes three reoviral replication steps, which are proteolytic disassembly, viral infectivity and efficient reovirus release.22 However, subsequent studies have shown that the situation is highly complex, and no definitive biomarker of sensitivity to reovirus has been identified as of yet.48 Similar to the canine MCT,39 the level of activated Ras in the canine lymphoma cell lines did not correlate to reovirus susceptibility in this study, (Fig. 2; Table 1). Indeed, it cannot be denied that there exist other mechanisms of reovirus-induced cell death yet to be explored. At the moment, ongoing studies are being conducted to elucidate the mechanism behind reovirus-induced cell death in canine cancers.
Reovirus serves as an attractive option for cancer therapy. As the wild-type reovirus is naturally cytotoxic and replicates specifically in cancer cells, the utilization of reovirus seems to be superior to the other genetically engineered oncolytic viruses. The minimal side effects that were reported in human clinical trials provide further reassurance of the safety of administration of reovirus. On top of that, the life cycle of reovirus takes place in the cytoplasm and does not include a stage of viral DNA synthesis.49 Therefore, it is very unlikely that reoviral genome fragments would integrate into the DNA and cause harmful mutations. Even though the primary mode of action of oncolytic viruses is to induce direct destruction of cancer cells, increasing evidence has suggested that these viruses further invoke the development of anti-tumour immune response.50 This immune response attacks the existing cancer cells and further protects the host against subsequent tumour challenges, even after the discontinuation of the therapy.51
From our studies, it seems that reovirus as a monotherapy has limited therapeutic efficacy in canine lymphoma in vitro. Therefore, a different approach using a combination of reovirus and chemotherapies can perhaps improve the anti-tumour activity in canine lymphoma cells. Clinical trials in humans have shown the synergistic effects of reovirus combination therapy such as docetaxel,52 carboplatin and paclitaxel.53 Besides exploiting the direct oncolytic effects of reovirus, gemcitabine has also been reported to enhance the efficacy of reovirus through anti-tumoral responses.54 Currently, we are investigating the effects of reovirus and chemotherapy agents in canine cancers as means to enhance oncolysis, especially in the clinical setting in the future.
Acknowledgements
We sincerely thank Dr. Hajime Tsujimoto (The University of Tokyo), Dr. Yasuhiko Okamura (Iwate University), Dr. Munekazu Nakaichi (Yamaguchi University), Dr. Barbara Rütgen (University of Veterinary Medicine Vienna), Dr. Maxey Wellman (The Ohio State University) and Dr. Steven E. Suter (North Carolina University) for the cell lines used in this study. We would also like to extend our appreciation to Dr. Malaika Watanabe and Dr. Mark Hiew Wen Han (Universiti Putra Malaysia) for their critical comments in writing this article.
Conflict of interest
All authors have no potential conflicts of interest to disclose except that Matt Coffey is the Chief Operating Officer of Oncolytics Biotech Inc. and has an ownership interest in that company.




