Autologous regulatory T cells in clinical intraportal allogenic pancreatic islet transplantation
ClinicalTrials.gov number: NCT04820270.
Summary
Allogeneic islet transplantation in type 1 diabetes requires lifelong immunosuppression to prevent graft rejection. This medication can cause adverse effects and increases the susceptibility for infections and malignancies. Adoptive therapies with regulatory T cells (Tregs) have shown promise in reducing the need for immunosuppression in human transplantation settings but have previously not been evaluated in islet transplantation. In this study, five patients with type 1 diabetes undergoing intraportal allogeneic islet transplantation were co-infused with polyclonal autologous Tregs under a standard immunosuppressive regimen. Patients underwent leaukapheresis from which Tregs were purified by magnetic-activated cell sorting (MACS) and cryopreserved until transplantation. Dose ranges of 0.14–1.27 × 106 T cells per kilo bodyweight were transplanted. No negative effects were seen related to the Treg infusion, regardless of cell dose. Only minor complications related to the immunosuppressive drugs were reported. This first-in-man study of autologous Treg infusion in allogenic pancreatic islet transplantation shows that the treatment is safe and feasible. Based on these results, future efficacy studies will be developed under the label of advanced therapeutic medical products (ATMP), using modified or expanded Tregs with the aim of minimizing the need for chronic immunosuppressive medication in islet transplantation.
Introduction
Pancreatic islet transplantation in type 1 diabetics is a low invasive therapeutic option that reduces the need for exogenous insulin and improves glycemic control, thus improving both morbidity and mortality [1]. The main drawback of this treatment is the need for chronic immunosuppressive medication to prevent graft rejection. Such drugs increase the susceptibility for infections and malignancies, and includes risks for drug toxicity and other significant side effects [2, 3]. While immunosuppressive regimens have improved over time, graft loss is still a major hurdle both in short term and long term [4]. Thus, alternative options to improve graft acceptance and reduce side effects are sought.
Regulatory T cells (Tregs) are a subset of CD4+ T cells with immunomodulatory properties crucial for prevention of autoimmune disease and excessive inflammation [5]. These properties have raised hope for their use in autoimmune disease and allogenic transplantation settings. Adoptive Tregs therapies have shown potential in preventing graft-versus-host disease (GVHD) in allogenic stem cell transplantation and for prolonging beta cell survival in newly onset type 1 diabetes [6-9]. Patients who have undergone liver and kidney transplantation coupled with Treg therapy show reduced need for standard immunosuppression [10, 11]. Also, increased Treg numbers in renal allografts has been associated with more favorable long-term outcome [12]. The main goal in the development of Treg therapies is to promote a tolerogenic state toward the transplanted graft and minimize the need for immunosuppressive medication. While standard immunosuppressive drugs can prevent graft rejection, some may hamper tolerance induction; commonly used immunosuppressants such as Basiliximab and Tacrolimus targets T-cell proliferation and function, which inhibits both effector T cells and Tregs [13, 14]. It is therefore important to both reduce the need for immunosuppressive drugs and design regimens that supports tolerance induction.
While no previous clinical trials of Treg therapy in islet transplantation have been published, studies are now being performed at major islet transplant centers. The first step is to evaluate the safety of the treatments via phase I/II trials, preferably including dose-ranging. If initial safety studies are successful, further studies may be performed evaluating the efficacy of the treatment and exploring whether the immunosuppressive medication can be reduced. Future trials may also attempt at increasing the effectiveness of the therapy, for example, by increasing Treg doses through cell expansion or by virally inducing cell modifications, which would classify the treatments as advanced therapy medicinal products (ATMPs).
In the present study we explored the safety and feasibility of infusing autologous polyclonal Tregs during intraportal allogenic islet transplantation in type 1 diabetics. Clinical features of the islet transplantation such as C-peptide, HbA1c levels, independence from exogenous insulin, hypoglycemic awareness and incidence of severe hypoglycemic events were registered.
Materials and methods
Patient selection
The study was approved by the Regional Ethics Committee (Dnr 2017/1805-32) Stockholm, Sweden, and was performed in accordance with local institutional and Swedish national rules and regulations. All participants were provided oral and written information and signed a written consent form prior to inclusion in the study. The inclusion criteria were as follows: age 18–65, debut of type 1 diabetes before age 40, treated with exogenic insulin >5 years, C-peptide <0.1 nmol/l after mixed meal tolerance test (MMTT) if not previously transplanted, failure to maintain acceptable glycemic control under optimal medical care, ability to understand and sign informed consent. Exclusion criteria were as follows: previous transplantations other than islet transplantation, BMI >30, daily insulin requirement >1 IU/kg/day, repeated abnormal liver function tests, unstable retinopathy, coagulation disorder, malignancy (except radically resected squamous cell carcinoma or basal cell carcinoma), unstable heart disease, active infection, serological evidence of HIV, hepatitis B or C infection, portal hypertension, pregnancy, breastfeeding, intent to become pregnant during the study period, immunization with panel reactive antibodies (PRA) >20%, positive cross-match test, known donor-specific antibodies (DSA) or patients with a condition, which the responsible physician deemed inappropriate to undergo transplantation.
Study design
The aim of the study was to assess the safety of combining autologous Treg infusion with intraportal allogeneic pancreatic islet transplantation. It was conducted at Karolinska University Hospital, Stockholm, and Uppsala University Hospital, Uppsala in Sweden. The study is registered at ClinicalTrials.gov with identifier NCT04820270. Procedures related to the islet transplantation were performed in accordance with guidelines from The Nordic Network for Clinical Islet Transplantation. Patients were followed 90 ± 14 days after transplantation. The primary endpoints were: bleeding in relation to the islet transplantation (a reduction of hemoglobin >20 g/l and intraperitoneal blood found at ultrasound examination postoperative day); portal vein thrombosis in relation to the islet transplantation visible in ultrasound examination; elevated transaminases above five times normal upper limits; infections requiring medical treatment; immunization, defined as occurrence of new anti-HLA-antibodies after transplantation; graft loss (MMTT stimulated C-peptide below 0.1 nmol/l). The secondary endpoints were: number of patients with systemic C-peptide levels >0.1 nmol/l fasting and >0.3 nmol/l after MMTT; number of patients without need for exogenous insulin treatment; percental reduction of HbA1c; result of Clarke hypoglycemia awareness score compared to baseline; number of patients with both a HbA1c ≤6.5%; diabetes control and complications trial (DCCT), and no incidences of severe hypoglycemic events; number of patients with an increase in (PRA) >10%.
Regulatory T-cell purification
Enrolled patients underwent leukapheresis. The leukapheresis product was stored overnight in room temperature before in vitro enrichment the following day (Supporting Information Fig. S1). A sterile enclosed CliniMACS system (Miltenyi Biotec, Bergisch-Gladbach, Germany) was used for Treg purification according to manufacturer’s instructions. Briefly, the cell product was first depleted from CD8 and CD19 positive cells through negative selection, then enriched for CD25 positive cells by positive selection, using CD8, CD19, and CD25 positive microbead reagents (Miltenyi Biotec). CliniMACS PBS/EDTA buffer (Miltenyi Biotec) supplemented with 0.25% human serum albumin was used for cell selection and cell preparation. Samples were analyzed for endotoxins and bacterial growth to ensure sterility. Release criteria were ≥60% CD4/CD25 positive cells and viability ≥70%. After purification the cell product was supplemented with cell freezing medium including 80% pooled plasma, 20% dimethyl sulfoxide (DMSO) and 20 IE/ml heparin. The product was cooled to −160 °C in a controlled rate freezer before cryopreservation in liquid nitrogen.
Islet isolation
Pancreases from brain-dead donors used for islet isolation were distributed through Scandiatransplant to The Nordic Network for Clinical Islet Transplantation. Islets were isolated according to clinical routine as previously described [15] at the Rudbeck Laboratory, Uppsala, Sweden.
Islet transplantation and regulatory T-cell infusion
Cryopreserved Tregs were thawed in a 37 °C water bath on the day of transplantation. The thawed cells were washed with sodium chloride solution supplemented with 2.5% albumin, followed by centrifugation and removal of the supernatant. Aliquots were taken for cell counting and flow cytometric analysis of phenotype and viability. Islets and Tregs were transported separated in platelet transfusion bags to the transplantation site and thereafter infused simultaneously by gravity through a percutaneous transhepatic cannula in the hepatic confluence of the portal vein [16]. Patients were treated with a modified Edmonton immunosuppressive regimen [17]. Basiliximab was used for induction and Tacrolimus/Everolimus preferred as maintenance.
Flow cytometric analyses
Cell sample analysis by flow cytometry was performed at multiple times before, during and after the CliniMACS-process to monitor the quality of the cell product. The expression of CD4, CD8, CD14, CD15, CD20, CD25, CD45, CD127, and FOXP3 was analyzed. Red blood cells were eliminated from the cell samples by the use of red blood cell lysis solution (Miltenyi Biotec) prior to antibody staining. Cell viability was analyzed using Via-Probe 7-Amino-actinomycin D (7-AAD) solution (Miltenyi Biotech) (Supporting Information Fig. S2). Cells were prepared for intracellular staining using FoxP3 Staining Buffer Set (eBioscience, Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. The following antibodies were used for analysis: CD8-APC, CD14-PE, CD15-PE, CD20-FITC, CD45-VioBlue, CD127-FITC, Treg-Detection-kit, all from Miltenyi Biotec; CD4-BV421 and CD25-PE from BioLegend, San Diego, CA, USA; FoxP3-Alexa Fluor 647 from BD Biosciences. All flow cytometric analyses were performed on a FACSVerse™ (BD Biosciences), and the data were analyzed by flowjo version 10.7.0 (FlowJo LLC, Ashland, OR, USA).
Statistical analysis
Data is expressed as mean ± standard deviation (SD) or range. Mean and SD were calculated using Microsoft Excel version 16.17 (Microsoft Corporation, Redmond, WA, USA).
Results
Selected patients
Five patients were enrolled in the study (Table 1), three males and two females, with a mean age of 50 years (±12). Three had undergone islet transplantations before (all with partial function) and came for a repeated transplant procedure. Two underwent their first transplantation.
| Patient | Gender | Transplant number | Weight (kg) | BMI | Daily insulin U/kg/day | HbA1c (IFCC) | Clarke score | Creatinine |
|---|---|---|---|---|---|---|---|---|
| RN | Male | 5 | 86 | 30.8 | 0.23 | 61 | nr | 86 |
| CA | Female | 2 | 69 | 25 | 0.3 | 44 | 3 | 77 |
| AG | Female | 4 | 59 | 21.3 | 0.3 | 44 | 6 | 90 |
| BJ | Male | 1 | 85 | 28.5 | 0.9 | 67 | 6 | 99 |
| AB | Male | 1 | 55 | 16.6 | 0.55 | 51 | 7 | 80 |
Regulatory T-cell purification
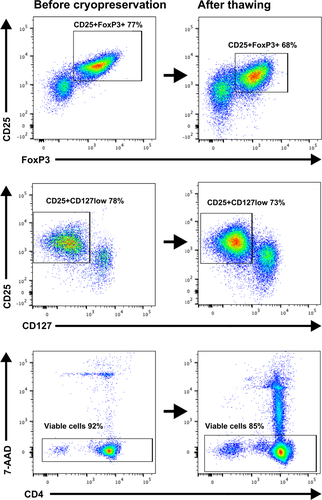
The leukapheresis yielded 183.73 (±44.43 SD) × 106 white blood cells per kilo bodyweight with a viability of 98.78% (±0.91 SD) (Table 2). After enrichment by CliniMACS 1.19 (±0.88 SD) × 106 T cells per kilo, bodyweight were isolated, with a CD4/CD25 positivity of 62.28% (±16.56 SD) and a viability of 81.54% (±6.28 SD) (Fig. 1). After storage by cryopreservation and thawing prior to transplantation, 0.63 (±0.42 SD) × 106 T cells per kilo bodyweight remained, which presented a cell loss of 45.36% (±27.43 SD). The final cell product showed a CD4/CD25 positivity of 65.60% (±11.21 SD), a CD4/CD25/FoxP3 positivity of 63% (±15.82 SD), and a viability of 85,4% (±6.82 SD). One patient presented a CD4/CD25 positivity of 34%, which did not reach the release criteria of ≥60% CD4/CD25 positivity. The patient was still included in the study and received the cell infusion based on the number of Tregs. Sterility was ensured with no signs of endotoxins or bacterial growth.
| Patient | Leukapheresis cell yield* | CliniMACS cell yield* | Transplanted cells* | CD4/CD25 positivity (%) | CD4/CD25/FoxP3 positivity (%) | Viability (%) |
|---|---|---|---|---|---|---|
| RN | 206.40 | 0.42 | 0.14 | 72 | 74 | 73 |
| CA | 106.48 | 0.49 | 0.19 | 44 | 34 | 93 |
| AG | 162.03 | 0.78 | 0.85 | 67 | 60 | 90 |
| BJ | 223.94 | 1.51 | 0.71 | 76 | 79 | 86 |
| AB | 219.82 | 2.78 | 1.27 | 69 | 68 | 85 |
- The latter three columns show flow cytometric data of the transplanted cells.
- * All cell counts are denominated as 106 cells per kilo bodyweight.
Primary endpoints
The primary endpoints were met for all patients in the study, with no thrombotic events, elevated transaminases, graft failure, or new donor-specific antibodies. One patient showed a small intraperitoneal bleeding day 1 after the transplantation but did not need any intervention or blood products. Another patient had mouth ulcers that led to readmittance and switch from Everolimus to Mycophenolate mofetil (MMF) two months after the transplantation. Other adverse events were limited to mild leucopenia and a urinary tract infection in one patient.
Secondary endpoints
All secondary endpoints were measured at 75 days post transplantation, except PRA levels that were measured at 90 days. All patients showed stimulated C-peptide levels above 0.3 nmol/l. No patients were considered insulin independent at day 75. The mean HbA1c was reduced from 7.0 (range 6.2–8.3) to 6.3% (range 5.7–6.6). The mean insulin doses were reduced from 32 (range 17–76) to 16 (range 8–30). No severe hypoglycemic events were reported. Three out of three patients with Clark score registered both pre and post-transplant presented a mean score reduction from 5.0 to 1.7. None of the patients showed an increase in PRA.
Discussion
This first-in-man study of autologous Treg infusion in allogenic pancreatic islet transplantation shows that the treatment is safe and feasible. This study also shows for the first time that Tregs can be infused in the portal vein and co-infused with pancreatic islets without adverse events. These findings may have important implications for islet transplantation specifically. In the majority of previously reported Treg therapies, the cell product is administered systemically by intravenous infusion in the periphery. Studies suggest that Tregs can home to inflamed regions of the transplanted graft and migrate to nearby secondary lymphoid organs, where they reduce alloimmune reactions locally [18]. In islet transplantation, intraportal infusion of both graft and Tregs may theoretically be preferable to allow for rapid migration of Tregs to local lymph nodes, where they can suppress antigen presentation and hamper T-cell activation. However, potential complications of the infusion must be taken into consideration, such as portal thrombosis or other coagulation events derived from the instant blood mediated inflammatory reaction (IBMIR). It is, therefore, important to evaluate the safety of intraportal co-infusion of islets and Tregs.
Today clinical islet transplantation is hurdled by graft loss and side effects from long-term immunosuppressive medications [2-4]. Novel methods to improve islet transplantation are needed, preferably treatments that both promote graft acceptance and minimize the need for immunosuppressive medications. The ultimate treatment would induce tolerance of the transplanted graft, which can be described as a state in which the immune system of the recipient of a tissue or organ does not reject the transplanted tissue. In humans, adoptive Treg therapies with ranging doses of 0.1–30 × 106 T cells per kilo bodyweight have shown promise in prevention of GVHD after allogeneic stem cell transplantation, for prolonging beta-cell survival in newly onset type 1 diabetes and in reducing the need for standard immunosuppression in kidney and liver transplantation [6, 7, 9-11]. Also, murine studies of islet transplantation show a vital role for Tregs in tolerance induction and prolonged graft survival after adoptive Treg therapy [19-21]. Thus, Treg-based therapies may hold promise of improving allogeneic islet transplantation.
In this study, autologous Tregs were co-infused with allogeneic pancreatic islets under a standard immunosuppressive regimen [17]. No severe adverse events were reported, only minor complications nonrelated to the Treg infusions. Importantly, no thrombotic events were reported. This suggest that co-infusion of Tregs and islets is safe and feasible, in line with previous studies of Treg therapies in various transplantation settings [9, 10, 22]. In this study dose ranges from 0.14 to 1.27 × 106 cells per kilo bodyweight were infused, which are rather small doses compared to other studies of adoptive Treg therapies. Cell expansion is commonly required to obtain large Treg numbers and previous trials in other transplantations settings suggest that doses between 0.1 × 106 and 20 × 106 polyclonal natural Tregs per kilo of bodyweight are needed to reach therapeutic levels [6, 7, 9, 23]. Studies show that T1D patients present the same frequency of Tregs as nondiabetics. However, some data that suggest that T1D Tregs have altered function and are less suppressive [24]. In contrast, a promising study of Treg therapy in T1D using ex vivo expanded autologous Tregs presented highly functional Tregs even after expansion [22]. Overall, caution is needed when introducing new therapies that modulate the immune system. While there is limited long-term data available regarding Treg therapies, immunosuppression can increase the risk of malignancies and infections as previously mentioned [2, 3]. Also, studies show that large number of Tregs at tumor sites are associated with poor prognosis [25]. Therefore, future trials with scaling Treg doses would be of interest for studying levels of Tregs both in blood and in transplant biopsies and correlate obtained findings with therapeutic efficacy and side effects.
A limitation of this study is the small patient cohort. This, in combination with patient heterogeneity and polypharmacy, makes potential effects of the treatment harder to assess. The trial was also complicated by the onset of the SARS-CoV-2 pandemic. When five patients had shown no signs of adverse events, we choose not to further enroll participants out of safety and logistical considerations. Studies of larger patient cohorts are needed, as well as efficacy studies that aimed to optimize the impact of the procedure. A potential way to improve the efficacy may be the use of donor antigen-specific Tregs, which have been found more effective than polyclonal Tregs [26]. A mouse study by Cabello-Kindelan et al. [27] of antigen-specific Treg infusions in islet transplantation showed robust Treg engraftment and induced remission in all mice. They also found a larger presence of islet-specific Tregs within the islets, compared to polyclonal Tregs. A hypothetical method for using of antigen-specific Tregs in islet transplantation is to culture and cryopreserve Treg clones with specificities to different HLA alleles. When a donor pancreas is available, selected HLA-matched Treg clones can be thawed and used for infusion during transplantation.
Other options to improve the efficacy of Treg therapies is by tailoring the immunosuppressive regimen. In this study no changes were made to the immunosuppressive regimen. By following regulatory demands, the trial was designed as a phase I/II study with focus solely on the safety of the procedure, not on optimizing or withdrawal of immunosuppressive drugs. Based on the results from this study, we aimed to make a following efficacy study in which the immunosuppressive medications are reduced. In the current study four immunosuppressive drugs were given to the patients: double-dose induction with Basiliximab, followed by continuous medication with Tacrolimus and Everolimus. One patient did not tolerate Everolimus and was switched to Mycophenolate mofetil (MMF) after two months. While these medications differ in their mechanism of action they all have potential effects on both effector T cells and Tregs: Basiliximab is a monoclonal antibody targeting the IL-2 receptor alfa-chain CD25, which is highly expressed on both activated effector T cells and Tregs [28]. While Basiliximab is effective for suppressing effector T cells, studies show transient effect on Tregs of unknown clinical importance [13]. Tacrolimus hampers calcineurin signaling in T cells and has pleiotropic suppressive effects on cell function and transcription, but is not known to promote Tregs [14]. Everolimus is an inhibitor of the intracellular kinase mammalian target of Rapamycin (mTOR), which can hamper effector T-cells expansion while preserving Tregs [29]. The mTOR inhibitors are well studied and commonly used for improving in vitro Treg expansions. MMF inhibits de novo purine synthesis, which hampers B- and T-cell proliferation. While its effect on human Tregs have not been elucidated, murine studies show that MMF treated Tregs preserve their function [30]. Notably, in this study the average MACS cell yield was less than a third in the previously transplanted patients who were on immunosuppression compared with the two nontransplanted recipients. Future studies that focus on tailoring an optimal immunosuppressive regimen for Treg therapy will be of interest.
In conclusion, this study shows that intraportal co-infusion of autologous polyclonal Tregs with allogenic islets in clinical islet transplantation is safe and feasible. These results lay the foundation for future efficacy studies and development of more advanced Treg therapies with the aim of minimizing the need for chronic immunosuppressive medication in islet transplantation.
Authorship
MB: performed study, analyzed data, wrote paper. MY: performed study, collected and analyzed data, revised paper. MM: collected and analyzed data. OK: study design, performed study, collected and analyzed data, revised paper. BvZ-M: performed study, collected and analyzed data. TL: study design, performed study, analyzed data, revised paper.
Funding
This study was supported by grants from the Swedish Medical Research Council (2019-01415), and by the Strategic Research Project in Diabetes, Karolinska Institute, Stockholm, Sweden.
Conflicts of interest
The authors of this manuscript have no conflicts of interest to disclose.
Acknowledgements
The authors thank Andrew Friberg, Anna Högvall, Rosanna Skoog and Sofie Ingvast for their assistance and efforts.
Open Research
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




