Association of graft survival with tacrolimus exposure and late intra-patient tacrolimus variability in pediatric and young adult renal transplant recipients—an international CTS registry analysis
Summary
Adolescent and young adult age is a high-risk window with an alarmingly increased likelihood of premature kidney graft loss due to immunological rejection. Using the large database of the Collaborative Transplant Study, we analyzed whether a more intense and less variable exposure to tacrolimus could counteract this young age-related enhanced immunoreactivity. Kidney graft recipients aged 12–23 years (n = 964) with a 1-year tacrolimus trough level between 4.0 and 10.9 ng/ml had a 5-year graft survival rate of 85.1%, significantly better than the poor 66.1% rate in patients with a trough level below 4.0 ng/ml who showed a 2.38-fold increased risk of graft loss in the multivariable analysis (P < 0.001). This association was not apparent in young children aged 0–11 years (n = 455) and less pronounced in adults aged 24–34 years (n = 1466). However, an intra-patient variability of tacrolimus (IPV) trough level ≥1.5 at post-transplant years 1 and 2 was associated with an increased graft loss risk in both 12- to 23-year-old and 0- to 11-year-old recipients (P < 0.001 and P = 0.045). Patients with high IPV made up as many as 30% of kidney graft recipients, indicating that a more intense and less variable exposure to tacrolimus could improve graft survival strongly in this high-risk group.
Introduction
Renal transplantation is the treatment of choice for children and adolescents with end-stage renal disease, because it improves long-term patient survival, reduces overall morbidity, and ensures near-normal cognitive and psychomotor function [1-4]. In recent decades, graft survival in the pediatric population has significantly improved [5-8]. Among multiple factors such as better operative procedures and improved selection of deceased kidney donors [8], this trend is also attributed to the widespread use of potent agents for induction and maintenance immunosuppressive therapy [5, 6].
Multiple studies, however, highlighted the adolescent age as a still existent high-risk window with an alarmingly increased likelihood of premature graft loss [9-13]. No recipient subgroups are exempt from the dramatic increase in graft loss during late adolescence and early adulthood, a high-risk window that modifies the relationship between typical recipient risk factors, such as donor type, panel-reactive antibodies, transplant history, insurance type and cause of renal disease, and graft loss [10]. The main contributing factor is certainly poor adherence to immunosuppressive medication [14, 15]; however, biologic differences related to a maturing immune system may also add to the increased rejection risk in this age group [16, 17].
Nonadherence to immunosuppressive medication is, due to its nature, challenging to assess. Therefore, investigators have focused on indirect indicators such as low trough levels of immunosuppressive agents [18, 19]. In addition, measurement of tacrolimus intra-patient variability (IPV), which is highly linked to long-term graft survival [13], has recently emerged as an indirect tool for estimating medication adherence in solid organ recipients [20]. In adult renal transplantation, Borra et al. [21] were the first to report that a high tacrolimus IPV was associated with reduced kidney transplant survival. In the pediatric patient population, one single-center case–control study recently reported that patients with late acute cellular rejection had a higher tacrolimus IPV than matched patients with stable graft function [22]. Two of the main limiting factors in studies regarding outcome in pediatric transplantation in relation to tacrolimus IPV is the small total number, due to relatively few pediatric kidney recipients and the monocentric study design, which reduces the generalizability of obtained results.
Contrary to broad scientific agreement about the risk associated with under-dosing of immunosuppressive agents, clinical data regarding optimal exposure or trough levels of immunosuppressive agents remain sparse, especially for maintenance therapy of graft recipients [23, 24]. In particular, there are no reliable data concerning the pediatric and adolescent population. We hypothesized that a more intense and less variable exposure to the main immunosuppressant tacrolimus could counteract the enhanced immune reactivity in the high-risk age group of adolescents and young adults and result in better graft survival. To this end, we investigated the association of tacrolimus trough levels beyond the 1st post-transplant year and of late tacrolimus IPV with long-term kidney graft survival as a robust endpoint in a large multicenter cohort of pediatric and young adult renal transplant recipients.
Materials and Methods
This is a retrospective study of pediatric and young adult renal transplant recipients reported to the Collaborative Transplant Study (CTS) database (www.ctstransplant.org) [25], who received a kidney transplant from a deceased donor between January 1, 2001, and December 31, 2017 (Fig. 1). Patients with multi-organ transplantations, missing follow-up data, graft loss within the first year post-transplant, and patients on mammalian target of rapamycin inhibitors were excluded. The patients were aged between 0 and 23 years at time of transplantation and were followed up to end of year 5 post-transplant. The upper age limit of 23 years was chosen as previous studies had suggested that renal graft survival in young adults is comparable to those of adolescent recipients [9-12]. For comparison, the subsequent age group of 24- to 34-year-old adult renal transplant recipients was also analyzed (Fig. 1).
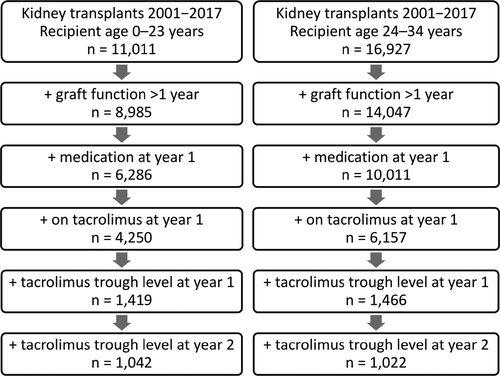
The CTS registry records information on follow-up at year 1 post-transplant and then annually. Patient data for this study were derived from over 100 centers in 31 countries. The work of the CTS is approved by the Ethics Committee of the Medical Faculty of Heidelberg University (No. 083/2005) and performed in accordance with the World Medical Association Declaration of Helsinki Ethical Principles in the currently valid version.
The first request for information on immunosuppression was made at post-transplant year 1 in patients with a functioning graft and then at years 2, 3, and 5. On the basis of this data collection, for the analysis of the effect of variability of recorded values on late graft survival, the values after the first post-transplant year are the best choice because they are obtained in a stable phase of medication after transplantation. Therefore, patients with a functioning graft at year 1 post-transplant, who were on tacrolimus medication during the first post-transplant year and on whom the information was available on dosage and tacrolimus C0 trough levels at year 1 and year 2, were analyzed (Fig. 1). The term “graft survival during post-transplant years 2–5” refers to patients with a functioning graft at 1 year post-transplant, who might loose their graft during the years 2–5 post-transplant.
We examined the intra-patient variability (IPV) of tacrolimus trough levels based on the post-transplant 1- and 2-year tacrolimus trough level. IPV was calculated as the ratio of the respective higher value divided by the respective lower value. Mantel–Cox log-rank (with trend) test was used for analysis of the association of both tacrolimus trough levels and intra-patient variability of tacrolimus trough levels with graft survival. In the investigated age range between 0 and 34 years, the annual mortality was 0.6% only, without significant differences between the subgroups. Because of this negligible difference between overall graft survival and death-censored graft survival, we report only data on overall graft survival. Multivariable Cox regression analysis was performed to account for the possible influence of the following confounders: transplant year, geographical region, transplant number, recipient and donor age and gender, original disease, HLA-A + B + DR mismatches, pretransplant antibodies, time and type of dialysis, cold ischemia time, induction therapy, immunosuppression at year 1 (type of antimetabolite, steroids), 1-year systolic blood pressure, and 1-year serum cholesterol. All confounders were suitably categorized, and the few missing values (<5%) were included in the analysis either with missing data indicators or by imputation methods. Unfortunately, confounders such as delayed graft function (DGF), estimated glomerular filtration rate, and proteinuria with a possible association could not be considered due to missing or, in the case of DGF, between the centers greatly varying information. A back-step elimination algorithm was used to exclude confounding factors with a threshold of P values >0.2. The interactions between confounders were also considered, and in the case of recipient age, further analyses were performed. Chi-squared (with trend) and Mann–Whitney U-test were used for the comparison of the demographic characteristics. The software package ibm spss Statistics version 25.0 (SPSS Inc., IBM Corporation, Somers, NY, USA) was used. P values below 0.05 were considered statistically significant.
Results
Study population
Between the years 2001 and 2017, the CTS registry lists 11 011 patients between the age of 0 and 23 years who had received a renal allograft from a deceased donor (Fig. 1). In 6286 patients (57%), data about graft function and medication at 1 year post-transplant were available. Of those, 4250 patients were on a tacrolimus-based immunosuppressive regimen at year 1 post-transplant. Corresponding tacrolimus trough levels were available in 1419 patients, in 1042 patients also at year 2 post-transplant. The patient group of 1419 patients was stratified into the age group 0–11 years (presumably pre-adolescent patients) and the age group 12–23 years (adolescents and young adults). The rationale for this stratification was that according to previous reports, graft survival in adolescents and young adults is significantly lower than in pre-adolescent patients [9-12]. The upper age limit of 23 years was chosen as previous studies had suggested that renal graft survival in young adults is comparable to those of adolescent recipients [9-12]. Twenty-four- to 34-year-old patients were analyzed for comparison (Fig. 1). Table 1 shows the respective demographic and disease characteristics of these three cohorts and Table 2 the number of graft failures after 1 year and after 2 years post-transplant. Graft survival during post-transplant years 2–5 in patients with known 1-year tacrolimus trough levels did not significantly differ from the remaining patients with different medication or missing tacrolimus trough levels, indicating that the analyzed patient cohort is representative for this patient population in general (Fig. S1).
| Characteristic | Unknown (%) | Recipient age | P | ||
|---|---|---|---|---|---|
|
0–11 years n = 455 |
12–23 years n = 964 |
24–34 years n = 1466 |
|||
| Transplant year | |||||
| 2001–2008 | – | 141 (31) | 297 (31) | 492 (34) | 0.95 |
| 2009–2017 | 314 (69) | 667 (69) | 974 (66) | ||
| Transplant number | |||||
| First transplant | 0.1 | 428 (94) | 861 (89) | 1,203 (82) | 0.002 |
| Retransplant | 25 (6) | 102 (11) | 263 (18) | ||
| Recipient sex | |||||
| Female | – | 184 (40) | 415 (43) | 636 (43) | 0.35 |
| Male | 271 (60) | 549 (57) | 830 (57) | ||
| Time on dialysis (months) | |||||
| No dialysis | 0.9 | 36 (8) | 60 (6) | 29 (2) | 0.24 |
| Mean ± SD | 27.0 ± 20.4 | 31.3 ± 32.0 | 62.3 ± 48.9 | 0.86 | |
| Original disease | |||||
| Glomerular | 0.6 | 90 (20) | 218 (23) | 342 (23) | <0.001 |
| Congenital | 160 (35) | 179 (19) | 120 (8) | ||
| Other | 202 (45) | 562 (59) | 996 (68) | ||
| Donor sex | |||||
| Female | 0.2 | 175 (39) | 371 (39) | 578 (39) | 0.99 |
| Male | 278 (61) | 590 (61) | 888 (61) | ||
| Donor age (years) | |||||
| Mean ± SD | – | 20.2 ± 15.5 | 24.8 ± 16.4 | 40.1 ± 15.0 | <0.001 |
| Cold ischemia time (h) | |||||
| Mean ± SD | 2.4 | 17.0 ± 6.5 | 19.3 ± 7.6 | 18.9 ± 7.7 | <0.001 |
| HLA-A + B + DR mismatches | |||||
| Mean ± SD | 4.1 | 3.5 ± 1.2 | 3.3 ± 1.4 | 2.8 ± 1.4 | 0.012 |
| 1-year systolic BP (mmHg) | |||||
| Mean ± SD | 2.4 | 108 ± 12 | 120 ± 15 | 126 ± 15 | <0.001 |
| 1-year tacrolimus TL (ng/ml) | |||||
| Mean ± SD | – | 6.8 ± 2.4 | 7.3 ± 2.6 | 7.7 ± 2.8 | <0.001 |
- BP, blood pressure; SD, standard deviation; TL, trough level.
- Chi-squared or Mann–Whitney test are used.
| Recipient age (years) | Years 2–5 post-transplant | Years 3–5 post-transplant | ||
|---|---|---|---|---|
| N | Number of graft failures | N | Number of graft failures | |
| 0–11 | 455 | 22 | 345 | 11 |
| 12–23 | 964 | 131 | 697 | 78 |
| 24–34 | 1466 | 161 | 1022 | 84 |
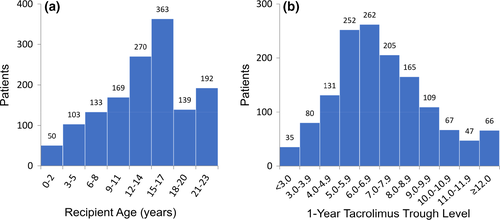
Figure 2a shows the distribution of recipient age at transplantation in patients with graft survival greater than 1 year and known 1-year tacrolimus trough level. The patient number in each 2-year age period increased with recipient age, peaked in the 15- to 17-year-old group and declined thereafter, most likely due to priority for pediatric patients in most organ allocation systems. Figure 2b displays the distribution of 1-year tacrolimus trough levels in this patient cohort. There was a near-normal distribution of data with most patients being in the range between 4.0 and 10.9 ng/ml; 8.1% showed a tacrolimus trough level < 4.0 ng/ml and 8.0% a trough level ≥ 11.0 ng/ml.
Graft survival in relation to recipient age and 1-year tacrolimus trough level
As illustrated in Fig. S2, overall graft survival at year 5 post-transplant differed significantly among the age groups; patients between 0–5 years or 6–11 years of age (presumably pre-adolescent children) had a significantly better graft survival during post-transplant years 2–5 than patients between 12–17 years or 18–23 years (P < 0.001), while graft survival in patients aged 24–29 and 30–34 years was intermediate. Associated differences between the age categories 0–5 and 6–11 years or 12–17 and 18–23 years were not statistically significant (P = 0.37 and P = 0.44, respectively).
Next, we stratified the patient cohort according to the 1-year tacrolimus trough level into the following three subgroups: (i) <4.0 ng/ml (low tacrolimus exposure), (ii) 4.0–10.9 ng/ml (standard tacrolimus exposure), and (iii) ≥11.0 ng/ml (high tacrolimus exposure). While in young patients (aged 0–11 years), 5-year graft survival was not significantly different among the three subgroups (Fig. 3a), and graft survival differed significantly in relation to the 1-year tacrolimus trough level in adolescents and young adults (12–23 years of age; Fig. 3b). Adolescents and young adults with a 1-year tacrolimus trough level between 4.0 and 10.9 ng/ml had a 5-year graft survival rate of 85.1% compared to the extremely low 66.1% rate in patients with a trough level of <4.0 ng/ml. Comparable results were obtained when the tacrolimus trough level was below 4 ng/ml at year 1 post-transplant, at year 2, or both at years 1 and 2 (results not shown). Also, comparable results were obtained when the 1-year tacrolimus trough level was >6.0 ng/ml or 4.0–5.9 ng/ml (results not shown). In adults 24–34 years of age, the difference in 5-year graft survival among the three subgroups was much less pronounced (Fig. 3c). When patients with a functioning graft at year 2 were analyzed, in all three age groups a low 1-year tacrolimus trough level <4.0 ng/ml was associated with a more than 2-fold higher incidence of treated rejection episodes during the second post-transplant year (Fig. S3, supplemental digital content), which supports the assumption that the mechanism of the negative association of a low 1-year tacrolimus trough level with graft survival is rejection-related.
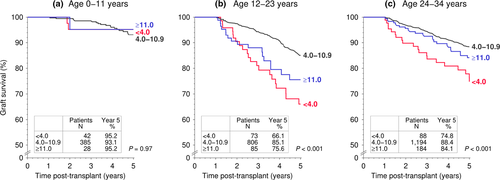
In adolescent and young adult patients, the risk of premature graft loss associated with a low 1-year tacrolimus trough level <4.0 ng/ml was 2.38-fold higher compared to a trough level of 4.0–10.9 ng/ml, whereas the risk was not significantly increased in recipients aged 0–11 years (Table 3). In 24- to 34-year-old adult patients, the risk of premature graft loss due to a low 1-year tacrolimus trough level <4.0 ng/ml was with 1.94-fold increase lower than the 2.38-fold increase in adolescent and young adult patients (Table 3). When the 12- to 23-year-old group was further stratified into adolescent (12–17 years of age) and young adult (18–23 years of age) patients, a comparably increased risk of premature graft loss was observed in both groups [12–17 years, hazard ratio (HR) 2.23; 18–23 years, HR = 2.85; Table 4]. A 1-year tacrolimus trough level ≥11.0 ng/ml in 12- to 23-year-old patients was associated with a 1.72-fold increased risk of premature graft loss compared to a trough level of 4.0–10.9 ng/ml; there was no significantly increased risk of a tacrolimus trough level ≥11.0 ng/ml in 0- to 11-year-old patients or in 24- to 34-year-old patients (Table 3, Fig. 3).
| Recipient age | N | HR | 95% CI | P value |
|---|---|---|---|---|
| 0–11 years | ||||
| Trough level | ||||
| <4.0 | 42 | 0.90 | 0.19–4.20 | 0.90 |
| 4.0–10.9 | 385 | 1 (ref) | – | – |
| ≥11.0 | 28 | 0.52 | 0.07–4.06 | 0.53 |
| Ratio | ||||
| <1.5 | 242 | 1 (ref) | – | – |
| ≥1.5 | 103 | 3.86 | 1.03–14.4 | 0.045 |
| 12–23 years | ||||
| Trough level | ||||
| <4.0 | 73 | 2.38 | 1.47–3.86 | <0.001 |
| 4.0–10.9 | 806 | 1 (ref) | – | – |
| ≥11.0 | 85 | 1.72 | 1.01–2.91 | 0.046 |
| Ratio | ||||
| <1.5 | 488 | 1 (ref) | – | – |
| ≥1.5 | 209 | 2.47 | 1.57–3.87 | <0.001 |
| 24–34 years | ||||
| Trough level | ||||
| <4.0 | 88 | 1.94 | 1.19–3.17 | 0.008 |
| 4.0–10.9 | 1194 | 1 (ref) | – | – |
| ≥11.0 | 184 | 1.37 | 0.88–2.13 | 0.17 |
| Ratio | ||||
| <1.5 | 713 | 1 (ref) | – | – |
| ≥1.5 | 309 | 1.76 | 1.14–2.74 | 0.012 |
- ref, reference.
- Hazard ratios (HR) with 95% confidence interval (CI) of low tacrolimus trough level <4.0 ng/ml and high ratio of tacrolimus trough levels at 1 and 2 years ≥1.5 are shown. The HR were adjusted for the following variables: recipient age, geographical region, recipient and donor gender, HLA-A + B + DR mismatches, primary renal disease, 1-year systolic blood pressure, immunosuppressive medication at year 1. Significant differences are indicated in bold.
| Recipient age | N | HR | 95% CI | P value |
|---|---|---|---|---|
| 12–17 years | ||||
| Trough level | ||||
| <4.0 | 49 | 2.23 | 1.21–4.12 | 0.010 |
| 4.0–10.9 | 535 | 1 (ref) | – | – |
| ≥11.0 | 49 | 1.52 | 0.72–3.22 | 0.27 |
| Ratio | ||||
| <1.5 | 321 | 1 (ref) | – | – |
| ≥1.5 | 152 | 2.11 | 1.23–3.61 | 0.006 |
| 18–23 years | ||||
| Trough level | ||||
| <4.0 | 24 | 2.85 | 1.27–6.41 | 0.011 |
| 4.0–10.9 | 271 | 1 (ref) | – | – |
| ≥11.0 | 36 | 2.24 | 1.00–5.01 | 0.050 |
| Ratio | ||||
| <1.5 | 167 | 1 (ref) | – | – |
| ≥1.5 | 57 | 4.05 | 1.63–10.1 | 0.003 |
- ref, reference.
- Hazard ratios (HR) with 95% confidence interval (CI) of low tacrolimus trough level <4 ng/ml and high ratio of tacrolimus trough levels at 1 and 2 years ≥1.5 are shown. The HR were adjusted for the following variables: recipient age, geographical region, recipient and donor gender, HLA-A + B + DR mismatches, primary renal disease, 1-year systolic blood pressure, immunosuppressive medication at year 1. Significant differences are indicated in bold.
Graft survival in relation to intra-patient variability of tacrolimus trough levels
We analyzed the association of intra-patient variability (IPV) of tacrolimus trough levels at post-transplant years 1 and 2 with subsequent graft survival during post-transplant years 3–5. As reported previously [13], a simple and practically easily applicable method for calculating IPV is determining the maximum ratio between two consecutive tacrolimus trough level values. An IPV of 30% corresponds hereby to a trough level ratio of 1.54 and an IPV of 45% to a ratio of 1.93. Therefore, we considered the ratio cutoffs 1.5 and 2.0 as a simple method to judge variability.
The majority of 0- to 23-year-old patients (730 of 1042, 70%) had a tacrolimus trough level ratio of <1.5, 201 patients (19%) a ratio between 1.5 and 1.9 and 111 patients (11%) a tacrolimus trough level ratio of ≥2.0 (Fig. S4). There was a highly significant (P < 0.001) association of IPV of tacrolimus trough level with graft survival during post-transplant years 3–5 (Fig. S4). We observed a stepwise reduction of 5-year graft survival with increasing IPV. Patients with a trough level ratio <1.5 had an excellent 5-year graft survival rate of 92.7%, whereas patients with a high trough level ratio ≥2.0 had a graft survival rate of only 79.9%. Intermediate graft survival rates were observed in patients with a ratio of 1.5–1.9. As illustrated in Fig. S5, it did not make a statistically significant difference whether the reason for IPV was a decrease or an increase of the tacrolimus trough level from year 1 to year 2.
Next, we analyzed the association of the IPV of tacrolimus trough levels with graft survival during post-transplant years 3–5 for the age groups 0–11, 12–23 and 24–34 years separately (Fig. 4). The percentage of patients with an IPV ≥1.5 was comparable in all three age groups with a rate of approximately 30%. In contrast to the association of low 1-year tacrolimus trough level, which was absent in 0- to 11-year-old recipients, IPV of tacrolimus trough levels was associated with graft survival both in the 0–11 and 12–23 years age group. The risk of graft loss increased 2.5-fold in 12- to 23-year-old patients with a tacrolimus trough level ratio ≥1.5 (multivariable Cox regression HR = 2.47, P < 0.001; Table 3) as compared to patients with a low tacrolimus trough level ratio <1.5. The IPV’s association with graft survival was evident in 0- to 11-year-old young children with a 3.86-fold and in 24- to 34-year-old adults with a 1.76-fold increased risk (P = 0.045 and 0.012, respectively; Table 3).
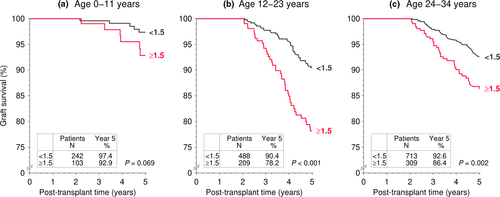
When the 12- to 23-year-old cohort was stratified into adolescent patients (12–17 years of age) and young adults (18–23 years of age), the association of IPV of tacrolimus trough levels with graft survival during post-transplant years 2–5 remained strong and was comparable in both groups; the corresponding hazard ratios of a tacrolimus trough level ratio ≥1.5 as a risk factor for graft loss were 2.11 for 12- to 17-year-old and 4.05 for 18- to 23-year-old patients (P = 0.006 and 0.003, respectively; Table 4).
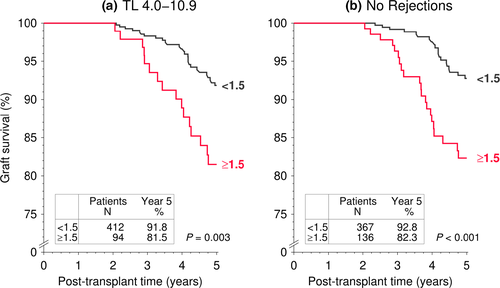
Next, we analyzed the association of IPV of tacrolimus trough levels with graft survival during post-transplant years 2–5 in the high-risk 12- to 23-year cohort in detail (Fig. 5). A high IPV of tacrolimus trough level in this group showed a negative association with graft survival even in the absence of known risk factors, such as tacrolimus trough level <4.0 ng/ml at years 1 and 2 post-transplant or previous rejection treatments during the first 2 post-transplant years (for both comparisons P < 0.001 as compared to patients with an IPV <1.5), indicating that a high IPV of tacrolimus trough level is an independent risk factor for graft survival. In pediatric and young adult patients (0–23 years of age), the combined exposure measures tacrolimus trough level >4 ng/ml both at year 1 and year 2 post-transplant and a tacrolimus IPV < 1.5 was associated with the best graft survival, while the combination of a tacrolimus trough level <4 ng/ml and a tacrolimus IPV ≥1.5 was associated with the worst graft survival (results not shown). The corresponding multivariable Cox regression analysis showed that the risk of premature graft loss associated with the combined exposure measures low tacrolimus trough level <4.0 ng/ml both at years 1 and 2 post-transplant and an IPV ≥1.5 was 3.52-fold higher compared to a trough level of ≥4.0 ng/ml and an IPV <1.5 (Table 5).
| Tacrolimus trough level and IPV | N | HR | 95% CI | P value |
|---|---|---|---|---|
| ≥4.0 ng/ml and <1.5 | 686 | 1 (ref) | – | – |
| ≥4.0 ng/ml and ≥1.5 | 210 | 2.36 | 1.43–3.87 | <0.001 |
| <4.0 ng/ml and <1.5 | 44 | 2.56 | 1.07–6.08 | 0.034 |
| <4.0 ng/ml and ≥1.5 | 102 | 3.52 | 2.00–6.22 | <0.001 |
- IPV, intra-patient variability.
- Hazard ratios (HR) with 95% confidence interval (CI) are shown.
Discussion
This is the first study that reports an association between tacrolimus exposure and graft survival in adolescents and young adults. In this observational registry study of a large cohort of 1419 pediatric or young adult renal transplant recipients and a control group of 1466 24- to 34-year-old recipients from more than 100 different transplant centers, we investigated the age-dependent influence of tacrolimus exposure on graft survival. In 12- to 23-year-old adolescents and young adults, a tacrolimus trough level below 4.0 ng/ml at year 1 was associated with an extremely poor 5-year graft survival of 66% and an approximately 2.4-fold increased risk of graft loss during the post-transplant years 2–5 as compared to patients with a trough level of 4.0–10.9 ng/ml. Such a strong influence of a low tacrolimus trough level was missing in younger children aged 0–11 years. Importantly, if the trough level was in the range of 4.0–10.9 ng/ml, also the group of 12- to 23-year-old recipients had a good 5-year graft survival of 85%, which was not very much different than the 88% survival rate observed in 24- to 34-year-old adult patients (Fig. 3). Hence, it appears that optimal tacrolimus exposure can at least partially counteract the enhanced immunoreactivity in this high-risk age group.
Numerous previous studies have shown that adolescents and young adults have the highest risk of graft failure, irrespective of age at transplant [9-12]. As reasons for this premature graft loss, late acute cellular rejections and de novo development of donor-specific HLA antibodies leading to acute and chronic antibody-mediated rejection [6, 19] have been described [19, 26-28]. In our study, a 1-year tacrolimus trough level <4.0 ng/ml was associated with a comparably increased incidence of treated rejection episodes during the second post-transplant year in all investigated age groups, while 5-year graft survival was not significantly worse in 0- to 11-year-old children. A possible explanation for this discrepancy is a worse rejection reversal outcome in adolescents and young adults despite adequate rejection treatment, leading to a disproportionately higher rate of premature kidney graft failure, as has been demonstrated by several observational long-term studies [28, 29].
While this enhanced immunoreactivity during adolescence and young adulthood is usually ascribed to socio-behavioral mechanisms and poor adherence to the immunosuppressive drug regimen [14, 15], biologic differences related to a maturing immune system may also contribute to the increased rejection risk in this age group [16, 17]. During the last years, the hypothesis of a more vigorous immune system during adolescent and young adult years has emerged, as the innate and adaptive immune system undergoes profound maturation during this phase [30]. Concerning the adaptive immune system, late childhood and adolescence comes with significant changes in the composition of peripheral blood mononuclear cell subsets [31, 32]. More recently, it has also been claimed that immune activation triggered by sex hormones during puberty could increase the risk of premature graft loss [33]. Irrespective of the specific reasons for the enhanced immunoreactivity, an obvious therapeutic strategy could be a more effective immunosuppressive therapy to improve outcome in these patients, beside a thorough patient education to prevent or improve nonadherence to immunosuppressive medication. Our observation of an association of a 1-year tacrolimus trough level of 4.0–10.9 ng/ml with better 5-year graft survival is a first step toward new age-specific immunosuppressive strategies to improve graft outcome in adolescents and young adults.
An indirect assessment of adherence is the measurement of tacrolimus IPV [6, 20]. A high IPV has been linked to late graft rejection and premature graft loss in kidney transplant recipients [20]. The negative association of high IPV with graft survival appears to be especially strong in adolescents and young adults (Fig. 4), which is in line with previous reports [13, 19-21]. Our results on graft survival in relation to IPV support these findings, as adolescents and young adults displayed the worst graft survival if they had an IPV ≥1.5, even in the absence of a 1-year tacrolimus trough level below 4 ng/ml or rejection treatments during post-transplant years 1 and 2. Importantly, patients with high IPV made up as many as 30% of adolescent and young adult kidney graft recipients. It is noteworthy, however, that the percentage of patients with a high IPV of ≥1.5 was comparable in all 3 age groups. These findings are in line with the results of other studies [18, 27]. Besides nonadherence, factors such as time of tacrolimus administration, interference of food and other drugs, and genetic polymorphisms of drug-metabolizing enzyme and transporters may also contribute to variable tacrolimus exposure.
The strengths of our study are the multicenter study design, the large sample size which allowed a statistically validated analysis, and the use of the robust end-point “graft survival.” However, there are also several limitations of our study. First, as this study is a retrospective registry analysis, we cannot state causalities but only associations. Our results are based on one or two consecutive measurements of tacrolimus trough levels in a two-year period; thus, below-critical trough levels of tacrolimus at some time between these measurements would not be taken into account, although being potentially clinically meaningful. A high tacrolimus IPV could also be due to iatrogenic changes in target trough levels, for example as a result of impaired graft function. However, our finding that a high IPV was associated with poorer graft survival even in the absence of a low tacrolimus trough level or a previously treated rejection episode is a strong indicator that IPV itself is independently associated with for poor graft survival. Also, high IPV could be associated with increased calcineurin inhibitor nephrotoxicity even in low tacrolimus trough level regimens [34, 35]. Transplant-associated infections like CMV or BKV infection could also have an association with high IPV (several intervals of over-immunosuppression) and poor graft survival [36, 37]. Another point is that patients included in this study underwent kidney transplantation between 2001 and 2017. In this period, the tacrolimus assays have likely changed in some centers. A problem with immunoassays such as the enzyme-linked immunosorbent assay and microparticle enzyme immunoassay is the cross-reactivity of the antibody with tacrolimus metabolites. Nowadays, the liquid chromatography–tandem mass spectrometry technique is being increasingly applied, yielding 15–25% lower tacrolimus concentrations in comparison with immunoassays as the cross-reactivity with tacrolimus metabolites is eliminated [38-40]. This might have been a potential factor contributing to a higher intra-patient tacrolimus level variability in some patients. Furthermore, the galenics of tacrolimus (immediate release vs. prolonged release) could have an impact on IPV, but the number of pediatric patients on prolonged release formulations of tacrolimus is too low to be influential in our analyses. Finally, this study included deceased donor kidney recipients only. We cannot exclude that the advantages of a live donor transplantation might have possibly counterbalanced the association between tacrolimus trough levels or intra-patient variability in tacrolimus trough levels and allograft outcomes.
In conclusion, our data indicate that a more consistent and less variable exposure to the main immunosuppressant tacrolimus later than 1 year post-transplant is associated with a better 5-year graft survival especially in adolescents and young adults, presumably by counteracting the enhanced alloreactivity in this high-risk age group.
Authorship
A.G., B.T. B.D. and C.S. participated in research design and performance of the research. B.D. and C.S. participated in data analysis. A.G., B.T., B.D., and C.S. participated in the writing of the paper.
Funding
The authors declare no funds were received for this study.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
We would like to give special thanks to the following 105 CTS centers from 31 countries which provided the information upon which this study is based: Aachen, Ankara, Augsburg, Belfast, Belo Horizonte, Berlin, Bern, Bochum, Bogota, Bonn, Buenos-Aires, Cali, Cape Town, Carshalton, Cologne, Dublin, Düsseldorf, Edmonton, Erlangen-Nuremberg, Essen, Florence, Fort Wayne, Frankfurt, Freiburg, Fulda, Gdansk, Geneva, Giessen, Glasgow, Göttingen, Goiania, Hamilton, Hannover, Heidelberg, Helsinki, Hong Kong (seven), Izmir (two), Jena, Jerusalem, Kaiserslautern, Katowice, Leuven, Liege, Linz, Liverpool, Ljubljana, Lübeck, Lyon, Maceio, Malatya, Manchester, Mannheim, Mar del Plata, Maracaibo, Marburg, Martin, Medellin (two), Mexico City, Milan, Modena, Moscow, Münster, Neiva, New York, Newcastle u Tyne, Osijek, Oviedo, Padua, Pamplona, Pato Branco, Pecs, Poitiers, Prague, Quebec, Regensburg, Reims, Rio de Janeiro (two), Rome, Rostock, Santander, Sao Paulo (two), St. Gallen, Strasbourg, Stuttgart, Tehran, Tel Aviv, Tübingen, Turin, Ulm, Uppsala, Valdivia, Valencia, Winnipeg, Würzburg, and Zurich. Open access funding enabled and organized by Projekt DEAL.