Glomerular C4d deposits can mark structural capillary wall remodelling in thrombotic microangiopathy and transplant glomerulopathy: C4d beyond active antibody-mediated injury: a retrospective study
Summary
Peritubular capillary C4d (ptc-C4d) usually marks active antibody-mediated rejection, while pseudolinear glomerular capillary C4d (GBM-C4d) is of undetermined diagnostic significance, especially when seen in isolation without concurrent ptc-C4d. We correlated GBM-C4d with structural GBM abnormalities and active antibody-mediated rejection in 319 renal transplant and 35 control native kidney biopsies. In kidney transplants, ptc-C4d was associated with GBM-C4d in 97% by immunofluorescence microscopy (IF) and 61% by immunohistochemistry (IHC; P < 0.001). Transplant glomerulopathy correlated with GBM-C4d (P < 0.001) and presented with isolated GBM-C4d lacking ptc-C4d in 69% by IF and 40% by IHC. Strong isolated GBM-C4d was found post year-1 in repeat biopsies with transplant glomerulopathy. GBM-C4d staining intensity correlated with Banff cg scores (rs = 0.45, P < 0.001). Stepwise exclusion and multivariate logistic regression corrected for active antibody-mediated rejection showed significant correlations between GBM duplication and GBM-C4d (P = 0.001). Native control biopsies with thrombotic microangiopathies demonstrated GBM-C4d in 92% (IF, P < 0.001) and 35% (IHC). In conclusion, pseudolinear GBM-C4d staining can reflect two phenomena: (i) structural GBM changes with duplication in native and transplant kidneys or (ii) active antibody-mediated rejection typically accompanied by ptc-C4d. While ptc-C4d is a dynamic ‘etiologic’ marker for active antibody-mediated rejection, isolated strong GBM-C4d can highlight architectural glomerular remodelling.
Introduction
Pioneering work by H. Feucht and colleagues on the complement degradation product C4d began a new era of research on antibody-induced graft injury 1-5. Over the past 15 years, C4d has attracted much interest, and has become an important biomarker for the diagnosis and classification of active antibody-mediated rejection in solid organ transplant biopsies 6-15.
In ABO compatible kidney allografts, C4d deposits along peritubular capillaries (ptc-C4d) follow a dynamic expression pattern 9. Ptc-C4d staining is found during active antibody-mediated graft rejection (AMR) with acute tissue injury, and vanishes following therapeutic intervention with reduction in circulating donor-specific antibodies. The ptc-C4d staining pattern is scored according to Banff guidelines on the interpretation of allograft pathology 11 and incorporated into diagnoses. However, C4d deposits in other anatomic compartments of the kidney, such as in glomeruli, are currently incompletely characterized and are not used to diagnose active rejection 9-11, 15. For example, mesangial C4d staining by immunofluorescence microscopy (IF) is interpreted to be ‘normal’ 16. Distinct granular intra-glomerular C4d staining in native and transplanted kidneys typically marks the presence of immune complexes and glomerulonephritides 17, 18. In renal allografts, linear or pseudolinear glomerular basement membrane C4d deposits (GBM-C4d) are often 19-27 but not always 28, associated with signs of chronic active AMR including transplant glomerulitis, ptc-C4d and transplant glomerulopathy. Pseudolinear GBM-C4d in native kidney biopsies was reported in pre-eclampsia and some but not all renal biopsies with thrombotic microangioapthy 29-31. Thus, C4d deposition along GBM segments has been described in different forms of glomerular injury.
Previously, we hypothesized that in contrast to ptc-C4d, isolated pseudolinear GBM-C4d does not mark active ongoing AMR but might rather indicate structural GBM remodelling. Subsequently, this hypothesis got support from Chua et al. 29. The aim of the current analysis was to evaluate the prevalence and significance of pseudolinear GBM-C4d in a large cohort of renal biopsies. Special emphasis was placed on GBM-C4d and architectural glomerular capillary wall remodelling with subendothelial new lamina densa formation and duplication: Can GBM-C4d mark structural changes rather than active and ongoing AMR as highlighted by ptc-C4d? The answer to this question furthers our understanding of the pathophysiological mechanisms of GBM remodelling and the role of complement in structural damage and repair.
Materials and methods
Study cohorts
We reviewed 319 consecutively obtained diagnostic kidney allograft biopsies collected during a 64-month study period between February 2008 and February 2013 from 219 patients. The majority of allograft recipients were transplanted at the University of North Carolina in Chapel Hill between December 1991 and December 2012. A total of 140 biopsies were taken within the first year post-transplantation (44%); 39 between the first and the second year (12%); 133 after 2 years (42%) and 7 without local knowledge of the specific transplantation date (2%). The study biopsy group contained 219/319 ‘index’ biopsies, that is first biopsy obtained during study period, and 100/319 additional follow-up biopsies from 61/219 patients (1–5 follow-up biopsies per patient during study period). Implantation biopsies, cases of glomerulonephritides or polyomavirus nephropathy, and specimens from ABO incompatible transplants were not included into the study cohort. A comparative control group consisted of selected native renal biopsies from nine patients with minimal change disease and 26 patients with thrombotic microangiopathy of various underlying etiologies and GBM remodelling (n = 2 scleroderma renal crisis, n = 2 infection including typical HUS, n = 4 malignant neoplasms, therapeutic intervention including radiation, bone marrow transplantation, n = 1 systemic lupus erythematosus, n = 9 severe hypertension, n = 2 atypical HUS, n = 1 pre-eclampsia, n = 5 undetermined). This study was approved by the Institutional Review Board of UNC (10-1353).
Clinical data
The following clinical data were collected from the UNC hospital database: patient demographics, year of transplantation, cross-match positivity, panel reactive antibody positivity, donor-specific antibody positivity at time of grafting and during follow-up until end of study period 3/2013, evidence of a hepatitis C infection (see Appendix S1 for further details on antibody testing). Post-transplantation patients with clinical suspicion of AMR were tested for donor-specific antibodies. In this study, ‘antibody-positive cases’ were defined as patients having detectable donor-specific antibodies for HLA-class I and/or class-II at least once during the entire post-transplantation time period starting at time of grafting and ending 1 March 2013 and ‘antibody-negative cases’ as patients without detectable alloantibodies at any time during follow-up.
Histological analysis
All biopsies sufficed for rendering a diagnosis according to Banff criteria 32. Sections were prepared for light microscopy (LM) and immunofluorescence microscopy (IF) according to standard protocols. Tissue for electron microscopy (EM) analysis was collected from all transplants older than 1-year postgrafting and from all cases during year-1 with proteinuria and/or haematuria. C4d staining: For IF, 3-μm cryostat sections were incubated with a monoclonal anti-human C4d antibody (Quidel A213, diluted 1:50) following routine procedures. For immunohistochemistry (IHC), performed in a randomly selected representative subgroup of study cases, formalin-fixed and paraffin-embedded tissue sections were pressure cooked at 125 and 90 °C for antigen retrieval and subsequently incubated with a polyclonal rabbit anti-human C4d antibody (ALPCO Diagnostics® Cat.# 004-BIRC4d, diluted 1:20 and incubated for 30 min at 37 °C).
Scoring of histology
Two experienced renal pathologists (VN, AG) who were blinded to the patients’ clinical data and C4d staining results scored all renal biopsies according to Banff ‘97 and ‘09 criteria 32, 33. In allograft biopsies, transplant glomerulopathy (TG) was defined according to Banff ‘97 criteria based on LM (cg scores 0–3). The same approach was arbitrarily used for scoring native control kidney biopsies including GBM duplications in cases of chronic thrombotic microangiopathy.
Scoring of immunostaining
Along peritubular capillaries, C4d staining by IF and IHC was scored according to Banff criteria as absent (0), minimal (1; <10% of peritubular capillaries staining), focal (2; 10–50%) or diffuse positivity (3; >50%) 11. For study purposes, ptc-C4d was also categorized as either positive (for IHC at Banff scores ≥1 and for IF at Banff scores ≥2), or as negative. GBM-C4d staining along peripheral glomerular capillaries with linear and/or dense granular deposits, termed ‘pseudolinear GBM-C4d’ for current study purposes, was assessed separately; mesangial C4d staining was not analysed. Cases with staining along the entire capillary wall circumference of at least one perfused glomerular capillary in at least one glomerulus by IF or IHC were arbitrarily classified as ‘GBM-C4d positive’ (minimum positive cut-off). GBM scoring was performed by VN and AG and recorded as consensus result. The GBM-C4d staining intensity was semi-quantitatively scored for IF and IHC on a scale from 0 to 3+ as 0 (absent or trace), weak (1+), moderate (2+) or strong (3+). The GBM staining pattern was scored as either focal (<50% of the total number of glomeruli) or diffuse (≥50% of the total number of glomeruli) and as either segmental (<50% of the glomerular tuft) or global (≥50% of the glomerular tuft). A case of acute AMR with C4d staining along ptc served as comparative positive staining control.
Scoring of ultrastructural GBM remodelling
Glomeruli (at least two per case) were studied by EM to investigate ultrastructural evidence of GBM remodelling and duplication. Three experienced renal pathologists (VN, AG, HKS), who were blinded to the patients’ clinical data and C4d staining status, evaluated low- and high-power digital EM images from cases with at least eight inflated glomerular capillary loops available for review. Ultrastructural GBM changes were grouped based on ‘round-table’ consensus into four categories: (i) circumferential GBM duplication with widening of the lamina rara interna and subendothelial new densa formation (one or more layers) involving at least one entire circumference of at least one glomerular capillary loop; other loops could show different changes including widening of the rara interna; (ii) noncircumferential GBM duplication with widening of the lamina rara interna and subendothelial new densa formation involving less than an entire circumference in at least one capillary loop, circumferential GBM duplications per definition absent; (iii) lamina rara interna widening, activation of endothelial cells including loss of fenestration and cytoplasmic thickening but no subendothelial new densa deposition (following descriptions given by Wavamunno et al. 34); and (iv) normal GBM.
Statistical analyses
The statistical analysis was performed using spss 20.0 software (SPSS, Chicago, IL, USA) and the SAS (University Edition) statistical software package (SAS Institute Inc., Cary, NC) for multivariate exact logistic regression. P < 0.05 was considered statistically significant.
Continuous variables were expressed as mean ± SD. For comparison of numerical and categorical clinical and histological data, the Student's t-test, one-way anova, Chi-square and Fisher's exact tests were utilized. Relationships between different categories of GBM duplication and GBM-C4d staining intensities were expressed using Spearman's rank correlation, supplemented by testing of group differences with anova F-test. Multivariate logistic regression and multivariate exact logistic regression were used to determine independent factors associated with GBM-C4d. Successively eliminating variables with nonsignificant p-values starting with the highest p-value did not indicate the presence of multicollinearity in the full multivariate model.
Clinical data – Testing of circulating donor-specific antibodies (see Appendix S1 for details).
Immune electron microscopy (see Appendix S1 for details).
Results
Renal allografts
Glomerular basement membrane changes
Transplant glomerulopathy (TG) by LM was observed in 52/319 biopsies (16.3%; Banff ‘97 score cg-1: 9/52, cg-2: 2/52, cg-3: 41/52), collected from 35/219 patients (15.9%). EM, performed on 198/319 biopsies, showed GBM duplication in 61/198 cases (40 circumferential, 21 noncircumferential); 31/61 biopsies had concurrent signs of TG by LM and 30/61 only revealed ultrastructural GBM duplication (10 circumferential, 20 noncircumferential). Eleven of 52 cases of TG seen by LM were not accompanied by EM changes as a reflection of differences in lesion sampling. In total, 82/319 transplant biopsies (25.7%) demonstrated GBM duplications by LM and/or EM. By ultrastructural examination, 15/198 cases revealed widening of the lamina rara interna without GBM duplication and 111/198 biopsies were normal both by LM and EM.
C4d staining
Immunofluorescence staining
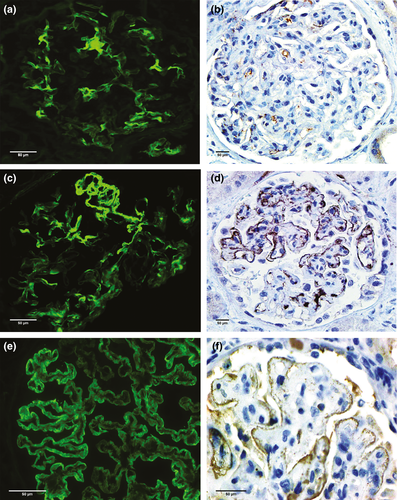
C4d staining along peritubular capillaries (ptc-C4d) by immunofluorescence microscopy (IF) was noted in 37/319 biopsies; in 12/37 with a focal distribution pattern (concomitant GBM-C4d deposits in all cases), and in 25/37 with a diffuse ptc-C4d pattern (concomitant GBM-C4d in 24/25 biopsies). Overall, pseudolinear GBM-C4d by IF was present in 196/319 transplant biopsies (61%, Table 1), 36/196 with concomitant ptc-C4d staining and 160/196 in isolation. The GBM-C4d staining intensity was strong in 30/319 (9%) and weak to moderate in 166/319 cases (52%). The staining pattern of biopsies with strong GBM-C4d staining was most frequently diffuse and global (24/30, 80%), whereas the staining of cases with weak-to-moderate staining was often focal and segmental (83/166, 50%) (Fig. 1).
| IF GBM-C4d | IHC GBM-C4d | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Absent (0+) | Weak (1+) | Moderate (2+) | Strong (3+) | Total | Absent (0+) | Weak (1+) | Moderate (2+) | Strong (3+) | Total | ||
| Degree of GBM duplication by LM (TG) | TG absent; cg0 | 120 (97) | 114 (90) | 27 (67) | 6a (20) | 267 (83) | 69 (87) | 7 (70) | 7 (47) | 3b (25) | 86 (74) |
| TG present; cg1 | 1 (1) | 5 (4) | 1 (3) | 2 (7) | 9 (3) | 2 (3) | 1 (10) | 2 (13) | 0 (0) | 5 (4) | |
| TG present; cg2 | 1 (1) | 1 (1) | 0 (0) | 0 (0) | 2 (1) | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | |
| TG present; cg3 | 1 (1) | 6 (5) | 12 (30) | 22 (73) | 41 (13) | 7 (9) | 2 (20) | 6 (40) | 9 (75) | 24 (21) | |
| Total | 123 (100) | 126 (100) | 40 (100) | 30 (100) | 319 (100) | 79 (100) | 10 (100) | 15 (100) | 12 (100) | 116 (100) | |
| Degree of GBM remodelling by EM | Normal GBM | 58 (77) | 47 (71) | 13 (43) | 2 (7) | 120 (60) | 37 (62) | 4 (50) | 1 (7) | 3 (27) | 45 (48) |
| Lamina rara interna widening | 9 (12) | 4 (6) | 4 (13) | 0 (0) | 17 (9) | 5 (8) | 0 (0) | 3 (21) | 0 (0) | 8 (9) | |
| Noncircumferential new lamina densa formation | 5 (7) | 8 (12) | 7 (23) | 1 (4) | 21 (11) | 9 (15) | 1 (13) | 1 (7) | 0 (0) | 11 (12) | |
| Circumferential new lamina densa formation | 3 (4) | 7 (11) | 6 (20) | 24 (89) | 40 (20) | 9 (15) | 3 (37) | 9 (64) | 8 (73) | 29 (31) | |
| Total | 75 (100) | 66 (100) | 30 (100) | 27 (100) | 198 (100) | 60 (100) | 8 (100) | 14 (100) | 11 (100) | 93 (100) | |
- GBM, glomerular basement membrane; LM, light microscopy; EM, electron microscopy; TG, transplant glomerulopathy by LM evaluated according to Banff ‘97 cg scoring criteria (cg-0 = no or only minimal TG by LM up to cg-3 = global, marked TG); IF, immunofluorescence microscopy; IHC, immunohistochemistry.
- Values are presented as number of biopsies (percentage).
- a Four of six cases with strong GBM-C4d by IF and no TG had GBM duplication by EM.
- b Three of three cases with strong GBM-C4d by IHC and no TG had concomitant ptc-C4d staining.
Immunohistochemical staining
ptc-C4d staining by immunohistochemistry (IHC) was noted in 31/116 cases; in 9/31 with a focal distribution pattern (concomitant GBM-C4d in 3/9 biopsies), and in 22/31 with diffuse staining (concomitant GBM-C4d in 16/22 biopsies). Overall, pseudolinear GBM-C4d by IHC was present in 37/116 transplant biopsies (32%, Table 1); 19/37 with concomitant ptc-C4d and 18/37 in isolation. The GBM-C4d staining intensity was strong in 12/116 (10%) and weak to moderate in 25/116 cases (22%). The staining pattern of biopsies with strong GBM-C4d was most frequently diffuse and global (n = 11/12, 92%), whereas the staining pattern of cases with weak-to-moderate staining was often focal and segmental (n = 10/25, 40%) (Fig. 1).
Both by IF and IHC, GBM-C4d staining was most pronounced under endothelial cells and podocytes; staining within thickened GBM segments was often less intense. The staining pattern was dense granular or linear, often detected side by side in the same glomerulus (termed ‘pseudolinear’; Fig. 1).
Glomerular basement membrane duplications and GBM-C4d
Light microscopy
TG was significantly associated with GBM-C4d staining both by IF 94% (49/52 TG) and IHC 67% (20/30 TG), P < 0.001 (Table 1). In particular, strong GBM-C4d expression corresponded with TG (24/30 biopsies, 80% with strong GBM-C4d by IF had TG and 9/12, 75% with strong GBM-C4d by IHC; P < 0.001). The GBM-C4d staining intensity by IF and IHC increased as Banff cg scores increased; this correlation was statistically significant (IF: rs = 0.453, P < 0.001; IHC: rs = 0.478, P < 0.001; Table 1). GBM-C4d in cases with TG was seen in isolation, that is without corresponding ptc-C4d, in 73% (36/49) of biopsies by IF and in 60% (12/20) by IHC.
Electron microscopy
Ultrastructural evidence of GBM duplication was significantly associated with GBM-C4d positivity (IF: 53 biopsies GBM-C4d positive/61 biopsies with GBM duplication by EM, 87%, P < 0.001; IHC: 22/40 cases, 55%, P < 0.01, Table 1). The C4d staining intensity correlated with the degree of ultrastructural GBM changes (IF: rs = 0.474, P < 0.001 and IHC: rs = 0.433; P < 0.001; Fig. 2). Cases with an unremarkable GBM by both LM and EM (n = 111) had no GBM-C4d staining by IF in 52% (85% by IHC), and weak-to-moderate GBM-C4d by IF in 47% (10% by IHC); strong GBM-C4d staining with normal GBM was exceptionally rare (1% by IF and 5% by IHC).
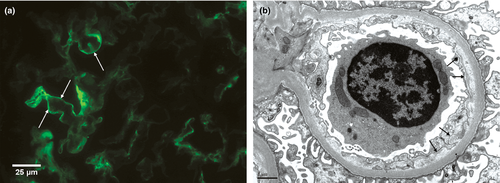
Also a combined cohort of 82 cases with GBM duplication either by LM (classical TG) and/or by EM demonstrated a significant association between GBM remodelling and GBM-C4d deposits (P < 0.001). In this group, GBM-C4d was seen in isolation without corresponding ptc-C4d in 62% (51/82) by IF and 31% (15/48) by IHC. By IF, the sensitivity and specificity of GBM-C4d for GBM duplication was 89% (73/82) and 48% (114/237), respectively. In comparison, isolated, strong GBM-C4d staining was less sensitive (22%, 18/82) but more specific (99%, 235/237). IHC, performed in 48/82 cases with GBM duplication and 68/237 cases without GBM duplication, had a sensitivity of 54% and a specificity of 84%. By IHC, isolated, strong GBM-C4d was most specific 100% (68/68) and least sensitive (8%, 4/48). Of note: in Table 1, four of six cases with strong GBM-C4d by IF had circumferential GBM duplication by EM only.
Isolated strong GBM-C4d occurred in temporal association with the development of GBM duplication and typical TG by LM. In early biopsies taken <1 year post-transplantation, TG by LM was present in 7/140 (5%) biopsies; isolated strong GBM-C4d was not found. In late biopsies, taken ≥1 year post-transplantation, TG by LM was present in 45/172 (26%) biopsies. Isolated strong GBM-C4d by IF was seen in 20/172 (12%) cases (in 16/45, 36% cases with TG), and by IHC in 4/28, 14% cases with TG.
In the cohort of 61 patients with available repeat biopsies, 3/61 had strong isolated GBM-C4d by IF and circumferential GBM duplication by LM and/or EM in their index biopsy. From these three patients, between one and three follow-up biopsies were obtained over 7–16months. All repeat biopsies had continuous isolated GBM-C4d staining of various intensity; there was no evidence of circulating donor-specific antibodies in 2/2 patients tested.
GBM-C4d staining and chronic active presumably antibody-mediated rejection: a stepwise exclusion analysis
To determine whether GBM-C4d staining was associated with GBM remodelling in cases lacking diagnostic evidence of antibody-mediated injury, we performed a stepwise exclusion analysis. In steps 1 and 2, we excluded cases with morphologic signs commonly associated with active antibody-mediated tissue injury and in step 3 additionally cases based on the donor-specific antibody status (Table 2).
| IF GBM-C4d | IHC GBM-C4d | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Absent (0+) | Weak (1+) | Moderate (2+) | Strong (3+) | Total | P-value | Absent (0+) | Weak (1+) | Moderate (2+) | Strong (3+) | Total | P-value | ||
| All cases | TG absent | 120 (98) | 114 (91) | 27 (68) | 6 (20) | 267 (84) | <0.001 | 69 (87) | 7 (70) | 7 (47) | 3 (25) | 86 (74) | <0.001 |
| TG present | 3 (2) | 12 (9) | 13 (33) | 24 (80) | 52 (16) | 10 (13) | 3 (30) | 8 (53) | 9 (75) | 30 (26) | |||
| Total | 123 (100) | 126 (100) | 40 (100) | 30 (100) | 319 (100) | 79 (100) | 10 (100) | 15 (100) | 12 (100) | 116 (100) | |||
| Step 1. Excluding cases with ptc-C4d | TG absent | 119 (98) | 98 (89) | 22 (71) | 4 (20) | 243 (86) | <0.001 | 61 (91) | 4 (80) | 2 (22) | 0 (0) | 67 (79) | <0.001 |
| TG present | 3 (2) | 11 (11) | 9 (29) | 16 (80) | 39 (14) | 6 (9) | 1 (20) | 7 (78) | 4 (100) | 18 (21) | |||
| Total | 122 (100) | 109 (100) | 31 (100) | 20 (100) | 282 (100) | 67 (100) | 15 (100) | 9 (100) | 4 (100) | 85 (100) | |||
| Step 2. Excluding cases with ptc-C4d and/or transplant glomerulitis | TG absent | 116 (98) | 96 (92) | 22 (76) | 4 (36) | 238 (91) | <0.001 | 61 (94) | 4 (100) | 2 (29) | 0 (0) | 67 (87) | <0.001 |
| TG present | 2 (2) | 8 (8) | 7 (24) | 7 (64) | 24 (9) | 4 (6) | 0 (0) | 5 (71) | 1 (100) | 10 (13) | |||
| Total | 118 (100) | 104 (100) | 29 (100) | 11 (100) | 262 (100) | 65 (100) | 4 (100) | 7 (100) | 1 (100) | 77 (100) | |||
| Step 3. Excluding cases with ptc-C4d, transplant glomerulitis, and/or DSAa | TG absent | 69 (100) | 45 (95) | 11 (79) | 1 (25) | 126 (94) | <0.001 | 33 (100) | 1 (100) | 0 (0) | 0 (0) | 34 (97) | 0.057 |
| TG present | 0 (0) | 2 (4) | 3 (21) | 3 (75) | 8 (6) | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 1 (3) | |||
| Total | 69 (100) | 47 (100) | 14 (100) | 4 (100) | 134 (100) | 33 (100) | 1 (100) | 1 (100) | 0 (100) | 35 (100) | |||
- Ptc-C4d = C4d staining along peritubular capillaries. GBM-C4d = pseudolinear C4d staining along the glomerular basement membrane. TG, transplant glomerulopathy by LM evaluated according to Banff ‘97 criteria; IF, immunofluorescence microscopy; IHC, immunohistochemistry; DSA, donor-specific antibodies.
- Values are presented as number of biopsies (percentage).
- a In this conservative approach ‘erroring on the side of DSA positivity’ a patient/case was defined as ‘DSA positive’ if either donor-specific antibodies were detected at any time during follow-up, or if the DSA status was undetermined/DSA data lacking.
Step 1) Exclusion of cases with peritubular capillary C4d staining
All biopsies with ptc-C4d deposits either by IF (37/319) or IHC (31/116) were excluded. GBM-C4d staining was seen in 36/39 (92%) remaining TG cases by IF and in 12/18 (67%) by IHC. Correlations in this subgroup closely reflected findings described above: GBM-C4d remained significantly associated with the presence of TG (P < 0.001), and typically, strong GBM-C4d staining indicated the underlying presence of GBM duplication.
Step 2) Additional exclusion of cases with transplant glomerulitis
Glomerulitis is often associated with active antibody-mediated rejection. All biopsies with transplant glomerulitis (and/or peritubular capillary C4d deposits as outlined in step 1) were excluded. By IF, GBM-C4d staining was seen in 22/24 (92%) remaining TG cases and in 6/10 (60%) by IHC. GBM-C4d staining remained significantly associated with TG (P < 0.001).
Step 3) Additional exclusion of cases with donor-specific antibodies
We chose a conservative approach and excluded all biopsies from patients who were positive for donor-specific antibodies at least once post-transplantation or who had an undetermined donor-specific antibody status (i.e. no data on donor-specific antibodies available). By IF, GBM-C4d staining was seen in 8/8 remaining TG cases and by IHC in 1/1.
Uni- and Multivariate analysis of GBM-C4d staining
By univariate analysis, the presence of GBM-C4d staining was significantly correlated with the presence of a duplicated GBM by LM and/or EM (combined group of n = 82 biopsies) and with signs of antibody-mediated rejection, that is presence of donor-specific antibodies, transplant glomerulitis and ptc-C4d (Table 3). By multivariate logistic regression, only the presence of a duplicated GBM by LM and/or EM and the presence of ptc-C4d independently correlated with GBM-C4d (variables with P < 0.25 in the univariate analysis were included as independent variables in the multivariate analysis; Table 4).
| Variable | IF GBM-C4d absent (n = 123) | IF GBM-C4d present (n = 196) | P-value |
|---|---|---|---|
| GBM duplications by LM or EM | 9 (7) | 73 (37) | <0.001 |
| DSA positivity | 32 (31) | 66 (46) | 0.013 |
| Positive cross-match at time of transplantation | 6 (6) | 9 (6) | 0.969 |
| PRA >30% | 9 (9) | 19 (12) | 0.368 |
| Transplant glomerulitis | 4 (3) | 29 (15) | 0.001 |
| IF ptc-C4d present | 1 (1) | 36 (18) | <0.001 |
| Female sex | 49 (40) | 94 (48) | 0.156 |
| Race | |||
| Caucasian | 47 (38) | 86 (44) | 0.557 |
| African American | 64 (52) | 95 (49) | |
| Other | 12 (10) | 15 (7) | |
| Hepatitis C virus infection | 8 (7) | 20 (12) | 0.187 |
- Values are presented as number of biopsies (percentage).
- IF GBM-C4d = pseudolinear C4d staining along the glomerular basement membrane observed by immunofluorescence microscopy. GBM, glomerular basement membrane; LM, light microscopy; EM, electron microscopy; DSA, donor-specific antibodies; PRA, panel reactive antibodies. IF ptc-C4d = C4d staining along peritubular capillaries observed by immunofluorescence microscopy.
| Variable | O.R. | O.R. 95% C.I. | P-value | |
|---|---|---|---|---|
| GBM duplication by LM or EM | 4.712 | 1.853 | 11.980 | 0.001 |
| Transplant glomerulitis | 1.306 | 0.349 | 4.887 | 0.692 |
| DSA positivity | 1.163 | 0.641 | 2.110 | 0.619 |
| IF ptc-C4d presenta | 17.656 | 3.616 | Infinity | <0.001 |
| Female sex | 0.851 | 0.486 | 1.490 | 0.573 |
| Hepatitis C virus infection | 1.480 | 0.567 | 3.861 | 0.423 |
- GBM, glomerular basement membrane; LM, light microscopy; EM, electron microscopy; DSA, donor-specific antibodies. IF ptc-C4d = C4d staining along peritubular capillaries observed by immunofluorescence microscopy.
- Variables with P < 0.25 in the univariate analysis (see Table 3) were included into the multivariable logistic regression analysis.
- a Exact conditional logistic regression was performed.
Native kidneys
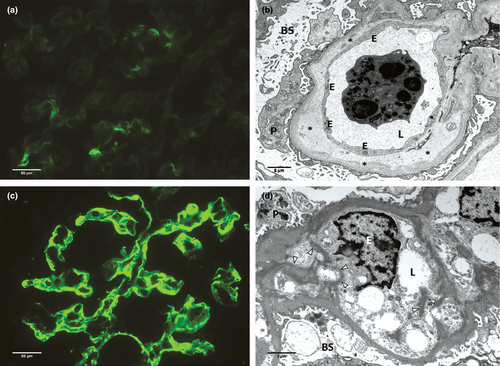
To investigate the association between GBM duplication and GBM-C4d staining in a setting without donor-specific antibodies, 35 native control biopsies were studied (n = 26 chronic TMA with GBM duplication, and n = 9 minimal change disease without GBM duplication). In native controls, GBM-C4d was significantly associated with GBM duplication, both by IF and IHC (IF: P < 0.001; IHC: P < 0.05; Fig. 3). In biopsies with minimal change disease, GBM-C4d was not observed. In biopsies with TMA, GBM-C4d was detected in 24/26 (92%) cases by IF, 14/24 with weak-to-moderate and 10/24 with strong GBM-C4d staining intensity (Fig. 3c,d). GBM-C4d staining intensity by IF was significantly correlated with the Banff cg GBM duplication scores (IF: rs = 0.764, P < 0.001; strong C4d expression was associated with a cg-3 Banff glomerular remodelling score in 10/10 cases). By IHC, GBM-C4d was present in 9/26 (35%) TMA controls. Ptc-C4d staining was not observed in any native kidney biopsy.
Morphologic correlate for GBM-C4d staining intensity in native and transplant kidneys
The degree of GBM-C4d staining was significantly associated with the degree of GBM duplication and the degree of subendothelial new densa multilamination. No GBM-C4d was noted in cases with only ‘watery’ widening of the lamina rara interna lacking new subendothelial densa formation/duplication (Fig. 3a,b). Such ultrastructural changes are seen in very early stages of microangiopathy-like glomerular injury. In comparison, strongest staining was found in glomeruli with multiple subendothelial layers of rudimentary new densa, often seen post recurrent glomerular endothelial injury. Also the accumulation of intracapillary matrix-like proteinaceous material, as seen in a case of pre-eclampsia, showed marked GBM-C4d staining (Fig. 3c,d).
Immunogold labelled glomerular C4d deposits
Immunogold staining signals were noted in subendothelial glomerular zones with GBM duplication and to a lesser degree also along visceral epithelial cells (Fig. S1).
Discussion
Our study sheds new light on the significance of C4d deposits along glomerular capillary walls. In a heterogeneous group of transplant and native kidney biopsies, we demonstrated for the first time that pseudolinear C4d accumulations along glomerular capillary walls were tightly associated with subendothelial new lamina densa formation and GBM duplication. GBM-C4d as a reflection of structural glomerular capillary wall remodelling was observed in native and transplant kidneys independent of the underlying etiology (Fig. 4). The GBM-C4d staining pattern mirrored the degree of glomerular capillary wall remodelling with no staining in cases showing only widening of the lamina rara interna but lacking subendothelial new lamina densa formation/duplication, focal and segmental staining in cases with minor GBM duplication and global staining in biopsies with widespread capillary wall restructuring. The strongest staining intensities were seen in cases with marked subendothelial new densa multilamination or intracapillary matrix accumulation as seen with repetitive and severe endothelial injury, including TMA in the setting of pre-eclampsia. GBM-C4d was also noted under podocytes, likely reflecting subepithelial capillary wall turnover and restructuring originating from visceral epithelial cells. By immunogold labelling, C4d deposits were mainly detected in architecturally altered subendothelial zones. Structural glomerular changes were reflected with the highest sensitivity by IF on frozen tissue samples, whereas IHC revealed highest specificity; overall, our C4d staining characteristics (IF versus IHC) were in agreement with previously published observations 35.
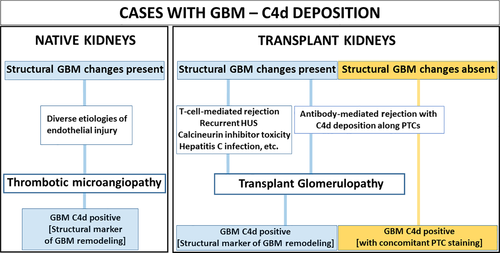
Glomerular C4d deposits can be seen in various settings and must be interpreted in the appropriate context. For example, weak linear GBM and more pronounced paramesangial C4d staining is found in normal glomeruli by IF as a reflection of physiologic complement and GBM turnover, lacking specific diagnostic significance but providing positive internal IF staining controls 16, 36. In our study, physiologic complement turnover might explain the high prevalence of linear GBM-C4d in normal glomeruli noted by IF. Such a glomerular C4d pattern is less apparent in formalin-fixed biopsies examined by IHC, likely due to differences in assay sensitivities and antibody affinities 35, 37. In the setting of glomerulonephritides distinct, finely granular C4d staining can be found marking the presence of immune complex deposits along the GBM and in mesangial zones 17, 18, 36. Post kidney transplantation, antibody-mediated rejection with complement activation commonly shows C4d along peritubular capillaries and often concomitant pseudolinear GBM-C4d staining, as reported here and elsewhere (Fig. 4) [17, 21-23, 27]. Ptc-C4d accumulation can occur and vanish within days 9, 33 and serves as dynamic ‘etiologic’ marker for antibody-induced graft injury. In contrast, pseudolinear GBM-C4d, in particular when seen in isolation without concurrent ptc-C4d, can be of different significance. We noted isolated GBM-C4d only in older grafts post year-1 when TG developed. Isolated GBM-C4d did not vanish but rather remained a constant finding over months based on the evaluation of repeat biopsies. The late occurrence and tight link to glomerular capillary wall remodelling suggest structural GBM alterations with duplication rather than active antibody-mediated rejection as a cause for complement accumulation along glomerular capillary walls. This notion is further supported by several of our findings. First, 60–70% of transplant biopsies with TG demonstrated isolated GBM-C4d rendering it a rather common finding. Second, GBM-C4d was significantly associated with GBM duplication by multivariate analysis, postexclusion of antibody-mediated graft injury, and importantly, in native kidney biopsies with thrombotic microangiopathies of various etiologies. Third, the degree of GBM-C4d staining was correlated with the degree of architectural changes. We, therefore, conclude that distinct, especially isolated global or segmental GBM-C4d staining can indicate glomerular basement membrane remodelling with new lamina densa formation. Its detection should stimulate a targeted search for thrombotic microangiopathy-like glomerular injury that might be in an early stage of development. As stains to detect C4d are standard practice, the approach outlined here is simple and can facilitate an early diagnosis when glomerular duplication is limited and progression under new therapies potentially preventable 38, 39. Whether ptc-C4d with concomitant GBM-C4d in cases of acute antibody-mediated rejection might represent – at least in some cases – a very early form of GBM remodelling is undetermined and requires future studies.
If GBM duplication is reflected by GBM-C4d, then can architectural changes along peritubular capillaries be reflected by ptc-C4d because multilamination of peritubular capillary basement membranes is a frequent finding in thrombotic microangiopathies and chronic rejection? 40. Although not specifically studied here, we think there are no data to support such speculation. For example, we found that native kidney specimens with chronic TMA revealed isolated GBM-C4d in 46% of cases; ptc-C4d was not seen. Potential differences in the response to injury in the microvascular of peritubular capillaries versus glomerular capillaries are currently not defined.
Complement is known to play a role in diseases affecting glomerular capillaries. Complement activation is associated with the atypical haemolytic uremic syndrome, a form of TMA caused by dysregulation of the alternative complement pathway (reviewed by George et al. 41). Also the complex lectin pathway of complement activation including serine proteases that have activating effects on endothelial cells and the complement cascade via C4 might be of pathophysiologic significance during glomerular capillary wall remodelling and duplication 42-45. The capacity of glomerular endothelial cells to synthesize complement factor C4 46 adds to the role that the complement system might play in this scenario. Recently, complement C4d and C5b-9 deposits were described in TMA and interpreted as possible diagnostic biomarkers in the clinical work-up of patients 29.
In the current study, we identify subendothelial and to a lesser degree subepithelial glomerular zones as ‘construction sites’ with GBM-C4d accumulation. We provide together with Chua et al. 29 strong evidence that complement is an integral building block during GBM remodelling in various settings, independent of a specific underlying etiology. However, it is beyond the scope of the current biopsy-based study to specifically address many aspects of pathophysiology or therapeutic intervention.
In conclusion, we show that pseudolinear C4d deposits along peripheral glomerular basement membranes can be seen in different settings: (i) as etiologic markers for active ongoing antibody-induced renal graft rejection, especially when seen with concurrent ptc-C4d, or (ii) as structural markers for microangiopathy-like GBM duplication in transplant and native kidneys, independent of the underlying remote or recent triggering event. In renal transplants, GBM-C4d as a structural marker becomes most apparent if detected with strong staining intensity and in isolation without concurrent ptc-C4d. Thus, GBM-C4d does not necessarily indicate a specific underlying disease etiology, and its presence should not be in-and-by itself interpreted as a reliable diagnostic marker for active ongoing antibody-mediated rejection (Fig. 4).
Authorship
All authors contributed significantly to the study design, execution, data analysis and writing of the manuscript.
Funding
The authors have declared no funding.
Conflict of Interest
The authors have declared no conflicts of interest.
Acknowledgements
The authors thank Victoria J Madden, Randolph Detwiler from UNC School of Medicine and all staff from the UNC Division of Nephropathology for superb assistance. We also thank Ingeborg Bajema, MD, from Leiden University Medical Center for critical review of the manuscript.