An in silico simulation of the frequency of administering HLA-incompatible low titer group O whole blood units when the donor pool includes unscreened female donors
Abstract
Background
As low titer group O whole blood (LTOWB) increases in popularity, blood centers are finding innovative ways of maintaining the supply. One potential way is collecting LTOWB from parous female donors without testing for HLA antibodies. This in silico simulation predicted the risk of an LTOWB unit containing an HLA antibody and the subsequent risk for an HLA-incompatible transfusion.
Methods
An LTOWB blood bank with 1 million units was simulated consisting of male, nulliparous, and parous female donors. The proportion of each donor type was modeled after the sex distribution at US blood centers. The parity of female donors was calculated based on the average number of live births per female depending on her age. HLA-alloimmunization risk was determined by her parity status. The HLA haplotypes of the simulated recipients were derived from the 100 most common HLA haplotypes in the US National Marrow Registry Program database. The proportion of different race/ethnic groups in the US was used to simulate 100,000 LTOWB recipients to whom between 1 and 10 units were administered.
Results
Overall, the risk of an LTOWB unit containing at least one HLA antibody was 12.2% and the rate of receiving an HLA-incompatible unit was 21.3%. The risk of receiving an HLA-incompatible unit rose from 4.8% after receipt of one unit to 36.5% after 10 units.
Conclusion
Blood collectors and hospitals should evaluate the potential TRALI risk against the benefit of a potentially expanded inventory of LTOWB before collecting plasma-containing products from non-HLA-tested parous female donors.
1 INTRODUCTION
Transfusion-related acute lung injury (TRALI) is an uncommon but serious adverse event following transfusion.1-3 While its exact pathophysiological mechanism is still unknown,4-6 donor human leukocyte antigen (HLA) or human neutrophil antigen (HNA) antibodies that bind to the recipient's cognate antigens are often implicated in these reactions.7-10 Therefore, many blood centers avoid collecting plasma-containing blood products, like plasma and low titer group O whole blood (LTOWB) units, from donors who either have or are likely to have these antibodies. It is known that the likelihood of developing HLA antibodies increases with each successive childbirth, thus, women with a pregnancy history are often excluded from donating plasma-containing blood products as a TRALI-mitigation step,11-15 or their plasma is tested for HLA antibodies and if negative, their plasma can be issued for transfusion. These risk mitigation steps have greatly reduced the risk of TRALI, with one multi-center study finding a TRALI rate of 2.88/100,000 distributed components in 2007 before these steps had been implemented. The TRALI rate was reduced to 0.60/100,000 distributed components in 2017,16 which was approximately 3 years after the Association for the Advancement of Blood and Biotherapies mandated that blood centers implement TRALI risk mitigation strategies for plasma and whole blood-derived products.17 Nulliparous women and men, whether having been transfusion recipients before donating blood or not, have a low baseline risk for HLA-alloimmunization,18 and so they are considered low-risk donors whose plasma is usually not screened for HLA class I and II antibodies before their donations are issued. At blood centers that screen donor plasma for HLA antibodies, screening for HNA antibodies is not common.
Testing parous women for HLA antibodies can be logistically cumbersome and expensive thereby potentially posing a barrier to collecting their plasma-containing blood products. With the increasing demand for products like LTOWB,19, 20 blood centers would like to be able to collect as many donors as possible without compromising recipient safety. Even though the risk of HLA-alloimmunization increases in proportion to increasing parity after at least four pregnancies the probability of HLA-alloimmunization is only approximately 32%.18 Thus, the majority of women with even the highest birthrates will still not be HLA-alloimmunized. Therefore, an in silico simulation was performed to determine how often an HLA-incompatible transfusion would occur if parous women were included in the LTOWB donor pool along with nulliparous women and men without testing their donations for HLA antibodies.
2 METHODS
2.1 Simulated blood donors
The simulation was built using the assumptions listed in Table 1. Briefly, the 2023 US National Blood Collections and Utilization Survey found that in 2021, 54.1% of all blood donors were female and 45.9% were male.21 Thus, a recipient had a slightly greater chance of receiving a unit from a female donor than a male donor. Males and nulliparous women were treated in this simulation as having a 0% risk for HLA alloimmunization; many blood centers that test for HLA antibodies amongst their donors would not test these types of donors. Thus, in this simulation, recipients of units from males and nulliparous women donors would not be at risk of an HLA mismatch. The occurrence of HNA was not modeled because they are not routinely tested amongst donors in the US.
| Variable | Assumption | Reference | Simulation validation | |
|---|---|---|---|---|
| Donor sex proportion | 54.1% female, 45.9% male | 19 | 54.1% female and 45.9% male | |
| Donor HLA alloimmunization risk | Male donors | Female donors | 16 | |
| 0% | 0 deliveries—0.0% | Class I: 0.0% | ||
| Class II: 0.0% | ||||
| 1 delivery—14.5% | Class I: 14.4% | |||
| Class II: 14.5% | ||||
| 2 deliveries—21.9% | Class I: 21.9% | |||
| Class II: 21.8% | ||||
| 3 deliveries—29.0% | Class I: 28.8% | |||
| Class II: 29.0% | ||||
| >3 deliveries—32.4% | Class I: 32.3% | |||
| Class II: 32.6% | ||||
| Average number of live births per woman | 1.657 | 20 | Not possible to estimate due to simulation design | |
| Average maternal age, SD (years) | First birth | 27.4, 6.2 | 20 | |
| Second birth | 29.9, 5.8 | |||
| Third birth | 31.1, 5.6 | |||
| Fourth birth | 32.1, 5.4 | |||
| Fifth birth | 33.0, 5.5 | |||
| Sixth birth | 34.2, 5.2 | |||
| Seventh birth | 34.2, 5.2 | |||
| Eigth birth | 36.4, 4.9 | |||
| HLA class I antibodies detected in donor units | Antibody | Percentage | 21 | |
| A2 | 12.03 | 11.70 | ||
| B44 | 8.27 | 8.15 | ||
| B60 | 8.27 | 8.08 | ||
| B7 | 7.52 | 7.36 | ||
| B57 | 6.77 | 6.77 | ||
| B62 | 6.77 | 6.79 | ||
| A24 | 6.02 | 6.03 | ||
| B35 | 4.51 | 4.51 | ||
| B8 | 4.51 | 4.58 | ||
| A3 | 3.76 | 3.84 | ||
| A11 | 3.01 | 3.09 | ||
| A31 | 3.01 | 3.13 | ||
| B27 | 3.01 | 2.98 | ||
| B48 | 3.01 | 3.08 | ||
| B58 | 3.01 | 3.14 | ||
| A1 | 2.26 | 2.30 | ||
| A32 | 2.26 | 2.33 | ||
| B39 | 1.5 | 1.54 | ||
| B56 | 1.5 | 1.54 | ||
| B61 | 1.5 | 1.53 | ||
| A25 | 0.75 | 0.74 | ||
| A26 | 0.75 | 0.81 | ||
| A29 | 0.75 | 0.74 | ||
| A33 | 0.75 | 0.73 | ||
| A36 | 0.75 | 0.78 | ||
| B18 | 0.75 | 0.75 | ||
| B55 | 0.75 | 0.71 | ||
| A68 | 0.75 | 0.74 | ||
| B49 | 0.75 | 0.81 | ||
| CW7 | 0.75 | 0.73 | ||
| HLA class II antibodies detected in donor units | DQ7 | 12.96 | 21 | 12.67 |
| DR52 | 12.96 | 12.57 | ||
| DR4 | 12.04 | 11.83 | ||
| DR53 | 12.04 | 11.74 | ||
| DQ8 | 9.26 | 9.36 | ||
| DR51 | 9.26 | 9.30 | ||
| DR15 | 6.48 | 6.56 | ||
| DQ5 | 4.63 | 4.84 | ||
| DQ6 | 4.63 | 4.75 | ||
| DQ2 | 3.7 | 3.79 | ||
| DR11 | 2.78 | 2.89 | ||
| DR7 | 2.78 | 2.92 | ||
| DQ9 | 2.78 | 2.89 | ||
| DR13 | 0.93 | 0.96 | ||
| DR17 | 0.93 | 0.96 | ||
| DR12 | 0.93 | 0.99 | ||
| DR16 | 0.93 | 0.99 | ||
| Number of HLA class I antibodies detected | Number of antibodies | Percentage | 21 | |
| 1 | 77.8 | 76.8 | ||
| 2 | 21.3 | 21.7 | ||
| 3 | 0.9 | 1.5 | ||
| Number of HLA class II antibodies detected | 1 | 51.6 | 21 | 51.5 |
| 2 | 34.4 | 35.3 | ||
| 3 | 10.9 | 10.3 | ||
| 5 | 3.1 | 2.9 | ||
| Proportion of each race/ethnicity to the overall US population (%) (for modeling recipients) | Race/ethnicity | Proportion | 23 | |
| White | 57.3 | 59.2 | ||
| Hispanic or Latino | 19.5 | 21.0 | ||
| Black or African American | 11.9 | 12.4 | ||
| Asian/Other Pacific Islander | 6.0 | 6.4 | ||
| American Indian and Alaska Native | 0.7 | 1.0 | ||
- Note: The table also demonstrates the performance of the simulation itself vis-à-vis the assumptions. Note that the assumed percentages of the five race/ethnic groups add to approximately 95% because certain respondents to the 2020 census were excluded (see text). In the simulation, only these five race/ethnic groups were simulated, hence their percentages add up to 100%.
For the simulated female donors who had at least one delivery, their parity rates were derived by using the ages of the approximately 36,000 unique female donors at a large regional blood collector in the southwest US over the 12-month period ending October 2024. Knowing the age of the individual female donors allowed for a calculation of the number of live births that each female donor was expected to have based on her age using data from the 2021 US National Vital Statistics Report.22 Data on the total fertility rate for women in the United States, that is, the average number of live births per woman, was also obtained from this report.22 For each degree of parity (1 live birth, 2 live births, 3 live births, >3 live births), the risk of HLA-alloimmunization was derived from the literature.18 For those female donors who were HLA-alloimmunized, the specificity/ies of the HLA class I and/or class II antibody/ies that they would have produced was derived from a study of the HLA antibodies found in 1054 units that were transfused to 164 randomly selected patients who did not have TRALI symptoms within 12 hours of the transfusion at two large American hospitals.23 That the frequency of HLA antibodies detected in these units came from randomly selected patients makes these antibody frequencies more generalizable to the donor pool at large compared to deriving these frequencies from, for example, patients who experienced a TRALI. The publication from which the specificity of these HLA antibodies was derived did not list the race/ethnicity of these donors, thus the donors in this simulation were not assigned to specific racial/ethnic groups. Alloimmunization to HLA class I and class II was treated independently meaning that the probability of having an antibody to either class depended solely on the female donor's probability of being alloimmunized, which was derived from her predicted degree of parity. The patterns of HLA antibody production, that is, the production of one HLA antibody versus the production of multiple HLA antibodies were also derived from the aforementioned study.23 Thus, a female donor who had at least one delivery might have one or more HLA class I and/or class II antibody/ies.
An LTOWB blood bank consisting of male, nulliparous, and parous female donors was constructed by performing the simulation 1 million times, thereby generating 1 million LTOWB units that were not all necessarily unique, using the above parameters.
2.2 Simulated transfusion recipients
To determine the HLA antigen haplotype frequencies amongst the simulated recipients, a list of the 100 most common high resolution HLA class I and II haplotypes in the US National Marrow Donor Program (NMDP) was obtained from the literature.24 The NMDP registry also includes donors from international donor centers, thus this list of haplotypes was derived from a total of 21 donor population groups. Each HLA haplotype was supplied with its frequency in each of the 21 donor population groups. The HLA haplotype frequencies for these 21 population groups were collated into four race categories including African American, Asian or Pacific Islander, Caucasian, Native American, as well as Hispanic ethnicity by the authors of the HLA haplotype study.24
Using data from the 2020 US census,25 the number of people who reported being from the Asian and the Other Pacific Island races was combined to be consistent with the categories of the NMDP HLA haplotype study. Once combined, the census data were used to determine each race/ethnic group's proportion of the total population of the United States. Respondents to the census who identified as being of Some Other Race, or who identified as being of multiracial/ethnic background were excluded. These exclusions accounted about 5% of the total US population.
The average American recipients' HLA haplotypes were produced by using the proportion of each race/ethnic group in the overall American population and the HLA haplotype frequencies for each race/ethnic group; HLA antigens of 100,000 transfusion recipients were simulated by selecting two HLA haplotypes from the distributions of HLA haplotypes by race/ethnic group and mapping the HLA haplotypes to their corresponding HLA antigens using an online tool.26
The simulation was performed by randomly assigning 10,000 of the simulated recipients to receive one through 10 LTOWB units (for a total of 100,000 simulated transfusion recipients), reflecting the number of LTOWB units that might be administered during trauma resuscitation. Once a unit was transfused it was not replaced in the simulated blood bank to replicate the fact that blood centers cannot immediately recollect another unit from the same donor (random sampling without replacement design).
The outputs of this simulation were the proportion of LTOWB units that contained at least one HLA antibody and the number of recipients who received an HLA-mismatched transfusion. The model was implemented in R version 4.4.1 and the script along with HLA haplotype information are shown in the Supplementary Material.
3 RESULTS
3.1 Validation of simulation
Table 1 provides the assumptions on which the model was built from the literature and also the performance validation of the simulation against these assumptions. For the parameters that required simulation, the model produced values that were nearly identical to the assumptions.
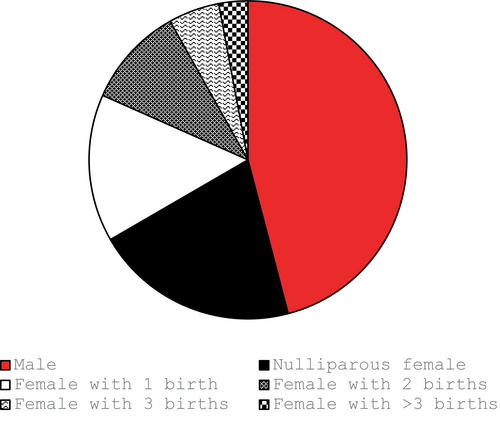
3.2 Rate of HLA antibodies in LTOWB units from male, nulliparous, and parous female donors
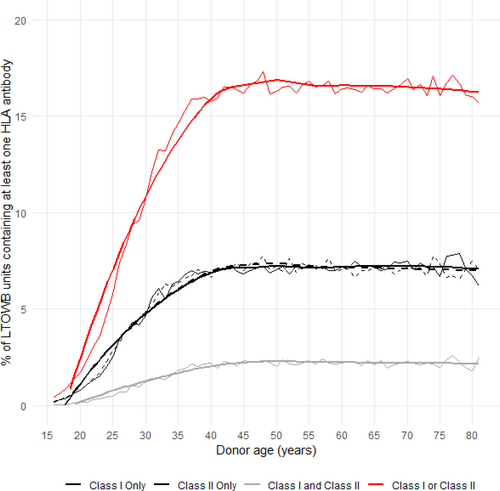
Figure 1 demonstrates the proportion of units donated by each of the different types of donors. The majority of units were donated by males and nulliparous females. Figure 2 demonstrates the probability that a donated unit would contain at least one HLA antibody to class I and/or class II antigens stratified by donor age. Overall, the probability of an LTOWB unit containing at least one HLA antibody was 12.2%. The rate of HLA antibody-containing units plateaued at approximately age 40 because, after that, few American women give birth, that is, have another major HLA antibody-inducing stimulus, and only parous females were capable of donating an HLA antibody-containing unit in this simulation. When female donors who had at least two pregnancies were excluded from donation, the probability of an LTOWB unit containing at least one HLA antibody decreased to 8.7%.
3.3 Rate of HLA-incompatible transfusions
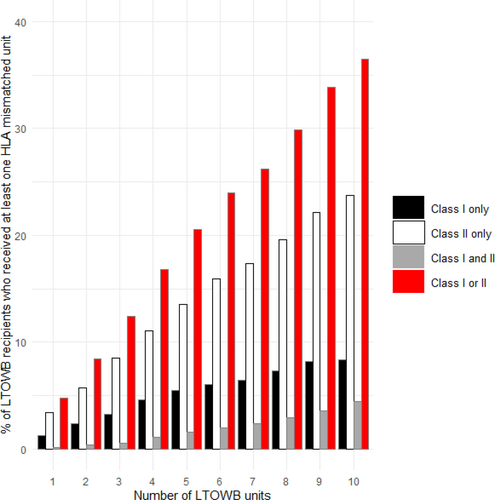
Using the average American recipients' haplotypes, which included HLA haplotypes based on the proportion of each race/ethnic group in the American population and the frequency of the HLA haplotypes for each race/ethnic group, the probability of receiving an HLA-incompatible unit with antibody(ies) to HLA class I, class II or both classes increased as the number of transfused LTOWB units increased (Figure 3). Overall, the probability of an HLA mismatch occurring for the average American recipient when receiving LTOWB from the simulated blood bank where units from all donors, including unscreened multiparous women, were included was 21.3%. The probability of an HLA mismatch increased from 4.8% after receipt of one unit to 36.5% after receipt of 10 units (Figure 3). When the units donated by females who had at least two pregnancies were excluded, the overall probability of an HLA mismatch decreased to 14.6%.
4 DISCUSSION
This in silico simulation demonstrated that the risk of causing an HLA mismatch when LTOWB units were collected from males, nulliparous females, and non-HLA antibody-tested parous females was approximately 21.3% for the average American recipients' haplotypes. Individual blood centers can use these data to inform their decision about how to proceed with collecting and issuing plasma-containing products, such as LTOWB, from parous females.
This simulation has several limitations. The antibody specificities were derived from a large study of HLA antibodies found in blood donor units at two American hospitals, so its generalizability to other centers in the United States or around the world is not certain because of the highly polymorphic nature of the HLA locus. The HLA haplotypes of the simulated recipients were derived from a review of a large US national database of HLA loci that also included donors from many different countries around the world. Using HLA haplotypes from donors both in the United States and in geographically diverse countries such as Japan, Korea, and the Caribbean for the simulated recipients in this study was designed to promote inclusivity and increase generalizability by attempting to reflect a wide range of potential transfusion recipients in the United States. Nevertheless, if specific HLA antibodies or haplotypes were not included in the data sources used to prepare this simulation, their effect on the rate of HLA-incompatible transfusions would not have been considered. As such, the overall rate of HLA-incompatible transfusions presented herein could be seen as a high-level estimation of the risk. On the other hand, the HLA haplotypes that were employed to design the recipients' haplotypes were based on high-resolution typing.24 However, there is significant complexity in the serologically-based HLA antibody nomenclature and in the DNA sequencing-based antigen mapping, and although a comprehensive mapping tool was used to map the HLA antigens from the haplotypes, it is possible that some antibody—antigen interactions were missed. Similarly, it is known that there is considerable overlap in the antigens to which HLA antibodies can bind, and modeling this complexity was beyond the scope of this simulation. Therefore, for these reasons, the overall 21.3% estimation of rate for an HLA-incompatible unit might actually be an underestimation of the rate of additional antigens that are bound by the antibodies were not included. Local blood collectors and hospitals should understand the nature of the HLA makeup of their local population of donors and patients before deciding on a TRALI risk-mitigation strategy.
It is important to consider that not all HLA-incompatible transfusions result in TRALI,23, 27-30 thus, even the patients in this simulation who were predicted to receive an HLA-incompatible transfusion might not experience TRALI. Therefore, the 21.3% rate of predicted HLA-incompatible transfusions does not necessarily equate to the expected TRALI rate, although these recipients could be considered to be at risk. It is known that HNA antibodies can also be etiological agents of TRALI. However, their potential contribution to causing this adverse event was not considered in this simulation because most blood centers do not test their donors for HNA antibodies. Thus, the simulated patients in this study received the same standard of care that would be present at most blood centers vis-à-vis HNA antibody testing.
While the frequency of HLA-alloimmunization by parity was derived from the literature,18 the actual parity of the female donors at this blood center was not known and was extrapolated from the mean age at which American women give birth over the course of their lives.22 The frequency of developing HLA antibodies was based solely on the predicted extent of the female donor's parity (which was based on her age at the time of donation) and did not include other potential stimulation events such as previous transfusion receipt or experiencing miscarriages; while it is known that transfusion is a weak stimulus for HLA-alloimmunization, losing two or more pregnancies confers a 4.5% risk for HLA alloimmunization.18 Similarly, transfused male donors have a 1.7% HLA alloimmunization rate.18 However, these rates are substantially lower than the HLA-alloimmunization risk for delivering at least one live baby (14.5%) and is even lower relative to the HLA-alloimmunization risk of having multiple deliveries,18 and blood centers that screen their donors for HLA antibodies do not usually screen these types of donors. Thus, the simulated recipients received the same standard of care as actual patients as it relates to donor HLA antibody screening.
Furthermore, the rate of HLA-alloimmunization per live birth was equally applied to both HLA class I and class II antibodies meaning that, for example, a female donor who was predicted to have delivered two live births had a 21.9% rate of developing an HLA class I antibody and a separate 21.9% rate of developing an HLA class II antibody,18 although the actual rate of class I- and class II-alloimmunization following parturition might not be equal in reality. Also, it is possible that some HLA antibodies are produced in combination with other specific HLA antibodies. While this simulation accounted for the proportion of patients who produced single or multiple HLA antibodies, the simulated donors were assigned antibody specificities based solely on the frequency of HLA antibodies in the population.23 Thus, specific HLA antibody combinations that might occur in nature did not necessarily occur in combination in this simulation. Furthermore, these antibodies were assumed not to evanesce, that is, the simulation assumed that once a female donor became alloimmunized, the antibody remained detectable and capable of binding to a recipient's cognate antigen. However, in reality, these antibodies are sometimes known to become undetectable thereby perhaps somewhat overestimating the risk of having an HLA-incompatible unit.
When units from the simulated blood bank were transfused, they were not replaced. This was done to simulate the reality of a hospital blood bank depleting its inventory as a resuscitation progresses. This was a reasonable assumption because it is unlikely that LTOWB donors could donate two units at once as is common for RBC units collected by apheresis. Therefore, it is unlikely that a second LTOWB unit from the same donor would be in a blood bank's inventory at the time that the first unit was issued. Furthermore, the simulation assumed that all of the donors were group O and had low titers of anti-A and -B thereby qualifying them to donate LTOWB. Thus, the simulation did not account for any potential interconnectedness between group O blood group, low anti-A and -B titers, and the propensity to become HLA alloimmunized or the nature of the HLA antibody produced, if any correlation between these parameters exists.
Lastly, if a blood center is collecting LTOWB units from non-HLA antibody-tested parous female donors then they might also be collecting other plasma-containing products from these donors such as plasma and apheresis platelet units. Thus, patients with life-threatening hemorrhage might also be exposed to HLA antibodies from the other blood products that they received during their resuscitation and later in on in their hospital course although this simulation only accounted for the exposure from LTOWB units.
This simulation of 100,000 transfusion recipients found that overall, 21.3% of these recipients would receive an HLA-incompatible unit thereby potentially placing those recipients at risk of TRALI. Blood collectors and hospitals should evaluate the potential TRALI risk against the benefit of a potentially expanded inventory of LTOWB before collecting plasma-containing products from non-HLA-tested parous female donors.
FUNDING INFORMATION
No funding was obtained to write this paper.
CONFLICT OF INTEREST STATEMENT
MHY has given paid lectures/reimbursed travel/consulting fees for: Grifols, Hemanext, Terumo, and Verax. MHY owns equity in Velico Medical, Inc. The other authors do not have any conflicts to report.




