Management of perioperative iron deficiency anemia as part of patient blood management in France: A budget impact model-based analysis based on real world data
Abstract
Objectives
Patient Blood Management (PBM) is defined as a patient-centered, systematic, evidence-based approach to improve patient outcomes by managing and preserving a patient's own blood, while promoting patient safety and empowerment. As a corollary, it also reduces the utilization of allogeneic blood components. However, demonstrating cost-effectiveness depends on the health insurance system considered. This analysis aims to estimate the one-year budget impact of PBM in four elective surgical areas, from French National Health Insurance and hospital perspectives.
Methods
A budget impact model was developed to estimate the difference in the cost of care between scenarios with and without PBM. The impact of hematopoiesis optimization (first pillar of PBM) was studied throughout the management of preoperative anemia and iron deficiency in four types of surgeries: orthopedic, cardiac & cardiovascular, vascular & thoracic, and urologic & visceral surgery. Estimation of model's parameters was based on data collected in 10 French hospitals, literature, and on data from the French national medico-administrative database.
Results
A total of 980,125 patients were modeled for all four therapeutic areas. Results shows that implementation of a PBM program could generate annual savings up to €1079 M from the French National Health Insurance perspective (€1018 M from the hospital perspective), and the sparing of 181,451 red blood cells units per year. The deterministic sensitivity analysis showed that PBM generates savings for both perspectives in most parameters tested.
Conclusion
Implementing PBM programs could result in important savings for the health care system in France.
Abbreviations
-
- DRG
-
- diagnosis related group
-
- ESA
-
- erythropoiesis stimulating agent
-
- ID
-
- iron deficiency
-
- IV
-
- intravenous
-
- LOS
-
- length of stay
-
- NCS
-
- national cost study
-
- PBM
-
- patient blood management
-
- PRBC
-
- packed red blood cells
1 INTRODUCTION
Patient Blood Management (PBM) is an evidence-based, patient-focused, multidisciplinary approach to improve patient outcomes.1 This strategy allows optimal conservation of the patient's own blood to improve the quality of care in terms of morbidity and mortality.1-3 PBM lies on three pillars1, 2, 4: (i) detection and management of anemia and iron deficiency (ID), (ii) minimization of blood loss and (iii) optimization of patient's tolerance to anemia. The first pillar is of crucial importance, preoperative anemia being an independent predictive factor for postoperative mortality and morbidity.5, 6 It is associated with poorer outcomes, increased length of hospital stay and subsequent higher hospital costs. The presence of iron deficiency and/or of anemia, is also associated with increased mortality, increased incidence of serious adverse events, major adverse cardiac events, transfusion, and prolonged hospital stay.3, 7-11
Despite the evidence and many national and international recommendations,1, 12 it is recognized that implementation of PBM could be wider.13 One of the barriers to PBM implementation is the estimated cost associated with preoperative anemia treatment and the belief that PBM is not cost-effective.14 Cost-effectiveness of PBM programs have been reported from local or regional experiments. In the state of Western Australia (>600,000 patients) implementation of PBM achieved product cost savings of AU$18 M with a 41% decrease in the number of blood units transfused over 5 years.15 Implementation of Pillar 3 (i.e., monitoring and feedback program as part of the implementation of restrictive transfusion triggers) in a PBM program at University Hospital of Zürich, Switzerland, allowed a total saving of €11.1 M over 4 years (2014–2017). The introduction of this program reduced allogeneic blood product transfusions by 35%, from 825 units per 1000 hospital discharges in 2012 to 536 units in 2017.16 To date, no study in France had evaluated the potential economic impact of implementing a national PBM program. Hence, this budget impact analysis aimed to model the economic impact of implementing preoperative hematopoiesis optimization in France, from both National Health Insurance and hospital perspectives, using real-life data collected in French hospitals.
2 METHODS
2.1 Model description
The objective of this budget impact analysis was to measure the difference between the costs of medical care with and without hematopoiesis optimization as part of a PBM program in France. Hence, two scenarios were compared: a scenario with no PBM (current practice) versus a scenario with PBM for all patients who could benefit from it (hematopoiesis optimization for patient with ID and/or anemia, using ferric carboxymaltose alone or with erythropoiesis-stimulating agents for orthopedic patients).
Hospital and French National Health Insurance perspectives were considered, and four elective surgeries areas were studied (orthopedic, cardiac & cardiovascular, vascular & thoracic, and urologic & visceral surgery).
- Average length of stay (LOS),
- Proportion of patient requiring blood transfusions,
- Number of RBC units transfused per patient,
- Proportion of patients treated with erythropoiesis stimulating agents (ESA) in orthopedic surgery and number of administrations,
- Proportion of patient treated with preoperative IV iron.
2.2 Estimation of PBM effect
We used the PERIOPES nationwide multicenter observational study17 to evaluate the current situation. Data from 10 French hospitals were collected between 2019 and 2021 (six university hospitals [Strasbourg, Nice, Saint-Etienne, Toulouse, Marseille, Besançon], two public non-university hospitals [Douai and Valenciennes], and two private hospitals [Paris Saint-Joseph and Gentilly]). Real-life data from these centers regarding transfusion rates, number of packed red blood cells (PRBC) units transfused and proportion of patients treated for ID and/or anemia were used in the current practice scenario without PBM.
The modeled population corresponds to the total number of patients identified in the Diagnosis Related Group (DRG) considered as relevant for a PBM program implementation. The number of patients per DRG was estimated from the number of stays observed for each DRG at a national level. The length of stay was based on national averages per DRG. The decrease in LOS in the PBM scenario was based on literature data.2
2.2.1 Proportion of patients assessed for their iron status and the proportion of patients treated with ferric carboxymaltose
Although the hospitals from which the data were collected did not have a well-established PBM program in place, non-routine ID diagnosis with or without treatment with ferric carboxymaltose could be observed for some patients. The proportion of patients treated with ferric carboxymaltose was used in the current practice scenario. In the modeled population of the PBM scenario, literature data were used to assess the prevalence of ID, and it was considered that 100% of the patients who underwent orthopedic, cardiac & cardiovascular, vascular & thoracic, and urologic & visceral surgery and were diagnosed with ID, were treated with ferric carboxymaltose.9, 18
2.2.2 Proportion of patients requiring blood transfusion and the number of PRBC units administered per patient
The proportion of patients transfused, and the average number of PRBC units transfused per patient used in the current practice scenario were obtained from the observational study.17 The decrease in transfusion rate and number of PRBC units transfused in the PBM scenario was based on literature data.2, 19-21
2.2.3 Proportion of patients requiring ESA and ESA dosing (orthopedic surgery only)
Preoperative treatment of anemia with ESA is indicated prior to major elective orthopedic surgery.12 The recommended dose of erythropoietin is 600 IU/kg per injection. Rineau et al. showed that following the implementation of a PBM program, a higher proportion of patients was treated with ESA preoperatively because of a systematic diagnosis of anemia with a lower dose of erythropoietin for patients receiving concomitant ferric carboxymaltose.22 Thus, the proportion of patients treated with ESA in Rineau et al. was used in the PBM scenario by considering two injections per treated patient.23
2.3 Cost parameters
The cost of iron status assessment includes both ferritin level and transferrin saturation. The cost was estimated to be €8.52 and was calculated as follows: [Act B21 (1213) + ActB17(2002) both are weighted according to the number of acts reimbursed in 2020 + quotation TB1.5 + security fee B5 + preanalytical fee B17] * reimbursement rate (60%)—deductible (€1.00). The iron status assessment was considered as an outpatient test and was therefore not considered from the hospital perspective. Anemia screening test was considered part of routine preoperative testing. Consequently, no extra cost for anemia screening was considered in the analysis.
The cost of preoperative ferric carboxymaltose treatment for ID patients included both administration and product costs. From National Health Insurance perspective, ferric carboxymaltose being an intra-DRG product, only the cost related to its administration were accounted for. The administration of preoperative intravenous iron was assessed by using the DRG 28Z17Z “sequential chemotherapy for non-tumor disorders” with a tariff of €369.46 in public hospitals (2021 tariff). From the hospital perspective, the product cost as well as the administration cost of ferric carboxymaltose were accounted for. The price of ferric carboxymaltose used in the analysis was the catalog price (€200.00 for a vial of 20 mL, 50 mg/mL) and the costs of the DRG 28Z17Z extracted from the National Cost Study in this perspective were €557.33 in public hospitals (National Cost Study, NCS 2020).
Postoperative iron IV treatment may be administered during the hospital stay following the surgery. Their cost is charged as intra-DRG. In practice, when a lower dose of iron is needed and multiple administrations are possible over time, an iron-sucrose product may be favored. The cost of the iron-sucrose product (between €1.90 and €17.00 per 100 mg/mL vial) being negligible with respect to the cost of hospital stay, postoperative iron injections were not accounted for in the analysis.
Costs associated with hospital stays are based on DRG tariffs for the National Health Insurance perspective (2021) and on the National cost reference for medical care for the hospital perspective (NCS 2020). The cost of each DRG was weighted by the size of the population to obtain the average cost of stay. The average LOS was used to calculate the cost of hospital stay per day. Hence, an average daily cost of stay was calculated depending on the surgery type. Hospital costs of stay are reported in Table S2 in the Appendix.
The DRG tariffs include the cost of RBC units. Thus, from the National Health Insurance perspective, the transfusion cost was not added to the costs of the hospital stay. The detailed hospitalization costs from the hospital perspective being available, it was possible to identify in the DRG costs (NCS, 2020) the part because of direct blood burden and replace it with the number of RBC units administered in both scenarios, considering their tariff in 2021 (€201.23). Consequently, it was possible to assess the difference in the transfusion cost between the two scenarios.
In orthopedic surgery, ESA was considered only from the perspective of National Health Insurance. Indeed, although ESA was prescribed in the hospital setting, dispensing and administration are performed in an outpatient setting. The tax-inclusive price of epoetin alpha/zeta was used for the analysis (Binocrit®, biosimilar of Eprex®, or Retacrit®, €251.96 for a 40,000 IU prefilled syringe and €12.60 for a 2000 IU prefilled syringe), plus the dispensing fees (€1.02).
Other pharmacological treatments administered in the perioperative setting (tranexamic acid, oral iron and filling solutions) are integral to the DRG. Hence, no extra cost related to these treatments was considered for the analysis. Folate and vitamin B12 are very rarely administered, and their costs are negligible, so we did not take them into account in the model.
2.4 Sensitivity analysis
A deterministic sensitivity analysis was conducted to assess the effect of uncertainty on the results. Table S3 in the Appendix lists the parameters where the values were successively modified by applying a variation of ±20% with respect to the reference value.
3 RESULTS
A total of 980,125 patients from the “Scan Santé” database were modeled for all four surgical areas, including 339,117 patients in orthopedic surgery, 33,087 patients in cardiac & cardiovascular surgery, 209,567 patients in vascular and thoracic surgery and 398,354 patients in urologic and visceral surgery. Table 1 displays the main parameters used for modeling both scenarios. All the parameters, including population size and data sources for each therapeutic area, are shown in Table S1 in the Appendix.
| Parameter | Orthopedic surgery (n = 339,117) | Cardiac & cardiovascular surgery (n = 33,087) | Urologic & visceral surgery (n = 398,354) | Vascular & thoracic surgery (n = 209,567) |
|---|---|---|---|---|
| Average duration of hospital stays without PBM (National Database) | 5.91 | 12.82 | 6.34 | 4.79 |
| Potential decrease in the average LOS with PBM (days) | −0.42 | −1.32 | −2.82 | −0.92 |
| Proportion of transfused patients without PBM (hospital study) | 14.1% | 47.6% | 6.6% | 18.0% |
| Potential decrease in the proportion of transfused patients with PBM in % | −55%2 | −50%2 | −60%19, 20 | −8%2 |
| Average number of RBC units per transfused patient without PBM (hospital data) | 2.6 | 2.4 | 2.9 | 2.9 |
| Potential decrease in the average number of PRBC per transfused patient with PBM in units | −0.22 | −0.92 | −0.519, 20 | −0.72 |
| Proportion of patients treated with ESA in orthopedic surgery without PBM (hospital data) | 5% | NA | NA | NA |
| Proportion of patients treated with ESA in orthopedic surgery with PBM | 10%23 | NA | NA | NA |
| Number of ESA injections in orthopedic surgery without PBM | 3 | NA | NA | NA |
| Number of ESA injections in orthopedic surgery with PBM | 223 | NA | NA | NA |
| Proportion of patients treated with ferric carboxymaltose with PBM | 75%18 | 75%18 | 75%18 | 21%10 |
Based on real-life data, the proportion of patients preoperatively treated with ferric carboxymaltose in the scenario without PBM was 10% in orthopedic surgery, 3% in urologic & visceral surgery, and 0% in cardiac & cardiovascular and vascular & thoracic surgeries. In the PBM scenario, we considered that all patients diagnosed with ID (i.e., 75% in orthopedic surgery, cardiac & cardiovascular surgery, urologic & visceral surgery according to Munoz et al (in their cohort of 2884 patients, 2163 (75%) had some ID)18 and 21% in vascular & thoracic surgery10) were preoperatively treated with ferric carboxymaltose.
Regarding the preoperative use of ESA in orthopedic surgery, hospital data showed that 5% of patients received it in current practice. In the scenario with PBM, Rineau et al. data were used (i.e., 10%) (Table 1).22
Average LOS gathered in the nationwide database and used for the current practice scenario ranged from 4.8 days (vascular & thoracic surgery) to 12.8 days (cardiac & cardiovascular surgery). According to the literature,2 the expected decrease in the average LOS due to PBM varied from 7% for orthopedic surgery to 44% for urologic and visceral surgery (Table 1).
Proportions of transfused patients for the current practice scenario varied between 7% (urologic & visceral surgery) and 48% (cardiac & cardiovascular surgery) and the mean number of PRBC units administered per transfused patient ranged from 2.4 (cardiac & cardiovascular surgery) to 2.9 (urologic & visceral surgery). The decrease in transfusion rate in the PBM scenario was based on literature data2, 19, 20 and varied between 8% and 60%, depending on the therapeutic area. The mean decrease in number of transfused PRBC units per patient due to PBM oscillated between −0.2 and 0.9 (Table 1).
For the 980,125 patients, total annual savings from National Health Insurance perspective would be €1079 M (Table 2, Figure 1). Depending on the surgical area, the estimated savings for National Health Insurance ranged from €48 M (cardiac & cardiovascular surgery) to €814 M (urologic & visceral surgery). Hospital stays were reduced by 1,472,153 days, which corresponds to a 25% decrease in all surgical areas combined (orthopedic surgery, −7%; urologic & visceral surgery, −44%).
| Surgical area perspective | Orthopedic surgery | Cardiac & cardiovascular surgery | Urologic & visceral surgery | Vascular & thoracic surgery | Total surgery |
|---|---|---|---|---|---|
| National Health Insurance total (€) | −€56.6 M | −€48.5 M | −€814.1 M | −€160.0 M | −€1079.2 M |
| Total in-hospital stay | −€133.7 M | −€57.1 M | −€908.6 M | −€176.1 M | −€1275.5 M |
| Total pharmacological | €75.0 M | €8.3 M | €91.4 M | €14.3 M | €188.9 M |
| Total biological test | €2.1 M | €0,3 M | €3.2 M | €1.8 M | €7.4 M |
| Hospital total (€) | −€45.5 M | −€47.6 M | −€732.6 M | −€191.9 M | −€1017.6 M |
| Total in-hospital stay | −€163.1 M | −€59.0 M | −€901.7 M | −€213.8 M | −€1337.7 M |
| Total pharmacological | €132.3 M | €16.6 M | €179.5 M | €28.3 M | €356.6 M |
| Total blood | −€14.6 M | −€5.1 M | −€10.4 M | −€6.3 M | −€36.5 M |
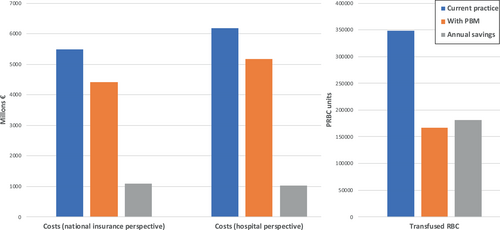
From a hospital perspective, these savings would be €1018 M for the study population and would range from €45 M (orthopedic surgery) to €733 M (urologic & visceral surgery) (Table 2, Figure 1). The 52% decrease in the number of PRBC units transfused after implementation of the PBM program resulted in savings of €37 M compared with the scenario without PBM. Based on the model, a PBM program implementation would spare 181,451 units of transfused PRBC units, with 74,674 patients transfused with a total of 166,989 PRBC units compared to 127,757 patients transfused with a total of 348,440 (Figure 1).
The deterministic sensitivity analysis showed that savings are generated for both perspectives for most of the parameters tested. The length of stay after the PBM implementation and the average cost per hospitalization day are the most important driving factors for the budget impact model. A ± 20% variation of these parameters in the National Health Insurance perspective would correspond to a budget impact of −€824 M € and −€1334 M (Table 3). In the hospital perspective, the budget impact after this variation would be −€750 M and −€1285 M (Table 4). The other parameters had a lower impact on the model results (Tables 3 and 4). To complete this sensitivity analysis, the combined effect of a ± 20% variation of all three parameters together after implementation of PBM (i.e., average LOS, proportion of patients transfused, and number of RBC units transfused) was performed and leads to a saving of €309 M from the health insurance perspective and €122 M from the hospital perspective. Compared with the main analysis, the implementation of PBM would spare 115,068 RBC units and avoid transfusion for 38,148 patients (vs. 181,451 RBC units spared and transfusion avoided for 53,053 patients).
| Parameter | Budget impact with the lower value (−20%) | Budget impact with the upper value (+20%) |
|---|---|---|
| Average length of stay after PBM implementation | −€824.1 M | −€1334.3 M |
| Average cost per hospitalization day | −€824.1 M | −€1334.3 M |
| Preoperative dose of IV iron | −€1079 M | −€897.3 M |
| Cost per iron IV administration | −€1115.6 M | −€1042.8 M |
| ESA - Proportion of patients treated after PBM implementation | −€1083.4 M | −€1075.1 M |
| Cost with ESA | −€1080.6 M | −€1077.8 M |
| Parameter | Budget impact with the lower value (−20%) | Budget impact with the upper value (+20%) |
|---|---|---|
| Average cost per hospitalization day | −€750.0 M | −€1285.1 M |
| Average length of stay after PBM implementation | −€750.0 M | −€1285.1 M |
| Preoperative dose of IV iron | −€1017.6 M | −€660.9 M |
| Cost per iron IV administration | −€1065.9 M | −€969.2 M |
| Cost per IV iron | −€1040.5 M | −€994.6 M |
| Cost per PRBC unit | −€1010.3 M | −€1024.9 M |
| Proportion of transfused patients after PBM implementation | −€1024.3 M | −€1010.8 M |
| RBC unit transfused after PBM implementation | −€1016.0 M | −€1019.1 M |
4 DISCUSSION
To our knowledge, this is the first published analysis estimating the budget impact of implementing hematopoiesis optimization as part of a PBM program in France. According to our model, and for the analyzed population of 980,125 patients, 181,451 RBC units would be spared (7.8% of total RBC units transfused in France in 202124) with an estimated annual savings of €1079 M from the National Health Insurance perspective and €1018 M from the hospital perspective. This analysis plaids for PBM, as its implementation would allow important savings for both National Health Insurance and hospital, and it would help reducing national transfusion dependency.13, 25
From the National Health Insurance perspective, the savings are mostly based on the reduction of the duration of hospital stay related to the implementation of the PBM program. The hospital stay was reduced by 1,472,153 days, corresponding to a 25% decrease in all surgical areas combined for the entire population. This leads to a reduced hospital costs and offsets additional costs associated with screening and treatment of anemia/ID. This analysis did not consider the possible reduced rate of hospital readmission (for a surgical complication) observed in anemic patients treated with iron compared to placebo and all the other related costs.15, 26
From the hospital perspective, the cost-savings in all surgical areas are 4 times higher than the cost increased from implementing hematopoiesis optimization. As in the case of the National Health Insurance perspective, savings from the hospital perspective originated from the decrease in the average LOS, corresponding to savings of €1338 M, but also to the 52% reduction in transfusions (€37 M). This could also be even more important, as the activity-based costs of transfusion could be estimated 3–5 times more than the actual product cost.27
It is important to highlight that we modeled the cost of implementing preoperative anemia treatment, but not the less costly cost of implementing the second and third pillars of PBM. Nevertheless, our results are in accordance with other studies reporting budget benefits related to the implementation of PBM programs in other countries. The savings induced by the decreased number of transfusions were evaluated, in these studies, to be US$2 M (approximately €1.8 M) for 101,794 patients, or also AUS$18 M (approximately €12 M) for 605,046 patients.15, 16 In our study with 980,125 patients the generated saving (by decreased blood use) is €37 M is very similar to these reports.
Although using only a selection of hospitals may have led to a partial representation of the practices observed in the studied surgeries, the use of real-life data from 10 French public/private hospitals that had not established a PBM program had the advantage of having transfusion rates comparable to those observed in current practice in most French hospitals, as opposed to others available in medico-administrative databases which tend to be underestimated, notably because of under-coding post-operative transfusions. However, it is possible that transfusion rates are lower in current practice in France.
One of the limitations of the observational study is that the proportion of ID patients is underestimated compared to what is observed in the literature.18, 28 This may be since diagnosis of iron deficiency was not routinely done in the study hospitals. It is likely that the proportion of ID patients diagnosed would be higher with the implementation of a PBM program protocolizing a systematic diagnosis of this condition. To overcome this underestimation, data from the literature were used to estimate the proportion of ID patients in France, considering a very conservative hypothesis (i.e., 75% of ID),18 we could have based our hypothesis on more recent data.28 In addition, we do not know whether (and if, to what degree) a change in the ID-prevalence and patient treatment ratio would be associated with a change in savings. We were neither able to differentiate the savings according to the cause of anemia (pure ID or functional ID).
The impact of PBM is likely to be influenced by patients' comorbidities. French data lacking, effects of a PBM program according to DRG severity levels could not be modeled in the current analysis.
In this analysis, the budget impact of the PBM implementation was only modeled according to the first pillar of this program, which consists of hematopoiesis optimization through the management of ID and anemia. Clinical data not being available, the impact of the other two pillars of PBM programs could not be modeled, but their costs are very low.
The main limitation of our analysis is the uncertain impact of PBM and optimizing preoperative hematopoiesis (which specifically includes the treatment of ID) on the average LOS, the main source of savings observed in the model. Indeed, it is likely that other factors influence the decrease of LOS, particularly with the encouraged development of day-case surgery. Similarly, LOS and transfusion rates mainly depend on the medical practice of each institution. Based on the medico-administrative database PMSI (Programme de médicalisation des systèmes d'information), the average LOS evolution over the 2018–2019 period (2020–2021 not used as reference due to potential impact of the COVID-19) shows a decrease in the mean LOS of 9.3% in orthopedic surgery for the DRG 08C48 (Hip replacements for conditions other than recent trauma) and 9.2% in digestive surgery for the DRG 06C04 (Major small bowel and colon procedures). For vascular and cardiac procedures, a slight increase in LOS was observed over the same period (between 0.1% and 1%). The potential decrease in the mean LOS used in the model, derived from the literature, could therefore be related not only to the implementation of hematopoiesis optimization in the framework of PBM, but also to other parameters. The benefit of treating anemia according to the different type of anemia is not known and have not been considered. Nevertheless, the deterministic sensitivity analysis showed that the PBM generates savings for both perspectives. With the lower value tested for the mean LOS and mean cost for hospitalization in the PBM scenario in all surgical areas combined, savings are still generated for both National Health Insurance (€824 M) and hospitals (€750 M). It is also worth mentioning that increasing the preoperative dose of IV iron generates additional costs for hospitals.
We used published data to estimate the reduction in transfusion rates after PBM implementation2, 19, 20 and to determine transfusion rates for the “current practice” scenario (in hospitals that did not have a well-established PBM protocol, but were motivated to implement it).17 It is possible that transfusion practices are more restrictive and/or that PBM implementation is less effective in other institutions in France. Nevertheless, the results of the sensitivity analysis show that varying the proportion of patients transfused does not change the budgetary impact. Data show that preoperative anemia and iron deficiency are associated with poorer outcomes, increased hospital length of stay, mortality, incidence of serious adverse events, major adverse cardiac events, transfusion, and prolonged hospital stay, whereas PBM programs are associated with a reduction in adverse outcomes. However, adverse events were not considered in the analysis, because French baseline data were not attainable. In addition, there is considerable uncertainty about the proportion of patients requiring medical transportation for hospitalization and preoperative iron injections. These transportation costs were not included in this analysis.
5 CONCLUSION
The results of this budget impact modeling confirm that the implementation of a PBM program, and especially the hematopoiesis optimization, could lead to significant savings for both the National Health Insurance and hospitals.
ACKNOWLEDGMENTS
The authors thank the investigators of the PERIOPES study, some of whose data were used for the purposes of this article. The authors would also like to thank Professor Marc Beaussier for his contribution to this work.
FUNDING INFORMATION
This work was founded by Vifor France.
CONFLICT OF INTEREST STATEMENT
Sigismond Lasocki received consulting fees, educational fees from VIFOR PHARMA, educational fees from MASIMO, consulting fees, educational fees from PFIZER. David Delahaye received during the past 3 years, lecture, and consulting fees from VIFOR PHARMA and PFIZER. Axel Hofmann received during the past 3-year honoraria or consulting fees and travel support from BMS/Celgene, PBMe SOLUTIONS, VIFOR PHARMA, WERFEN. He also received travel support from IFPBM. David Fuks, Pierre-Henri Savoie, Claude Dussart and Pascal Paubel declare no conflicts of interests.