Predicting organ functioning with and without blood transfusion in critically ill patients with anemia
Abstract
Background
To develop a model for the prediction of the (most likely) effect of red blood cell (RBC) transfusion on subsequent organ functioning in nonbleeding critically ill patients with hemoglobin concentrations between 6 and 9 g/dL.
Study Design and Methods
We conducted a retrospective cohort study using electronic health care data of nonbleeding patients admitted between November 2004 and May 2016 at the intensive care unit (ICU) of the Leiden University Medical Center, The Netherlands. We analyzed the associations between transfusion (yes/no) and next-day SOFA scores (Sequential Organ Failure Assessment—as a measure for organ functioning) for all observed combinations of hemoglobin values (between 6 and 9 g/dL) and concurrent clinical variables.
Results
Data of 6425 ICU admission of 5756 critically ill patients with 28,702 hemoglobin values between 6 and 9 g/dL (transfusion decision moments) of which 22.1% were followed by a transfusion were analyzed. The adjusted average difference between the next-day SOFA score of transfused versus not-transfused patients was 0.08 (95% confidence interval [CI] −0.03 to 0.18). At singular transfusion decision moments, the score predicted a beneficial effect of transfusion on next-day SOFA score for some subgroups and medical conditions and a harmful effect in other occasions.
Conclusions
Among these critically ill patients with hemoglobin concentrations between 6 and 9 g/dL the population average effect of transfusion on the next SOFA score was negligible. Further, our results support caution in clinical decision-making regarding transfusion of critical ill, nonbleeding ICU patients.
1 INTRODUCTION
Currently available quantitative evidence merely underscores the absence of an effect of red blood cell transfusion on outcome in critically ill patients. Randomized clinical trials have focused on critically ill patients with hemoglobin concentrations between 7 and 9 g/dL. These trials typically compared a liberal transfusion threshold (transfusion when hemoglobin drops below 9 g/dL) with a restrictive transfusion threshold (postpone transfusion until hemoglobin drops below 7 g/dL). Most of these trigger trials have shown that transfusions, on average, do not affect clinical outcomes of critically ill patients with hemoglobin concentrations between 7 and 9 g/dL.1
Some have argued that the observed absence of an effect of transfusion in randomized trials may be, among other things, the result of beneficial effects on clinical outcome in some patients and harmful effects in others.2 After all, effects of blood transfusion not only depend on the severity of anemia (hemoglobin concentration), but also on the medical condition of the transfusion recipient (e.g., conditions with altered oxygen demand and/or oxygen delivery). Doctors, therefore, might base the decision to transfuse also on diagnosis, APACHE score and physiological parameters.3, 4
The uncertainty about effects of blood transfusion in individual critically ill patients with hemoglobin concentrations below 9 g/dL and the assumption that the effect on clinical outcomes depends on myriad aspects of the medical condition of the patient, produces considerable practice variation.5-7
Practice variation facilitates the comparison of treatment strategies in real-world data.8 To learn which treatment strategy is most likely beneficial, one can compare similar past patients who have or have not been transfused. Due to intensive monitoring of critically ill patients in the ICU and electronic storage of all this information, it is increasingly possible to identify adequate numbers of past patients with similar medical conditions.
With this in mind, we set out to develop a model to predict whether transfusion was associated with better subsequent organ functioning in critically ill patients with hemoglobin concentrations between 6 and 9 g/dL, taking continuously changing physiological parameters into account.
2 MATERIALS AND METHODS
2.1 Setting and study design
We created a research database by gathering electronically available real-world data from critically ill patients who had been admitted to the 29-bed tertiary university mixed intensive care unit (ICU) at the Leiden University Medical Center (LUMC), the Netherlands.9 Electronic health records were introduced in 2004 and were fully implemented in all ICU units by 2006. The ICU in the LUMC is supervised by board certified critical care physicians, with different medical specialty backgrounds, together with a team of residents and fellows. The local protocol for blood transfusion, which has been in use since 2006, recommends transfusing red blood cells when the hemoglobin concentration is lower or equal to approximately 7.2–8.0 g/dL depending on the clinical condition of the patient (e.g., conditions with increased myocardial oxygen demand). There was no computerized provider order entry system or clinical decision support for transfusion practices. Ethical approval was obtained by the Medical Ethical Committee of the LUMC, which waived the need for requesting patients' informed consent. The study is reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
2.2 Study population
For our database, we established a cohort of adult critically ill patients with at least one hemoglobin concentration ≤10 g/dL during their stay in the ICU between November 2004 and May 2016. We excluded Jehovah's witnesses, because they were likely to have refused blood transfusions, via free text searches in the electronic medical health record. Other exclusion criteria were: significant acute bleeding (at least five red blood cell transfusions within a period of 24 h) during ICU admissions and no data in the Mediscore database, encompassing the Dutch National Intensive Care Evaluation (NICE) registration.10 Patient information was de-identified prior to analysis, as our dataset did not contain names, geographic subdivisions, birthdates or medical record numbers.
2.3 Transfusion decision moments, exposure and outcome definition
The database comprised separate records of all hemoglobin values of the included critically ill patients. Transfusion decision moment was defined as the moment at which a hemoglobin concentration was between 6.0 and 9.0 g/dL; that is the range of hemoglobin values for which transfusion may be beneficial, depending on the presence of other clinical characteristics. Along with hemoglobin concentration, that is, at each of those transfusion decision moments, we documented: (1) the patients' values of characteristics as assessed during the ICU stay before and at the same moment, (2) whether the patient had received a red blood cell transfusion within the next 6 h, and (3) the latest and the next SOFA scores. A complete list of the variables is presented in Box S1.
We excluded decision moments that occurred after the first 10 days of the ICU admissions because medical conditions and prognosis during prolonged admissions differ considerably from medical conditions during the first 10 admission days.11 We excluded decision moments when the patient was discharged from the ICU within 6 h after the hemoglobin measurement, because these patients were not eligible to be exposed to transfusion within 6 h. We also discarded decision moments for which it was unknown whether or not the patients had died the day after that decision moment.
Exposure was defined as transfusion of one or more units of red cells at the ICU in the period between a hemoglobin measurement and 6 ho thereafter.
The outcome was organ functioning the next day at midday as assessed by the Sequential Organ Failure Assessment (SOFA) score, further referred as “next-day SOFA score.” The SOFA score is a validated score to determine the extent of a patient's organ function or rate of failure during the stay in an ICU.12 Besides, the SOFA score is a good indicator of prognosis.13 All clinical variables were extracted from the electronic health records, the laboratory information system and the Mediscore database.
2.4 Statistical analyses
Transfusion decision moment was the unit of analysis. Our aim was to develop a final model that could be used to predict at every single transfusion decision moment the most likely next-day SOFA score both with and without a transfusion. The analysis was done in three steps, fitting different types of models. First, a model to estimate the population average effect of transfusion, adjusted for potential confounders. Second, a series of models were fitted to explore effects of transfusion in various subgroups. Third, a model to estimate effects of transfusion for each individual decision moment.
The first model was a multivariable linear regression model with next-day SOFA score as dependent variable and transfusion (within 6 h yes/no) together with potential confounders as independent variables (listed in the footnote of Table 1). The selection of these confounding variables was based on prior knowledge from the literature and on a survey among intensive care physicians.7 We repeated this analysis, stratified by hemoglobin concentration.
| Patient characteristics | Total unique patients (n = 5756) |
|---|---|
| Age (years)—mean (SD) | 63 (15) |
| Gender—no. (%) male | 3420 (59.4) |
| Weight (kg)—median (IQR) | 76 (67–85) |
| Height (m)—mean (SD) | 1.73 (0.10) |
| BMI (kg/m2)—mean (SD) | 26 (5) |
| Number of admissions during study period—no. (%) | |
| 1 | 4775 (83.0) |
| 2 | 746 (13.0) |
| 3 | 170 (3.0) |
| 4 | 43 (0.8) |
| 5 | 17 (0.3) |
| 6 | 2 (0.0) |
| 7 | 3 (0.1) |
| Chronic diagnosis—no. (%) | |
| Diabetes | 671 (11.7) |
| Immunologic insufficiency | 469 (8.2) |
| Chronic renal insufficiency | 379 (6.6) |
| Hematological malignancy | 306 (5.3) |
| Cardiovascular insufficiency | 238 (4.1) |
| Metastasized neoplasm | 217 (3.8) |
| Cirrhosis | 220 (3.8) |
| COPD | 215 (3.7) |
| Chronic respiratory insufficiency | 139 (2.4) |
| Chronic dialysis | 92 (1.6) |
| Aids | 12 (0.2) |
| Admission characteristics | Total admissions (n = 6425) |
| Type of admission—no. (%) | |
| Medical | 3211 (50.0) |
| Emergency surgery | 2495 (38.8) |
| Elective surgery | 719 (11.2) |
| Referring facilitya—no. (%) | |
| Ward | 4629 (72.1) |
| Emergency department | 949 (14.8) |
| Other high care department | 423 (6.6) |
| Other hospital, ICU | 277 (4.3) |
| Other hospital, ward | 120 (1.9) |
| Other | 8 (0.1) |
| APACHE II scorea—median (IQR) | 18 (14–23) |
| APACHE II admission subgroup diagnosisa—no. (%) | |
| Cardiovascular | 3501 (54.5) |
| Respiratory | 1001 (15.6) |
| Gastrointestinal | 831 (12.9) |
| Neurological | 513 (8.0) |
| Sepsis | 352 (5.5) |
| Renal | 110 (1.7) |
| Hematological | 68 (1.1) |
| Metabolic | 49 (0.8) |
| Mechanically ventilated within 24 h | 5104 (79.4) |
| Diagnosis at admission—no. (%)b | |
| Confirmed infection | 675 (10.5) |
| Acute renal failure | 537 (8.4) |
| Cardiopulmonary resuscitation | 323 (5.0) |
| Myocardial infarction before CABG | 274 (4.3) |
| Dysrhythmia | 233 (3.6) |
| Intracranial mass | 178 (2.8) |
| Cerebrovascular disease | 177 (2.8) |
| Gastro-intestinal blood loss | 103 (1.6) |
| Thrombolytic therapy after acute MI | 13 (0.2) |
| Burns | 7 (0.1) |
- a Only valid measurements presented, therefore percentages do not add up to 100%. Data on missing values can be found in the supplemental material.
- b Multiple diagnoses possible. IQR interquartile range SD standard deviation BMI body mass index COPD chronic obstructive pulmonary disease MI myocardial infarction CABG coronary artery bypass grafting.
In the second step, we explored effects of transfusion in subgroups of each of the clinical characteristic separately; for every variable a new multivariable linear regression model was constructed, including all potential confounders and an interaction term between transfusion and the particular selected variable. For continuous variables we combined the effect of red cell transfusion and the additive interaction effect. Bonferroni's method was used to adjust p-values for multiple testing.
In the third step, we constructed a multivariable linear regression model with next-day SOFA score as dependent variable and as independent variables: transfusion (within 6 h yes/no), all selected confounding variables (listed in Box S1) and interaction terms for transfusion (within 6 h yes/no) with each of the confounding variables. Using this model, we predicted next-day SOFA scores with and without red cell transfusion for all decision moments. The difference between the predicted next-day SOFA score with and the predicted next-day SOFA score without transfusion was interpreted as the most-likely effect of transfusion at each particular transfusion decision moment.
Different strategies were used for handling of missing values. Details of these strategies and the percentage of missing data can be found in the supporting information. Robust standard errors were used to account for clustering of outcomes within ICU patients in all models. All reported P values are 2-sides, and P < .05 was considered statistically significant. All statistical analyses were performed in Stata/SE version 16.1.
2.5 Sensitivity analyses
The time between seeing the value of a hemoglobin concentration, that is, the transfusion decision moment, and the actual transfusion may have taken longer than 6 h, which may have led to misclassification of exposure to transfusion (within 6 h). To examine whether this influenced our results, we repeated our analyses with transfusions within 8 h after the transfusion decision moment as exposure of interest.
To evaluate whether next-day SOFA score may have been too soon to observe an effect of transfusion, we additionally evaluated the effect of transfusion on the SOFA scores 2 days after the decision moments.
3 RESULTS
3.1 Population
Between November 2004 and May 2016, 19,315 patients, representing 22,817 admissions, were admitted to the ICU. A total of 11,681 (51.2%) ICU admissions met the inclusion criteria. After excluding patients for the reasons given in Figure 1, data of 6425 ICU admissions of 5756 critically ill patients could be included for analysis. Demographic and baseline characteristics of the patients and ICU admissions are summarized in Table 1.
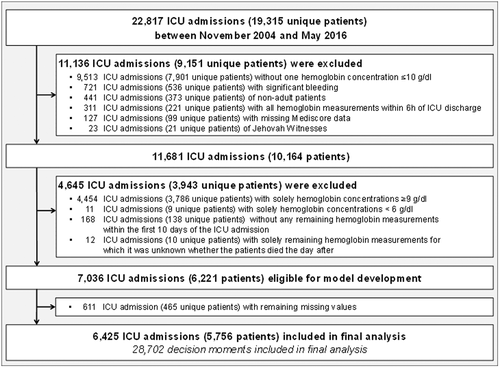
3.2 Laboratory and clinical characteristics at transfusion decision moments and subsequent transfusions
Table 2 presents clinical characteristics at the decision moments according to whether or not patients had received a transfusion within 6 h after the hemoglobin measurement. A total of 28,702 hemoglobin values were between 6 and 9 g/dL and the moments at which they were observed were labeled transfusion decision moments. Of these transfusion decision moments, 6335 (22.1%) were followed by a transfusion within 6 h. The proportion of decision moments followed by a transfusion decreased with increasing hemoglobin levels; 873 of 1305 (66.9%), 3575 of 8026 (44.5%), and 1887 of 19,371 (9.7%) for hemoglobin levels between 6 to 7, 7 to 8, and 8 to 9 g/dL, respectively.
| Clinical characteristics | Transfusion within 6 h after Hb measurement | ||
|---|---|---|---|
| Yes | No | P value | |
| n = 6335 (22.1) | n = 22,367 (77.9) | ||
| Hemoglobin concentration (g/dL)—mean (SD) | 6.3 (0.6) | 8.3 (0.5) | <.001 |
| First day ICU admission—no. (%) | 2270(35.8) | 3751(16.8) | <.001 |
| Previous hemoglobin concentration (g/dL)—mean (SD) | 8.4 (0.9) | 8.5 (0.8) | <.001 |
| Previous day's SOFA score—median (IQR) | 7 (7 to 9) | 7 (5 to 9) | <.001 |
| Time to previous hemoglobin measurement (h)—median (IQR) | 6.6 (3.1 to 11.2) | 10.3 (5.7 to 23.8) | <.001 |
| Standard (scheduled) hemoglobin measurement—no. (%) | 1770 (27.9) | 11,635(52.0) | <.001 |
| Age—mean (SD) | 62.7 (14.5) | 61.3(14.8) | <.001 |
| Male gender—no. (%) | 3913 (61.2) | 14,005(62.6) | .551 |
| Type of admission—no. (%) | |||
| Emergency surgery | 2497 (39.4) | 6929 (31.0) | <.001 |
| Medical | 3075 (48.5) | 12,785 (57.2) | |
| Elective surgery | 763 (12.0) | 2653 (11.9) | |
| APACHE II score—mean (SD) | 20.4 (7.6) | 20.8 (7.5) | .003 |
| APACHE II admission diagnosis subgroup—no. (%) | |||
| Cardiovascular | 3575 (54.4) | 10,038 (44.9) | <.001 |
| Sepsis | 412 (6.5) | 1546 (6.9) | |
| Gastrointestinal | 962 (15.2) | 3379 (15.1) | |
| Hematological | 143 (2.3) | 428 (1.9) | |
| Metabolic | 21 (0.3) | 175 (.8) | |
| Neurological | 302 (4.8) | 1768 (7.9) | |
| Renal | 97 (1.5) | 318 (1.4) | |
| Respiratory | 823 (13.0) | 4715 (21.1) | |
| Mechanically ventilated—no. (%) | 3778 (59.6) | 12,913 (57.7) | .032 |
| Respiratory rate (breath/min)—no. (%) | |||
| Bradypnea (<10) | 154 (2.4) | 355 (1.6) | <.001 |
| Normal | 4234 (66.8) | 13,227 (59.1) | |
| Tachypnea (>20) | 1947 (30.7) | 8785 (39.3) | |
| Central venous oxygen saturation monitored—no. (%) | 20 (0.3) | 60 (0.3) | .615 |
| PaO2/FiO2 ratio—no. (%) | |||
| Not measured | 2725 (43.0) | 8641 (38.6) | <.001 |
| <30 | 1465 (23.1) | 6526 (29.2) | |
| >30 | 2145 (33.9) | 7200 (32.2) | |
| Lactate concentration (mmol/L)—no. (%) | |||
| Low | 4159 (65.6) | 17,481 (78.2) | <.001 |
| High (>2.2) | 2176 (34.4) | 4886 (21.8) | |
| Arterial pH—no. (%) | |||
| Acidosis (<7.35) | 1983 (31.3) | 4835 (21.6) | <.001 |
| Normal | 3141 (49.6) | 11,302 (50.5) | |
| Alkalosis (>7.45) | 1211 (19.1) | 6230 (27.9) | |
| Troponin (μg/L)—no. (%) | |||
| Not measured | 3295 (52.0) | 14,716 (65.8) | <.001 |
| Normal (<0.050) | 518 (8.2) | 1311 (5.9) | |
| High (0.050–1.000) | 1690 (26.7) | 4301 (19.2) | |
| Extremely high ≥1.000) | 832 (13.1) | 2039 (9.1) | |
| Mean systolic pressure (mmHg)—no. (%) | |||
| Low (<90) | 688 (10.9) | 1261 (5.6) | <.001 |
| Normal | 3565 (56.3) | 11,339 (50.7) | |
| High (>120) | 2082 (32.9) | 9767 (43.7) | |
| Mean heart rate (bpm)—no. (%) | |||
| Bradycardia (<60) | 144 (2.3) | 500 (2.2) | .043 |
| Normal | 4177 (65.9) | 15,192 (67.9) | |
| Tachycardia (>100) | 2014 (31.8) | 6675 (29.8) | |
| Diuresis (mL/h)—no. (%) | |||
| <30 | 1811 (28.6) | 5195 (23.2) | <.001 |
| 30–50 | 873 (13.8) | 2758 (12.3) | |
| 50–150 | 2491 (39.3) | 9138 (40.9) | |
| >150 | 1160 (18.3) | 5276 (23.6) | |
| Thrombocytes (×109/L)—no. (%) | |||
| Severe thrombocytopenia (<50) | 763 (12.0) | 2718 (12.2) | <.001 |
| Thrombocytopenia (50–150) | 3288 (51.9) | 9357 (41.8) | |
| Normal | 2093 (33.0) | 8994 (40.2) | |
| Thrombocytosis (>450) | 191 (3.0) | 1298 (5.8) | |
| Prothrombin time (s)—no. (%) | |||
| Shortened (<12) | 128 (2.0) | 549 (2.5) | <.001 |
| Normal | 1698 (26.8) | 7134 (31.9) | |
| Prolonged (>15) | 4509 (71.2) | 14,684 (65.7) | |
- Abbreviations: FiO2, fraction of inspired oxygen; Hb, hemogloblin; IQR, interquartile range; paO2, arterial partial pressure of oxygen; SOFA, sepsis-related organ failure assessment score.
3.3 Population average effect of transfusion on next-day SOFA score
Table 3 presents next-day SOFA scores according to transfusion along with crude and adjusted differences in next-day SOFA scores between decision moments with and without transfusions. Among patients who had not received a transfusion within 6 h after a decision moment, the mean next-day SOFA score was 6.96 (95% CI 6.84 to 7.09); among those who were transfused within 6 h after the decision moment it was 7.25 (95% CI 7.08 to 7.63); crude difference 0.29 (95%CI 0.15 to 0.43). After adjustment for confounding the average difference between SOFA score after transfusion versus no transfusion, the “average treatment effect in the population”, was 0.08 (95% CI −0.03 to 0.18). For hemoglobin concentrations between 6 and 7 g/dL, the difference in next-day SOFA score between those transfused and not-transfused was −0.09 (95%CI −0.46 to 0.28); for hemoglobin 7 to 8 and 8 to 9 g/dL, it was 0.01 (95%CI −0.13 to 0.15), and 0.17 (95%CI 0.00 to 0.34), respectively.
| Hemoglobin concentration at transfusion decision moment | SOFA score 1 day after transfusion decision moment | ||||
|---|---|---|---|---|---|
| n (%) | Without transfusion mean (95% CI) | With transfusion mean (95% CI) | Crude difference (95%CI) | Population average treatment effect adjusted difference (95%CI)a | |
| 6–7 g/dL | 1305 (4.6) | 7.35 (6.75 to 7.94) | 7.55 (7.24 to 7.87) | 0.21 (−0.40 to 0.81) | −0.09 (−0.46 to 0.28) |
| 7–8 g/dL | 8026 (28.0) | 7.19 (6.98to 7.39) | 7.39 (7.19 to 7.58) | 0.20 (−0.03 to 0.43) | 0.01 (−0.13 to 0.15) |
| 8–9 g/dL | 19,371 (67.5) | 6.90 (6.77 to 7.02) | 6.86 (6.61 to 7.11) | −0.03 (−0.28 to 0.21) | 0.17 (0.00 to 0.34) |
| Overall | 28,702 (100.0) | 6.96 (6.84 to 7.09) | 7.25 (7.08 to 7.43) | 0.29 (0.15 to 0.43) | 0.08 (−0.03 to 0.18) |
- Note: Separate models were fitted in each of the three hemoglobin concentration groups.
- Abbreviations: CI, confidence interval; SOFA, sequential organ failure score.
- a Adjusted for hemoglobin measurement first day ICU admission, previous hemoglobin concentration, previous SOFA score, time to previous hemoglobin measurement, routine (scheduled) hemoglobin measurement, age, gender, type of admission, APACHE II score, APACHE II admission diagnosis subgroup, mechanically ventilation, PaO2/FiO2 ratio, lactate concentration, arterial pH, troponin, mean systolic pressure, mean heart rate, diuresis, thrombocytes, and prothrombin time.
Table S2 presents the estimated effects of red cell transfusion on the next-day SOFA according to the different levels of the separate clinical variables. After Bonferroni correction (P < .05/23), the following statistically significant transfusion effects were observed: transfusion at the first day of ICU admission was associated with a worse next-day SOFA score compared with no transfusion (difference, 0.34; 95% CI, 0.14 to 0.53); on other ICU admission days the difference was −0.04 (95% CI, −0.21 to 0.06). Transfusion in mechanically ventilated patients compared with patients without mechanically ventilation was also associated with a worse next-day SOFA score (difference, 0.19; 95% CI +0.05 to 0.32), representing a negative effect of transfusion on subsequent organ functioning. A similar effect was found for patients with high lactate levels (difference 0.22; 95% CI 0.04 to 0.41).
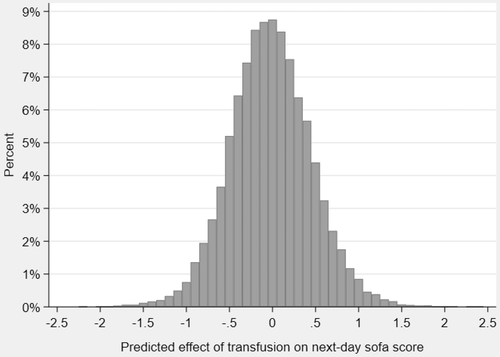
3.4 Predicted effect of transfusion on next-day SOFA score at each individual decision moment
The predicted effect of transfusion on next-day SOFA-score at each individual decision moment ranged from a decrease in next-day SOFA score (beneficial effect) of −2.2 to an increase (harmful effect) of 2.4. Figure 2 shows the predicted, most likely effects of transfusion at individual decision moments. Table S3 presents all regression coefficients of the final model used to predict the effect of transfusion on next-day SOFA score for individual decision moments.
3.5 Sensitivity analyses
Transfusion within 8 h after the decision moments: Of the total of 28,702 hemoglobin measurements between 6 and 9 g/dL, 7350 (25.6%) resulted in RBC transfusion within 8 h. The proportion of decision moments that resulted in red blood cell transfusion decreased with increasing hemoglobin levels; 954 of 1305 (73.1%), 4110 of 8026 (51.2%) and 2286 of 19,371 (11.8%) for hemoglobin levels between 6 to 7, 7 to 8, and 8 to 9 g/dL, respectively. The effects of transfusion that had occurred within 8 h (instead of 6 h) after the transfusion decision moment on next-day SOFA score were similar to the those observed for transfusions within 6 h after the decision moment (Table S4).
48-h SOFA score: Table S5 shows results of sensitivity analyses for the effect of transfusion on 48-h SOFA scores. The effect of transfusion on 48-h SOFA score was similar to the observed effect of transfusion on next-day SOFA score
4 DISCUSSION
To the best of our knowledge, the present study is the first to enable estimation of the most-likely transfusion effect at individual transfusion decision moments. The model, estimating a most likely effect of transfusion on subsequent organ functioning taking different subgroups and changing physiological parameters into account, showed a beneficial effect in some and a harmful effect on subsequent organ functioning in others. The population average effect of transfusion among nonbleeding critically patients with hemoglobin concentrations between 6 and 9 g/day on subsequent organ functioning was more or less zero. This was confirmed in most of the potentially relevant subgroups, including hemoglobin concentrations between 6 and 7, and 7 and 8 g/dL. In the subgroup of hemoglobin concentration between 8 and 9 g/dL a small but harmful effect was found.
Our findings corroborate those of others showing that transfusion, on average, may have limited beneficial effects among critically ill patients with hemoglobin concentrations above 7 g/dL.1 In addition to the population-average effect of transfusion, the present study also estimated transfusion effects in subgroups of clinical variables. The subgroup results suggest that on average, transfusions did not positively influence organ functioning, as assessed with next-day SOFA scores, in most of the subgroups. In contrast, in some subgroups, transfusion was associated with a statistically significant harmful effect on subsequent organ functioning. Mechanically ventilated patients who had been transfused had worse next-day SOFA scores than mechanically ventilated patients who had not been transfused, which was independent of other determinants of organ functioning. This could be a true effect of red cell transfusion, (e.g., transfusion associated circulatory overload) or the result of residual confounding by indication.14
To appreciate these findings several aspects of our study need to be discussed. First, a unique feature and strength of our study is that we compared transfused and nontransfused decision moments. This enabled the estimation of a most likely effect of transfusion on next-day organ functioning compared with no-transfusion, as opposed to comparing the effect of transfusion strategies based on hemoglobin thresholds.
Second, the comparison of transfused versus nontransfused decision moments comes with the possible disadvantage that a patient who did not get a transfusion on the day of the decision moment, may get a transfusion on the next day, and vice versa. This would lead to underestimation of the effect of transfusion. To prevent that, we decided to measure the outcome the day immediately after the transfusion.
Third, we used next-day SOFA score to measure the effect of transfusion on organ functioning. One might argue that transfusions have other effects, effects that are not measured with a SOFA score. We chose SOFA score, because transfusions are given to increase oxygen delivery to organs and optimize the circulation, in order to prevent deterioration of organ functioning. SOFA score is a validated measure for organ functioning.15 There is no established minimal clinical important difference for change in SOFA score, but several observational studies found an association between changes in SOFA score of 1 or 2 points and mortality.16-18 Also, the timing of SOFA score 1 day after the transfusion may have been too early to observe an effect of the transfusion. Yet, effects of transfusion on anemia-related symptoms in patients with other indication, for example cancer, are generally observed 1 day after the transfusion.19 And, we observed the same absence of effect in our sensitivity analysis in which we studied SOFA scores 2 days after transfusion.
Fourth, our results may be the result of residual confounding (by indication), because patients who receive transfusions differ from those who are not transfused. We adjusted for almost anything that doctors consider when they assess the need for transfusion, nevertheless, there may still be unknown, unmeasured confounding.
Fifth, the complexity of our final regression model reduces the risk of bias, but could have resulted in high variance (overfitting). Based on the large number of subjects in our study, we assume that overfitting is not a major problem, as a previous simulation study has shown that only a few subjects per variable is required for accurate estimation of regression coefficients and confidence intervals.20 However, our model was not externally validated to test the performance.
In conclusion, our results suggest that red cell transfusion for patients with hemoglobin concentrations between 6 and 9 g/dL has on average no effect on the next-day SOFA score. This lack of effect on subsequent organ functioning was also found in most subgroups of clinical characteristics. Further, this study demonstrates the limits of retrospective analyses of patient care data. No such analysis, no matter how large and carefully assembled, curated, and analyzed, can answer individual clinical care questions definitively. With that understood, our data do support caution in clinical decision-making regarding transfusion of critical ill, nonbleeding, ICU patients.
AUTHOR CONTRIBUTIONS
Floris J. Kranenburg: Designed research, performed research, collected data, analyzed and interpreted data, performed statistical analysis, and wrote the manuscript. Sesmu M. Arbous: Designed research, performed research, supervised statistical analyses, interpretation of results and writing of the manuscript. Camila Caram-Deelder: Performed statistical analysis, analyzed and interpreted data and revised the manuscript. Hein Putter: Performed statistical analysis, interpreted data and revised the manuscript. Saskia le Cessie: Performed statistical analysis, interpreted data and revised the manuscript. Johanna G. van der Bom: Designed research, performed research, supervised statistical analyses, interpretation of results and writing of the manuscript.
ACKNOWLEDGMENTS
We wish to thank A. van Galen for the data extraction from electronic health records. Additionally, we would like to thank the scientific committee of the CCTR for their feedback on our study protocol and statistical analysis plan.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ETHICS STATEMENT
The study was approved by the Medical Ethical Committee of the LUMC, which waived the need for requesting patient's informed consent.




