Improved outcomes and reduced costs associated with a health-system–wide patient blood management program: a retrospective observational study in four major adult tertiary-care hospitals
Abstract
BACKGROUND
Patient blood management (PBM) programs are associated with improved patient outcomes, reduced transfusions and costs. In 2008, the Western Australia Department of Health initiated a comprehensive health-system–wide PBM program. This study assesses program outcomes.
STUDY DESIGN AND METHODS
This was a retrospective study of 605,046 patients admitted to four major adult tertiary-care hospitals between July 2008 and June 2014. Outcome measures were red blood cell (RBC), fresh-frozen plasma (FFP), and platelet units transfused; single-unit RBC transfusions; pretransfusion hemoglobin levels; elective surgery patients anemic at admission; product and activity-based costs of transfusion; in-hospital mortality; length of stay; 28-day all-cause emergency readmissions; and hospital-acquired complications.
RESULTS
Comparing final year with baseline, units of RBCs, FFP, and platelets transfused per admission decreased 41% (p < 0.001), representing a saving of AU$18,507,092 (US$18,078,258) and between AU$80 million and AU$100 million (US$78 million and US$97 million) estimated activity-based savings. Mean pretransfusion hemoglobin levels decreased 7.9 g/dL to 7.3 g/dL (p < 0.001), and anemic elective surgery admissions decreased 20.8% to 14.4% (p = 0.001). Single-unit RBC transfusions increased from 33.3% to 63.7% (p < 0.001). There were risk-adjusted reductions in hospital mortality (odds ratio [OR], 0.72; 95% confidence interval [CI], 0.67-0.77; p < 0.001), length of stay (incidence rate ratio, 0.85; 95% CI, 0.84-0.87; p < 0.001), hospital-acquired infections (OR, 0.79; 95% CI, 0.73-0.86; p < 0.001), and acute myocardial infarction-stroke (OR, 0.69; 95% CI, 0.58-0.82; p < 0.001). All-cause emergency readmissions increased (OR, 1.06; 95% CI, 1.02-1.10; p = 0.001).
CONCLUSION
Implementation of a unique, jurisdiction-wide PBM program was associated with improved patient outcomes, reduced blood product utilization, and product-related cost savings.
The term patient blood management (PBM) was coined in 2005 to help bring about a realignment of transfusion practice from product focus to patient focus.1, 2 PBM is an evidence-based bundle of care that optimizes medical and surgical patient outcomes by clinically managing and preserving a patient's blood.2-5 In 2010, the World Health Assembly recommended PBM to its member states by resolution; as a result, PBM is high on international health agendas.6-8 PBM is most effective when it is part of a multidisciplinary program.6, 9, 10 The Australian national Patient Blood Management Guidelines contain an evidence-based recommendation that health care services should establish a multidisciplinary, multimodal PBM program; and The Australian Commission on Safety and Quality in Health Care has recently listed the National Patient Blood Management Collaborative as a top national priority and also has included PBM in hospital accreditation.11-13
A programmatic approach to PBM has been associated with improved patient and economic outcomes and reductions in transfusion.14-21 However, those programs focused on specific surgical procedures, implemented selected PBM strategies, or were confined to individual institutions. Several challenges to wider implementation of PBM have been identified, namely, clinicians' resistance to change; a broader systems approach; needed resources; engagement of senior leadership across the health system; changes across the whole patient pathway, including primary care; translating evidence-based guidelines into clinical practice; and linking patient, laboratory, and transfusion databases to report on outcomes.6, 22-25
After the successful implementation in 1990 of a blood-conservation program in one of the state's private hospitals,1, 26 in 2008, the Western Australia Department of Health initiated a 5-year project to implement a comprehensive, sustainable health-system–wide PBM program.6, 27 This was fundamentally a quality, safety, and effectiveness initiative with resource and economic implications. Implementing a PBM program across an entire health system required a redesign of clinical processes and culture change at all levels of the health care organization.25, 28 Implementation was based on a predefined program design incorporating successful hospital-based models and change management principles. Its primary aim was “improving medical and surgical patient outcomes while achieving significant cost savings” by applying PBM principles.6, 27
Western Australia is an Australian state with a population of approximately 2.6 million, with 74% living in the capital, Perth. There are five major public tertiary-care hospitals, four adult and one pediatric, which consume almost 60% of the blood products issued to the state. In the financial year 2008-2009, the Western Australia red blood cell (RBC) issuance rate was 31.8 per 1000 population, one of the lowest reported rates in the developed world (issuance rates for Germany, Denmark, and United Kingdom were 57.3, 60.0, and 36.3 per 1000, respectively, and the transfusion rate in the United States was 48.8 per 1000).6, 29 However, from financial years 2002-2003 to 2008-2009, total RBC unit issues to the Western Australia state rose 12% and were projected to continue rising over the next 4 years, largely due to the rapidly growing and aging population.6 Data showed that 88% of all RBC transfusions were one to three units, suggesting that considerable numbers of transfusions could be preempted with PBM,30 thus avoiding the unintended negative consequences31, 32 and inherent risks of transfusion22, 33 along with the associated costs.34 With commencement of the program, the upward issuance trend was arrested and decreased each year thereafter despite average annual increases of 3% in population and 6% in hospital discharges. In the fincancial year 2015-2016, the RBC issuance rate per 1000 population decreased to a low of 19.4 (Fig. 1).
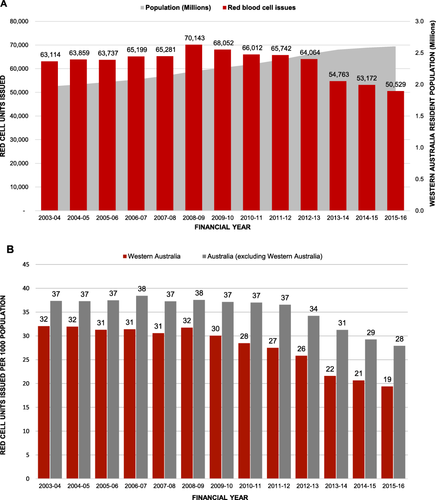
(A) Data on RBC issues and resident population for the State of Western Australia from 2002-2003 to 2013-2014. RBC issuance data published and unpublished National Blood Authority (Australia) data printed with permission. Issuance of RBCs was progressively increasing in Western Australia. With the introduction of the Patient Blood Management Program in 2008-2009, this upward trend was arrested, and issuance has decreased each year despite an annual population increase. (B) RBCs issued per 1000 population in Western Australia compared with the Australian national average. Published and unpublished National Blood Authority (Australia) data printed with permission. [Color figure can be viewed at wileyonlinelibrary.com]
This retrospective, observational study assessed what impact the jurisdiction-wide PBM program had on key outcome measures in all emergency and elective acute-care adult inpatients admitted to the four major adult metropolitan tertiary hospitals where the majority of emergency care and high-complexity procedures and interventions are performed, including the major trauma, burns, and obstetrics referral services for Western Australia (see Appendix S1, available as supporting information in the online version of this paper).
MATERIALS AND METHODS
Program design
Details of program structure, rationale, and implementation are discussed elsewhere.6, 27 The program incorporated principles of the Kotter model35, 36 for successful change management, including: motivation for change, executive and clinical leadership, multidisciplinary clinical team engagement, clinical strategies, education and communication initiatives for clinicians and patients, feedback on practice change and embedding the changes with policies and procedures (see Appendix S1, Summary Table).6 A literature review identified a triad of independent but modifiable risk factors for adverse patient outcomes: namely, anemia, blood loss, and blood transfusion.6 Mitigation of these risk factors by the application of the three-pillar concept of PBM could achieve improved patient outcomes with the corollary of reducing RBC, fresh-frozen plasma (FFP), and platelet transfusions and costs.3, 6, 27, 34, 37 Clinical implementation was built around a three-pillar, nine-field matrix with principles applied equally to surgical and nonsurgical patients (Fig. 2).6, 27 The strategies employed included the following:
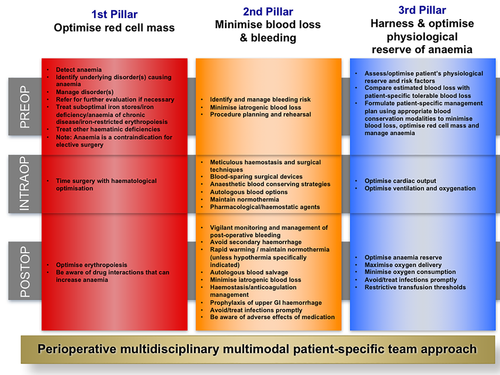
The three-pillar, nine-field matrix of patient blood management. This matrix was designed for the Western Australia Patient Blood Management Program to assist in the clinical implementation of multiple patient blood management (PBM) strategies. These strategies are considered in the perioperative period in a patient and procedure-specific context. (Reformatted from Hofmann A, Friedman D, Farmer S. Western Australia Patient Blood Management Project 2008-2012: Analysis, Strategy, Implementation and Financial Projections. Perth, Western Australia: Medicine and Economics; 2007:1-215.27) The principles of this matrix were also applied to nonsurgical patients before, during, and after treatment. [Color figure can be viewed at wileyonlinelibrary.com]
Pillar 1. Optimize RBC mass
Systems were re-engineered to facilitate timely preintervention patient assessment and optimization of hemoglobin and iron stores, and the use of intravenous iron and other hematinics for postoperative and in-hospital anemia as well as anemia and iron deficiency in pregnancy and primary care.26, 38
Pillar 2. Minimize blood loss
Educational and clinical initiatives were undertaken to reduce blood loss, including preintervention bleeding risk assessment and management, surgical hemostasis workshops and symposia, use of blood-preserving anesthetic techniques, hemostatic agents, autologous blood salvage, viscoelastic coagulation testing with targeted therapy in critical bleeding and coagulopathy, and minimize laboratory blood sampling volumes.26, 38
Pillar 3. Optimize the patient-specific tolerance of anemia
No specific transfusion threshold values were established for the program. Transfusion decisions were encouraged to take into account patient-specific clinical and physiological factors and accept evidence-based, more restrictive but individualized thresholds.38 A single-unit RBC transfusion policy was adopted in symptomatic, nonactively bleeding anemic patients.26, 38
Implementation of the program was planned in stages, with PBM staff to be appointed in Year 1 at the five tertiary-care hospitals and further state leadership and hospital and health service staff to be appointed in Years 2 and 3.27 However, the global financial crisis resulted in delays and cancellations of staff employment and modifications to implementation and program structure.1, 6 PBM staff were appointed only in the four adult tertiary-care hospitals, and 2013-2014 was the first full year in which the program operated with these positions filled.
Patient involvement
Patients were not involved in the design of this study or in the development of outcome measures. However, patient education initiatives were incorporated into program implementation, including educational information on the website, a patient fact sheet, a hospital open day, and patient informed consent/refusal (see Appendix S1).
Data sources
The data included in this study were sourced from the Western Australia PBM data system. This automated reporting system does not use a probabilistic linkage. Details of the linking are published elsewhere.39 The system links data from five core hospital information systems: laboratory (ULTRA LIS), transfusion (ULTRA TM), patient administration (TOPAS and WebPAS), theatre (surgical) management (TMS), and emergency department (EDIS), creating a detailed view of patient characteristics and outcomes associated with anemia and transfusion practices. The study included all emergency and elective multiday stay inpatients aged 16 years and older who were admitted to the four adult tertiary-care hospitals in Western Australia between July 2008 and June 2014, including major trauma, burns, obstetrics, hematology and oncology, gastroenterology, and all major surgery, including cardiac and major organ transplantation surgery. Because of changes in hospital configuration in early 2015, it was not possible to make comparisons with the baseline year beyond June 2014.
The study was reviewed by the Department of Health Western Australia Human Research Ethics Committee and complied with the national guidelines for research.
Outcome measures
Five primary key program-performance indicators and four primary hospital-wide patient outcome measures were selected before our analysis. Performance indicators were: mean RBC, FFP, and platelet units transfused per inpatient discharge; mean pretransfusion hemoglobin; proportion of single-unit RBC transfusions; proportion of elective surgical patients admitted anemic (hemoglobin level <13.0 g/dL for men and <12.0 g/dL for women); and cost of blood product acquisition. Operating room transfusions and patients identified as bleeding were excluded from the single-unit and pretransfusion hemoglobin measures. Costs were based on the yearly prices charged by the National Blood Authority (Australia) under the National Blood Agreement and were calculated by product type (see Appendix S1). Conversion to US dollars was based on the average yearly exchange rate.
The patient outcome measures selected were in-hospital mortality, hospital length of stay, 28-day all-cause emergency readmissions, and hospital-acquired complications; namely, infection and composite acute myocardial infarction (AMI)-stroke (a composite chosen because of the low incidence of each). Hospital-acquired complications were identified using data coded according to the International Statistical Classification of Diseases and Related Problems, Tenth Revision, Australian Modification (ICD-10). These codes include a condition-onset flag to distinguish between hospital-acquired diagnoses and comorbidities present on admission in routinely coded administrative data. We defined emergency readmissions as any inpatient who was readmitted within 28 days of discharge. A predefined secondary measure selected was an estimation of gross savings based on published calculations of activity-based cost of transfusion.
Because the purpose of the study was to measure the potential impact of the PBM program over time, the key exposure of interest was the discharging Australian financial year (July 1 to June 30), with July 1, 2008 to June 30, 2009 as the baseline comparator.
Statistical analysis
A multivariate analysis was performed and adjusted for the following potential confounders: hospital, patient age, sex, admission type (elective or emergency), Diagnosis-Related Group (DRG) category (medical, surgical, or other), indigenous status, and patient comorbidities. These confounders were chosen to control for the possible effects of any changes in patient case-mix on patient outcomes over the study period. Continuous variables were not grouped into categories. Patient comorbidities were given a score based on the Charlson Comorbidity Index (CCI). The ICD-10 version of the CCI, as described by Quan and colleagues was applied, with hospital-acquired diagnoses excluded.40
Poisson regression analysis was used to evaluate the rate of blood units transfused per discharge over time and to calculate the rate ratios. Linear regression was used to test the relationship between the year of discharge and the mean pretransfusion hemoglobin level. Logistic regression was used to test changes in the proportion of single-unit RBC transfusions, elective surgical patients admitted anemic, in-hospital mortality, hospital-acquired complications, and emergency readmissions. A zero-truncated, negative binomial regression was used to model the data for analysis of the impact on hospital length of stay. A robust variance adjustment was applied in the regression models to account for correlation between multiple admissions for the same patient.
RESULTS
Overall, 605,046 inpatient admissions were included in the 6-year study. The mean patient age was 55.4 years (standard deviation, 21.7 years), and 51.4% of patients (n = 311,214) were women. Of these admissions 7.8% of patients (n = 47,382) received at least one unit of RBCs, FFP, or platelets, for a total of 152,636 RBC units, 46,030 FFP units, and 28,089 platelet units transfused (Table 1).
| RBCs | FFP | Platelets | Combined | |||||
|---|---|---|---|---|---|---|---|---|
| Specialty* | Units | % of total | Units | % of total | Units | % of total | Units | % of total |
| Hematology | 25,544 | 16.7 | 2,248 | 4.9 | 13,101 | 46.6 | 40,893 | 18.0 |
| Tracheostomy† | 18,335 | 12.0 | 11,783 | 25.6 | 3,029 | 10.8 | 33,147 | 14.6 |
| Gastroenterology | 18,934 | 12.4 | 4,449 | 9.7 | 1,640 | 5.8 | 25,023 | 11.0 |
| Gastrointestinal surgery | 12,097 | 7.9 | 4,457 | 9.7 | 980 | 3.5 | 17,534 | 7.7 |
| Orthopedics | 15,112 | 9.9 | 1,170 | 2.5 | 402 | 1.4 | 16,684 | 7.4 |
| Cardiothoracic | 8,908 | 5.8 | 4,786 | 10.4 | 2,246 | 8.0 | 15,940 | 7.0 |
| Trauma | 6,665 | 4.4 | 2,583 | 5.6 | 504 | 1.8 | 9,752 | 4.3 |
| Vascular surgery | 6,310 | 4.1 | 1,428 | 3.1 | 311 | 1.1 | 8,049 | 3.5 |
| Urology | 4,984 | 3.3 | 1,279 | 2.8 | 239 | 0.9 | 6,502 | 2.9 |
| Miscellaneous surgery | 4,341 | 2.8 | 1,247 | 2.7 | 836 | 3.0 | 6,424 | 2.8 |
| Cardiology | 4,589 | 3.0 | 735 | 1.6 | 375 | 1.3 | 5,699 | 2.5 |
| Immunology | 3,022 | 2.0 | 636 | 1.4 | 1,534 | 5.5 | 5,192 | 2.3 |
| Respiratory medicine | 3,457 | 2.3 | 489 | 1.1 | 551 | 2.0 | 4,497 | 2.0 |
| Rheumatology | 425 | 0.3 | 3,424 | 7.4 | 73 | 0.3 | 3,922 | 1.7 |
| Gynecology | 3,022 | 2.0 | 616 | 1.3 | 144 | 0.5 | 3,782 | 1.7 |
| Obstetrics | 2,582 | 1.7 | 474 | 1.0 | 157 | 0.6 | 3,213 | 1.4 |
| Nephrology | 1,340 | 0.9 | 1,377 | 3.0 | 159 | 0.6 | 2,876 | 1.3 |
| Medical oncology | 2,379 | 1.6 | 254 | 0.6 | 213 | 0.8 | 2,846 | 1.3 |
| Neurosurgery | 1,632 | 1.1 | 758 | 1.6 | 430 | 1.5 | 2,820 | 1.2 |
| General medicine | 1,976 | 1.3 | 377 | 0.8 | 222 | 0.8 | 2,575 | 1.1 |
| Other | 6,982 | 4.6 | 1,460 | 3.2 | 943 | 3.4 | 9,385 | 4.1 |
| Total | 152,636 | 100.0 | 46,030 | 100.0 | 28,089 | 100.0 | 226,755 | 100.0 |
- a Shown are the top 20 specialties (based on diagnosis-related group) by total number of RBCs, FFP units, and platelet units transfused between July 2008 and June 2014.
- b This specialty group refers to patients who had a tracheostomy and/or ventilation for ≥96 hours.
Mean units transfused per 1000 discharges
Mean units transfused are presented by year in Fig. 3. RBC, FFP, and platelet units transfused per 1000 discharges decreased 41% compared with the baseline year (rate ratio [RR], 0.59; 95% confidence interval [CI], 0.58-0.60; p < 0.001). This decrease included reductions of 41% in RBC units (RR, 0.59, 95% CI 0.58-0.60; p < 0.001), 47% in FFP units (RR, 0.53; 95% CI, 0.51-0.55; p < 0.001), and 27% in units of platelets transfused (RR, 0.73; 95% CI, 0.70-0.76; p < 0.001).
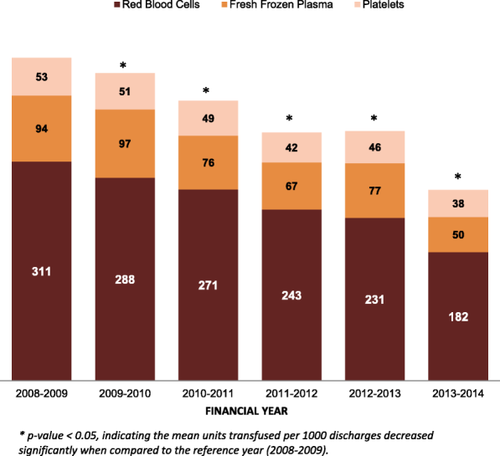
Mean units of blood transfused per 1000 discharges. Shown are the mean units of RBCs, FFP, and platelets transfused per 1000 discharges. An asterisk denote a p value < 0.05, indicating that the mean units transfused per 1000 discharges decreased significantly compared with the reference year 2008-2009. [Color figure can be viewed at wileyonlinelibrary.com]
Pretransfusion hemoglobin, single-unit RBC transfusions, and patients admitted anemic
The mean RBC pretransfusion hemoglobin level decreased from a baseline mean level of 7.9 g/dL to 7.3 g/dL (p < 0.001) in Year 6. The proportion of single-unit RBC transfusions increased from 33.33% to 63.69% (p < 0.001). The proportion of elective surgical patients admitted anemic decreased from 20.81% to 14.42% (p = 0.001).
Product-acquisition cost savings
Historic data and forward projections at program design predicted that, without practice change, product utilization would continue to increase. Adopting a conservative approach, if the annual rate of transfusion remained at baseline year levels, then an additional 50,115 units of blood would have been transfused over the study period, comprising 35,423 RBC units, 10,721 of FFP units, and 3970 platelets units. Based on product-acquisition cost, the calculated savings from this reduction is $18,507,092 in Australian dollars (AU$) and $18,078,258 in US dollars ($US).
Estimation of activity-based cost of transfusion savings
Activity-based costs of RBC transfusion reportedly are threefold to fivefold higher than-acquisition costs,41, 42 and it has been determined that the cost of FFP is more than nine times the cost of product acquisition.43 Activity-based costs of platelets are currently unknown. Using these published calculations, the gross savings in this 6-year study are estimated at between AU$80M and AU$100M (between US$78M and US$97M).
Hospital-wide patient outcomes
Unadjusted and adjusted patient outcomes are presented by year in Table 2. Unadjusted in-hospital mortality decreased from 2.0% in 2008-2009 to 1.7% in 2013-2014 (p < 0.001). After adjusting for potential confounders, this represented a 28% reduction (p < 0.001).
| Outcome variable* | 2008-2009 | 2009-2010 | 2010-2011 | 2011-2012 | 2012-2013 | 2013-2014 |
|---|---|---|---|---|---|---|
| In-hospital mortality | ||||||
| Unadjusted rate, % | 2.03 | 2.10 | 1.85 | 1.76 | 1.77 | 1.65 |
| Adjusted OR (95% CI)† | Ref | 1.01 (0.94-1.08) | 0.95 (0.89-1.02) | 0.89 (0.83-0.95) | 0.81 (0.76-0.87) | 0.72 (0.67-0.77) |
| Hospital length of stay | ||||||
| Unadjusted mean, days | 5.91 | 5.95 | 5.72 | 5.51 | 5.46 | 5.26 |
| Adjusted rate ratio (95% CI)† | Ref | 1.05 (1.03-1.07) | 1.01 (0.99-1.03) | 0.96 (0.94-0.98) | 0.92 (0.90-0.94) | 0.85 (0.84-0.87) |
| 28-Day readmissions | ||||||
| Unadjusted rate, % | 11.42 | 11.82 | 12.74 | 13.57 | 13.27 | 12.42 |
| Adjusted OR (95% CI)† | Ref | 1.03 (0.99-1.07) | 1.14 (1.10-1.18) | 1.21 (1.17-1.26) | 1.16 (1.12-1.20) | 1.06 (1.02-1.10) |
| Hospital-acquired infectionc | ||||||
| Unadjusted rate, % | — | 2.34 | 2.04 | 1.78 | 1.80 | 1.95 |
| Adjusted OR (95% CI)† | — | Ref | 0.92 (0.85-0.99) | 0.80 (0.74-0.87) | 0.77 (0.71-0.83) | 0.79 (0.73-0.86) |
| Acute myocardial infarction-stroke‡ | ||||||
| Unadjusted rate, % | — | 0.50 | 0.48 | 0.40 | 0.42 | 0.36 |
| Adjusted OR (95% CI)† | — | Ref | 1.02 (0.86-1.20) | 0.84 (0.71-1.00) | 0.85 (0.71-1.00) | 0.69 (0.58-0.82) |
- a Shown are the unadjusted and adjusted patient outcomes by financial year over the period of the patient blood management program.
- b Ratios and 95% confidence intervals are adjusted for hospital, age, sex, Diagnosis-Related Group (DRG) category, admission type, indigenous status, and comorbidities.
- c Data were available for two of the four hospitals.
- CI = confidence interval; OR = odds ratio; Ref = reference category.
The mean length of stay decreased significantly over the study period (5.9 days vs 5.3 days; p < 0.001). After adjusting for confounders, the mean length of stay was reduced by 15% (p < 0.001).
The proportion of all-cause, 28-day emergency readmissions increased from 11.4% to 12.4%. After adjustment, this represented a 6% increase (p = 0.001).
Hospital-acquired complications were analyzed for two of the study hospitals, because data were incomplete for the others. Data also were missing from the baseline year, because reporting began in the year 2009-2010. The unadjusted incidence of hospital-acquired infections in the two hospitals decreased from 2.3% in 2009-2010 to 2.0% in 2013-2014, representing a 21% reduction after adjusting for confounders (p < 0.001). The incidence of AMI-stroke decreased from 0.5% to 0.4%, representing a 31% reduction after adjusting for confounders (p < 0.001).
DISCUSSION
The implementation of a health-system–wide PBM program was associated in four adult public tertiary hospitals within that health system with significant reductions in hospital mortality, length of stay, RBC, FFP, and platelet transfusions, considerable product-acquisition and estimated activity-based transfusion cost savings, and an increase in all-cause emergency readmissions. There were significant reductions in infection and AMI-stroke at the two institutions that coded hospital-acquired complications.
Reduced blood product utilization in this study was associated with product-acquisition cost savings of AU$18.5M (US$18.1M). However, gross savings include activity-based costs of transfusion,41-44 which are estimated in this 6-year study at between AU$80M and AU$100M (US$78M and US$97M). These costs, both acquisition and activity-based, are jointly borne by the state and federal governments.6 A one-time investment of AU$4.5M was made to cover the health-system–wide, 5-year change management and implementation process. This included funds for external PBM experts to plan, coordinate, and guide the project; a state PBM Medical Director (0.1 full-time equivalent); a state PBM Clinical Nurse Coordinator (0.5 full-time equivalent); Department of Health project officers (providing administrative and data support, including creating a sustainable PBM data and reporting system); and honoraria and travel support for national and international key opinion leaders in PBM to clinically support the implementation process. This budget covered various other items, including attendance at conferences, educational sessions, and study tours.
Relation to other studies
The present study is novel, in that it reports on multicenter, hospital-wide outcomes associated with the world's first comprehensive health-system–wide PBM program. The program employed multiple, evidence-based PBM clinical strategies and used culture change methodology, systems and patient pathway re-engineering, and continuous automated data collection and feedback. Its findings are consistent with and strengthen the findings of others who have examined the impact of either individual PBM strategies or PBM programs in selected patient groups.14-17, 20, 45 Goodnough and colleagues reported reductions in mortality and length of stay associated with their single-hospital–wide intervention to implement more restrictive RBC transfusion practices, with millions of dollars in cost savings.17 Their findings correlate with a systematic review and meta-analysis of randomized control trials by Salpeter and colleagues demonstrating that trials with more restrictive transfusion thresholds significantly reduced cardiac events, infection, rebleeding, and mortality compared with trials that used less restrictive thresholds.46 Large numbers of risk-adjusted observational studies have demonstrated an independent dose-dependent association between RBC transfusion and increased morbidity, including infection, AMI-stroke, and hospital length of stay, and mortality.38, 47-58 There is limited evidence for the efficacy of FFP transfusions in most clinical situations, likely contributing to overuse and adverse patient outcomes.38, 59 Platelet transfusions may be associated with adverse events, there is low-quality evidence to guide practice, and they are often inappropriately used.23, 38, 60
Although we observed an already relatively restrictive mean RBC transfusion threshold become more restrictive, the Western Australia program was not primarily an appropriate or restrictive transfusion initiative.30 It adopted a comprehensive application of the three pillars of PBM.26, 30 These pillars aim to modify the triad of risk factors for adverse patient outcomes and preempt what could otherwise result in an “appropriate” transfusion.30 When an RBC transfusion is indicated, a single-unit policy may reduce the exposure dose.
Increases in anemia and blood loss are independently associated with poorer outcomes and increase the likelihood of transfusion.15, 61, 62 PBM programs in surgical patients have shown an association between improved anemia management and reduced blood loss, and improved outcomes. Kotze and colleagues observed significantly reduced preoperative anemia incidence and blood loss in their orthopedic surgery program with an associated reduction in transfusions.15 Gross and colleagues noted significantly reduced blood loss and increased mean discharge hemoglobin levels despite a more restrictive transfusion threshold in cardiac surgery.16 Program implementation was associated with significant reductions in RBC, FFP, and platelet transfusion as well as product costs. Both programs were associated with improved patient outcomes.
It is not possible to determine the effect of any one strategy used within the Western Australia PBM program; likely a combination of strategies contributed to the modification of risk factors. The multifaceted multimodal multidisciplinary team approach adopted has been shown to be effective in change management and clinical improvement.63 Up-to-date, evidence-based education and practice feedback played key roles (Appendix S1). Evaluation forms completed at more than 60 departmental PBM road shows revealed that 82% of responders said there was information that was new to them, 69% said they would change their clinical practice based on evidence presented, and 13% said they would not change their practice; the main reason provided for the latter response was that the information reinforced their already conservative practice. The provision of benchmarking data to hospitals, departments, and individual clinicians also gave strong motivation for practice improvement.6, 39, 64
The current study differs from others in finding an increase in all-cause emergency readmissions.15-17, 21 Loftus and colleagues reported a significant reduction in 30-day readmissions in a multicenter study of patients undergoing total hip and knee arthroplasty.21 Gross and colleagues observed a nonsignificant reduction in 30-day readmission in a single-center study of patients undergoing cardiac surgery.16 In a single center study of primary hip and knee arthroplasties, Kotze and colleagues identified a significant reduction in 90-day readmissions.15 Goodnough and colleagues, in a single-center study of a hospital-wide initiative, found that 30-day readmission rates remained stable.17 Although this measure is commonly used in clinical research, it has limitations in interpretation. Future subgroup analyses may provide insights into which clinical contexts have higher or lower readmission rates. However, because this outcome measures all-cause emergency readmissions, we are unable to determine the number of patients who were readmitted for reasons unrelated to their previous admission. Given this limitation, we recommend caution, as have others, in interpreting this result as an indicator of quality of care.65
Strengths and weaknesses of this study
Because this was an observational study in which a linked data system was used, it has both strengths and limitations. A strength is that exposures, outcomes, and covariates were sourced from validated hospital data systems, which undergo regular quality audits. It also included a large, multicenter population of all acute-care, adult inpatients. A limitation is that there was no control group, because we sought to measure change over time, and comparisons were based on a baseline year. We were also unable to compare outcomes with other Australian jurisdictions, because we had no access to equivalent patient outcomes data. Comparisons with other Australian jurisdictions would have been problematic because during the same period other states had implemented various PBM initiatives, Australia released its national PBM guidelines, and PBM was included in national hospital accreditation.
An observational study in itself cannot establish a causal link between program implementation and outcomes of interest. Nor can outcomes be attributed completely to the PBM program, as other hospital initiatives may have played a role. However, the greatest reduction in blood utilization was in the only year in which all study hospitals had their PBM Medical Director and PBM Nurse—positions that have been identified as key to a successful PBM program.6
Improvements in patient outcomes, including length of stay, mortality, and hospital-acquired complications, were significant even after adjusting for potential confounders, reducing the likelihood that they were due to changes in patient mix. We adopted a similar approach to that used by Goodnough and colleagues, who reported concurrent hospital-wide outcomes associated with a single-hospital–wide intervention.17
Implications for clinicians and policymakers
Blood transfusion is one of the most frequently performed therapeutic procedures; it crosses many medical disciplines, has been identified as one of the top five overused therapies, and is associated with negative patient outcomes and increased costs.17, 31, 37, 66, 67 Therefore, the impact of PBM programs may be substantial considering the global health sector's challenge to improve patient outcomes with increasingly restricted funding.
Unanswered questions and future research
Cost-saving calculations in this study were based on blood product utilization but did not include the impact of the program on other modalities, for example, iron therapy in anemia management, and cryoprecipitate (or fibrinogen concentrate) and antifibrinolytics such as tranexamic acid in coagulopathy. A comprehensive, cost-effectiveness analysis would include these modalities and the savings associated with reduced complications and hospital length of stay.37 This assessment was beyond the scope of this study and will be the subject of future analysis.
One way to confirm the findings of this and other studies on the impact of PBM would be to conduct a cluster, randomized controlled trial to assess complex, process-of-care interventions. However, such an assessment may not be possible for ethical reasons alone; currently, it would appear to be almost impossible in developed countries with wide uptake of PBM. For example, many authoritative international bodies—including the AABB, the Joint Commission, and the Advisory Committee on Blood Safety and Availability in the United States; European Union Patient Blood Management, the National Health Service Blood and Transplant, Department of Health, and National Blood Transfusion Committee in the United Kingdom; the Ontario Nurse Transfusion Coordinators (ONTraC) Program in Canada; and the Australian Commission on Safety and Quality in Health Care and the National Blood Authority in Australia—have various PBM initiatives and are promoting it as a standard of care to improve outcomes.4-6, 8, 68-70 However, replicating the current findings across another jurisdiction would provide additional evidence for the overall benefits of such a program.
CONCLUSIONS
In a health system with one of the world's lowest RBC issuance rates per 1000 population and an already relatively restrictive mean RBC transfusion threshold, a comprehensive, jurisdiction-wide PBM program in the study hospitals was associated with significant hospital-wide reductions in morbidity, mortality, length of stay, blood product use, and costs. The decreasing blood product issuance rates highlight the finding that, since conclusion of the 5-year project, the culture generated by the PBM program in Western Australia has been sustained. These findings may have considerable implications for health systems across the globe, including high-income and low-income countries.
ACKNOWLEDGMENTS
We thank the PBM Medical Directors and PBM Clinical Nurse Consultants (Coordinators); PBM Committee members and administrators; Department of Health support staff; and the many physicians, nurses, pharmacists, scientists, and internet technology staff who contributed to the success of the Program. Special thanks to Amanda Esson and Aqif Mukhtar.
CONFLICT OF INTEREST
Axel Hofmann reports personal fees from Vifor Pharma AG and TEM International GmbH, outside the submitted work. Simon Towler reports nonfinancial support from the Medical Society for Blood Management and the National Blood Authority (Australia), outside the submitted work. Kevin M. Trentino reports nonfinancial support from the Medical Society for Blood Management and The Health Roundtable, outside the submitted work. Shannon L. Farmer reports personal fees from Thieme (Stuttgart, Germany) and Elsevier Science USA and nonfinancial support from the National Blood Authority (Australia), the Medical Society for Blood Management, and The Health Round Table, outside the submitted work. Michael Leahy reports honorarium from Vifor Pharma. The remaining authors made no disclosures.