Inactivation of Zika virus in plasma with amotosalen and ultraviolet A illumination
Abstract
BACKGROUND
Zika virus (ZIKV) is an arthropod-borne virus (arbovirus) transmitted by mosquitoes. The potential for ZIKV transmission through blood transfusion was demonstrated during the ZIKV outbreak that occurred in French Polynesia from October 2013 to April 2014. Pathogen inactivation of blood products is a proactive strategy that provides the potential to reduce transfusion-transmitted diseases. Inactivation of arboviruses by amotosalen and ultraviolet A (UVA) illumination was previously demonstrated for chikungunya, West Nile, and dengue viruses. We report here the efficiency of this process for ZIKV inactivation of human plasma.
STUDY DESIGN AND METHODS
Plasma units were spiked with ZIKV. Viral titers and RNA loads were measured in plasma before and after amotosalen and UVA photochemical treatment.
RESULTS
The mean ZIKV titers and RNA loads in plasma before inactivation were respectively 6.57 log TCID50/mL and 10.25 log copies/mL. After inactivation, the mean ZIKV RNA loads was 9.51 log copies/mL, but cell cultures inoculated with inactivated plasma did not result in infected cells and did not produce any replicative virus after one passage, nor detectable viral RNA from the second passage.
CONCLUSION
In this study we demonstrate that amotosalen combined with UVA light inactivates ZIKV in fresh-frozen plasma. This inactivation process is of particular interest to prevent plasma transfusion-transmitted ZIKV infections in areas such as French Polynesia, where several arboviruses are cocirculating.
ABBREVIATIONS
-
- CHIKV
-
- chikungunya virus
-
- DENV
-
- dengue virus
-
- TCID50
-
- 50% tissue culture infectious dose
-
- WNV
-
- West Nile virus
-
- ZIKV
-
- Zika virus
Zika virus (ZIKV) is an arthropod-borne virus (arbovirus) of the genus Flavivirus, family Flaviviridae. ZIKV was first isolated in 1947 from a Rhesus monkey from the Zika forest in Uganda.1 Until 2007, only sporadic cases have been recorded in Africa and Asia.2-6 The first reported outbreak of ZIKV outside Africa and Asia occurred in 2007 on the North Pacific island country of Yap Island (Federated States of Micronesia).7 ZIKV then caused the largest outbreak ever recorded from October 2013 to April 2014 in French Polynesia, South Pacific, with an estimated 28,000 cases.8-10 In 2015, the first documented outbreak of ZIKV in the Americas occurred in Brazil11 in which dengue (DENV) and chikungunya (CHIKV) viruses also circulate. The most common clinical manifestations of ZIKV infections are rash, conjunctivitis, fever, and arthralgia,7 but severe neurologic complications have also been reported in French Polynesian patients.12
The ZIKV natural transmission cycle involves mosquitoes. ZIKV has been isolated from several Aedes (Ae.) mosquito species, with Ae.13 and Ae.14 being confirmed as competent vectors. In French Polynesia, Ae. aegypti and Ae. polynesiensis are suspected to have contributed to ZIKV transmission during the 2013 to 2014 outbreak.8 Non–vector-borne transmissions of ZIKV by sexual intercourse15, 16 and perinatal infection17 have also been reported. The potential for ZIKV transmission through blood transfusion has been demonstrated during the French Polynesian outbreak as 2.8% of blood donors, who were asymptomatic at the time of donation, were found positive for acute ZIKV infection using specific reverse transcription–polymerase chain reaction (RT-PCR).18
Prevention of transfusion-transmitted ZIKV infections is challenging because most of the cases are asymptomatic and are not detected during medical questionnaire,18 and nucleic acid testing (NAT) for ZIKV is often not routinely available. An alternative strategy to NAT is pathogen inactivation, a proactive method designed to reduce or abolish infectivity of pathogens in blood products.19 Several processes have been developed for the inactivation of pathogens during the preparation of fresh-frozen plasma (FFP) and platelet (PLT) concentrates.19 Among them, a photochemical treatment using a psoralen (amotosalen, S-59), in combination with ultraviolet A (UVA) illumination, has been shown to inactivate a broad range of viruses, bacteria, and protozoans.20-24 Amotosalen intercalates into double-helical structures of DNA and RNA and forms covalent adducts with pyrimidine bases upon UVA illumination. These adducts prevent nucleic acid replication and transcription to occur and also inhibits DNA repair mechanisms.
Inactivation of arboviruses in plasma by treatment with amotosalen and UVA light has been previously demonstrated for CHIKV,25 arbovirus of the Alphavirus genus, and for West Nile virus (WNV)25 and DENV,26 arboviruses of the Flavivirus genus. However, to our knowledge, the use of this process to inactivate ZIKV in plasma has never been investigated.
To evaluate the efficacy of amotosalen and UVA light treatment for inactivation of ZIKV in human plasma, we performed a spiking experiment of plasma units with ZIKV and compared the viral titers and viral RNA loads before and after inactivation, in accordance with the recommendations for evaluation of pathogen reduction efficacy.27 Moreover, we measured the reduction of viral RNA loads induced by inactivation and compared it to the viral RNA loads of sera collected in French Polynesia in 2013 and 2014 from ZIKV-infected, but asymptomatic blood donors18 to validate the use of amotosalen and UVA light to prevent transfusion-transmitted ZIKV infections.
MATERIALS AND METHODS
The handling of infectious material (virus culture, infection of plasma bags, viral and RNA load determination) was performed at the “Institut Louis Malardé” (Tahiti, French Polynesia). Inactivation of the infected plasma units was performed at the blood bank center of French Polynesia (Tahiti). The schematic flow diagram of the experimental design is illustrated in Fig. 1.
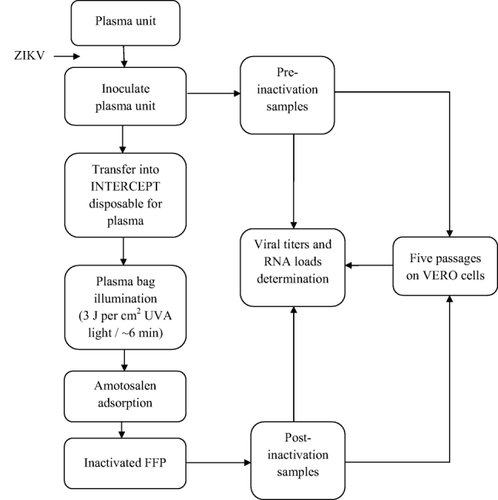
Schematic flow diagram of the experimental design.
Virus
The ZIKV strain (PF13/251013-18) was isolated from the serum of a French Polynesian patient infected in 2013. ZIKV was propagated in African green monkey kidney cells (VERO) grown at 37°C in an atmosphere of 5% CO2 in minimum essential medium supplemented with 2% fetal bovine serum (FBS), 1% penicillin-streptomycin, and 3% sodium bicarbonate 7.5% (Life Technologies, Carlsbad, CA). From the cell culture supernatants, four viral concentrates (between 1.25 and 7 mL each) were obtained as previously described26 and stored at −80°C with 20% FBS.
Plasma and serum collection
Plasma units were collected from American blood donors by the Interstate Blood Bank of Chicago (Chicago, IL) to eliminate risk of ZIKV antibodies. To assess the absence of previous infections by Flaviviruses known to circulate in the United States (DENV and WNV), each plasma unit was tested with a dengue immunoglobulin (Ig)G capture kit (Platelia, Bio-Rad, Hercules, CA) and a classic WNV IgG kit (Serion Elisa, Abcam, Cambridge, UK), to detect IgG antibodies against DENV and WNV, respectively. Only plasma units found negative for both DENV and WNV IgG were selected for the study. The 26 sera of French Polynesian ZIKV-infected and asymptomatic blood donors were obtained from the blood bank center of French Polynesia as previously reported.18
Infection of plasma units with ZIKV
Four plasma units (A, B, C, and D) were inoculated with ZIKV as previously described.26 A sample from each infected plasma unit (preinactivation sample) was then collected and stored at −80°C until the determination of viral titers and RNA loads.
Inactivation process
Inoculated Plasma Units A, B, and C were treated with amotosalen combined with UVA illumination as previously described,26 whereas inoculated Plasma Unit D was not inactivated and was the positive control. After transfer into a container with a compound absorption device that removes the residual amotosalen and the free photoproducts, samples from each inactivated plasma unit (inactivated samples) and from the positive control (noninactivated sample) were collected and stored at −80°C until the determination of viral titers and RNA loads.
Detection of replicative ZIKV and viral titration
For the detection of replicative ZIKV, all pre-, post-, and noninactivated samples were inoculated in triplicate on VERO cells in 24-well plates, and five successive passages were performed as previously described.26 For each passage, inoculated cells were maintained at 37°C in an atmosphere of 5% CO2 for 4 to 6 days. After each passage, indirect immunofluorescence assay was performed as previously reported26 to detect ZIKV inoculated cells, using anti-flavivirus mouse antibodies 4G2 (Institut Pasteur, Paris, France) at a dilution of 1:50 in phosphate-buffered saline (PBS, Biomérieux, Marcy l'Etoile, France).
For viral titration, triplicate 10-fold dilutions of preinactivation, postinactivation, and control samples were inoculated on VERO cells in 96-well plates. Inoculated cells were maintained at 37°C in an atmosphere of 5% CO2 for 6 days and then were washed twice with 400 µL of PBS using a microplate washer (Bio-Rad). Cells were fixed in cold acetone for 10 minutes at room temperature and air-dried. Fifty microliters of antibodies 4G2 diluted 1:2000 in diluent buffer containing PBS with 5% skimmed milk (Régilait, Saint-Martin-Belle-Roche, France) and 0.3% Tween 20 (Merck, Darmstadt, Germany) were distributed in all wells. Plates were stored at 30°C for 1 hour, and then cells were washed four times with 400 µL of wash buffer (PBS with 0.3% Tween 20). Fifty microliters of goat anti-mouse IgG conjugated to horseradish peroxidase (Santa Cruz Biotechnology, Santa Cruz, CA) diluted 1:2000 in diluent buffer were distributed in all wells. Plates were stored at 30°C for 1 hour, and then cells were washed four times with 400 µL of wash buffer. Cells were incubated at room temperature in the dark for 5 minutes with 50 µL of peroxidase substrate tetramethylbenzidine before the addition of 50 µL of tetramethylbenzidine stop solution (KPL, Inc., Gaithersburg, MD). Absorbance was read at a wavelength of 450 nm (OD450) using a microplate photometer (Thermo Scientific, Waltham, MA). Infectious wells were counted for each dilution and viral titers were expressed as 50% tissue culture infectious dose (TCID50/mL) using the method of Reed and Muench.28
ZIKV RNA quantitation
For all plasma (pre-, post-, and noninactivated samples), cell supernatant, and serum samples, RNA extraction was performed from 200 µL of each sample using an extraction system (easyMAG, bioMérieux), and real-time RT-PCR was performed in a thermocycler (CFX96, Bio-Rad) as previously described.29 A standard curve using 10-fold serial dilutions of a ZIKV RNA transcript, obtained from the strain PF13/251013-18 with an in vitro transcription system (Riboprobe System-T7, Promega, Madison, WI) and quantitated using a RNA assay kit with a fluorometer (Quant-iT and Qubit, respectively, Invitrogen, Carlsbad, CA), was included within the RT-PCR run to estimate the copy number in samples. The limit of detection for this assay is 25 to 100 viral RNA copies.29 Results were expressed in log copies/mL.
RESULTS
Detection of replicative ZIKV and ZIKV titration
Viral titers in preinactivation samples (A, B, and C) ranged from 6.46 to 6.63 log TCID50/mL (mean, 6.57 log TCID50/mL; Table 1). The culture of preinactivation and noninactivated (D) samples produced replicative viruses during successive passages, whereas no replicative virus was detected in inactivated samples, even after five passages.
| Replicative ZIKV after | |||||||
|---|---|---|---|---|---|---|---|
| Plasma samples | Initial viral titers | First passage | Second passage | Third passage | Fourth passage | Fifth passage | Log reduction |
| A | |||||||
| Preinactivation sample | 6.46 | +a | + | + | + | + | >6.46 |
| Inactivated sample | −b | − | − | − | − | − | |
| B | |||||||
| Preinactivation sample | 6.63 | + | + | + | + | + | >6.63 |
| Inactivated sample | − | − | − | − | − | − | |
| C | |||||||
| Preinactivation sample | 6.61 | + | + | + | + | + | >6.61 |
| Inactivated sample | − | − | − | − | − | − | |
| D (control) | |||||||
| Preinactivation sample | 6.14 | + | + | + | + | + | |
| Noninactivated sample | 6.28 | + | + | + | + | + | |
- a Positive immunofluorescence.
- b Negative immunofluorescence.
ZIKV RNA quantitation
Viral RNA loads in preinactivation and inactivated samples, respectively, ranged from 10.11 to 10.41 log copies/mL (mean, 10.25 log copies/mL) and from 9.41 to 9.67 log copies/mL (mean, 9.51 log copies/mL; Table 2). Viral RNA load (copies/mL) and viral infectivity (TCID50/mL) values are different because not all virus particles are infectious. After the first passage of inactivated samples on VERO cells, ZIKV RNA loads ranged from 3.63 to 4.07 log copies/mL (mean, 3.86 log copies/mL), and from the second to the fifth passage, viral RNA remained undetectable indicating the absence of replicative virus following inactivation. The 26 sera from French Polynesian blood donors showed ZIKV RNA loads ranging from 3.40 to 6.91 log copies/mL (mean, 4.85 log copies/mL; Table 3).
| RNA loads after | ||||||
|---|---|---|---|---|---|---|
| Plasma sample | Initial RNA loads | First passage | Second passage | Third passage | Fourth passage | Fifth passage |
| A | ||||||
| Preinactivation sample | 10.22 | 10.31 | 10.08 | 10.36 | 10.17 | 10.48 |
| Inactivated sample | 9.67 | 4.07 | NDa | ND | ND | ND |
| B | ||||||
| Preinactivation sample | 10.41 | 10.30 | 10.17 | 10.26 | 10.22 | 10.42 |
| Inactivated sample | 9.44 | 3.63 | ND | ND | ND | ND |
| C | ||||||
| Preinactivation sample | 10.11 | 10.43 | 10.19 | 10.30 | 10.20 | 10.65 |
| Inactivated sample | 9.41 | 3.87 | ND | ND | ND | ND |
| D (control) | ||||||
| Preinactivation sample | 10.36 | 10.26 | 10.02 | 10.32 | 10.02 | 10.28 |
| Noninactivated sample | 9.90 | 9.89 | 9.99 | 9.95 | 10.18 | 10.01 |
- a ZIKV RNA not detected.
| Sample IDs | RNA loads |
|---|---|
| 27/11/13-66 P2 | 5.70 |
| 27/11/13-71 P1 | 5.25 |
| 28/11/13-86 P1 | 3.95 |
| 28/11/13-86 P2 | 3.76 |
| 28/11/13-95 P2 | 5.33 |
| 28/11/13-95 P3 | 4.15 |
| 29/11/13-59 P3 | 4.96 |
| 04/12/13-106 P1 | 5.65 |
| 06/12/13-79 P1 | 4.06 |
| 09/12/13-117 P2 | 6.91 |
| 09/12/13-121 P2 | 5.54 |
| 09/12/13-193 P1 | 6.72 |
| 10/12/13-36 P2 | 6.16 |
| 20/12/13-59 P2 | 4.63 |
| 20/12/13-63 P2 | 3.73 |
| 20/12/13-70 P2 | 3.47 |
| 20/12/13-78 P1 | 5.35 |
| 23/12/13-38 P1 | 5.15 |
| 24/12/13-43 P2 | 5.34 |
| 31/12/13-34 P1 | 4.49 |
| 03/01/14-34 P3 | 6.19 |
| 03/01/14-38 P1 | 3.40 |
| 09/01/14-116 P3 | 4.24 |
| 09/01/14-122 P1 | 3.45 |
| 24/01/14-44 P2 | 4.71 |
| 13/02/14-128 P2 | 3.90 |
DISCUSSION
Members of the AABB's Transfusion Transmitted Diseases Committee identified emerging infectious disease agents that pose a real or theoretical threat to transfusion safety, due to their presence in blood during the donor's asymptomatic phase; their survival or persistence in blood during processing and storage; and the fact they must be recognized as responsible for a clinically apparent outcome in some recipients who become infected.30, 31 Arboviruses are considered as threats for the blood supply since evidence of their transfusion transmissibility has been found. WNV is the best documented transfusion-transmitted arbovirus with 23 patients that were confirmed to have been infected in 2002 in the United States through transfused red blood cells (RBCs), PLTs, and FFP.32 DENV infections after transfusion with RBCs or FFP have been reported in 2002 in Hong Kong,33 and in 2007 in Puerto Rico34 and Singapore,35 the latter two resulting in cases of DENV hemorrhagic fever. In addition, the potential for CHIKV transmission by blood transfusion has been demonstrated in the Caribbean in 2014.36
ZIKV is an emerging pathogen that has caused several outbreaks in the Pacific region since 2007,9, 37 and the potential for ZIKV transfusion-transmitted infection has been demonstrated in French Polynesia.18 Symptoms of ZIKV infections are typically mild, but severe neurologic complications can occur12 and raise the question of the threat posed by ZIKV for the blood supply. Although no posttransfusion ZIKV infection has ever been reported, the detection of a high number of ZIKV asymptomatic infections among blood donors (42/1,505) during the 2013 to 2014 outbreak in French Polynesia18 revealed the risk of transfusion-associated transmission of this virus. Subsequently, the European Center for Disease Control recommended that the blood safety authorities be vigilant regarding the risk of Zika fever, including deferral of blood donors with travel history in areas with ongoing circulation of ZIKV.38
During outbreaks, several strategies have been implemented to prevent transfusion-associated transmission of arboviruses. During the CHIKV outbreaks in 2005 to 2007 on Reunion Island39 and in 2007 in Italy,40 local blood donations were interrupted, and blood products were imported from blood bank centers elsewhere. In geographically isolated areas such as French Polynesia, importation of blood products from foreign blood bank centers is not routinely possible. In addition, the deferral of blood donors that have spent time in epidemic areas, as was recommended in Europe, is impossible. During the French Polynesian outbreak, blood products were kept in quarantine during 1 week, and blood donors were asked to contact the blood bank center in case of Zika fever symptoms. However, this procedure was not effective for asymptomatic infected blood donors.
Specific ZIKV NAT was implemented in routine practice during the French Polynesian outbreak,18 on the basis of protocols implemented to prevent WNV transmission by transfusion in North America. In the United States, NAT has been routinely used since 2003 for the detection of WNV in donated blood products, after cases of posttransfusion infections that occurred in 2002.41 Some limitations are to be considered when using NAT: first, it does not detect all infected blood donations, especially when nucleic acid loads are low and when sera are tested in large pools; second, due to its nucleotide sequence specificity, NAT cannot be used to screen a wide range of pathogens with one run, necessitating the use of multiple assays if several pathogens are cocirculating in the same area; third, it requires molecular biology expertise or access to a validated screening facility; and fourth, testing several pathogens by NAT is very expensive and time-consuming. Failures of NAT have also been reported. Indeed, since implementation of systematic screening of blood donations in the United States for the detection of WNV, several cases of transfusion-associated transmission of WNV have been documented.42-44 In addition, one fatal case of WNV infection after probable transfusion-associated transmission, with a blood donation that was nonreactive by individual NAT, was reported in 2012.45
In contrast to NAT, the pathogen inactivation process is nonspecific and it can inactivate a broad spectrum of pathogens including bacteria, viruses, and protozoan.20-24 This process is particularly suitable in areas with endemic circulation of pathogens and/or with cocirculation of multiple pathogens. Photochemical treatment is efficient for pathogen inactivation in PLT and plasma blood components, but it cannot be used on RBCs because UVA light is absorbed by hemoglobin and poorly penetrates through RBCs.19 Thus, until the introduction of a new pathogen inactivation technology that can be used in RBCs concentrates, both NAT and inactivation strategies should be considered to reduce the risk of transfusion-associated transmission diseases, depending on the pathogen targeted, its mode of circulation, and the type of blood product used for transfusion.
Photochemical treatment of plasma with amotosalen and UVA light has been previously shown to inactivate more than 6.8 logs of WNV25 and more than 5.61 logs of DENV.26 The efficacy of the treatment of plasma with amotosalen and UVA light to inactivate ZIKV should be specifically demonstrated, even though other members of the Flaviviridae family have been shown to be inactivated by the same process.
In our study, we inoculated plasma units with ZIKV and monitored the ZIKV inactivation by both viral culture and RT-PCR. As a large part of the French Polynesian population had been immunized against ZIKV during the 2013 to 2014 outbreak,9 the plasma samples used for our experiments were collected from American blood donors to avoid virus neutralization by existing ZIKV IgG antibodies. In addition, because cross-reactions exist within the Flavivirus genus,46 DENV and WNV IgG-negative plasma units were selected for the experiments.
Immediately after inactivation, we found no replicative viruses in plasma samples. To ensure that there were no remaining infectious viral particles, we performed successive passages on VERO cells and, even after five passages, we detected no replicative viruses. According to the recommendations of the Food and Drug Administration (FDA) for evaluation of pathogen reduction efficacy, the pathogen inactivation process should, ideally, have the ability to reduce the pathogen load in a blood product by 6 to 10 log copies/mL.27 Our results showed that amotosalen and UVA light treatment of ZIKV inoculated plasma samples was able to inactivate a mean viral RNA load of 10.25 log copies/mL, which meets the criteria set by the FDA.
After inactivation, we still detected high viral RNA loads in plasma samples. It was previously demonstrated that amotosalen and UVA light treatment, although abolishing viral infectivity by blocking viral RNA replication and transcription via adduct formation on nucleic acids, does not impair RT-PCR detection of DENV and viruses belonging to alpha- and poxvirus genera.26, 47 Similarly, even though ZIKV particles became noninfectious after photochemical inactivation, amotosalen-modified viral RNAs could still be detected by RT-PCR. Indeed, it has been shown that RT-PCR amplification of nucleic acid fragments of less than 300 bp are not inhibited48 and our RT-PCR protocol generated amplicons of 76 bp.29 After one passage on VERO cells, the mean viral RNA load dramatically decreased, and from the second passage, ZIKV RNA was no longer detected, confirming the absence of viral replication due to the lack of infectious particles after inactivation with amotosalen and UVA light. The fact that replicative viruses were still detected in the noninactivated sample (positive control) confirmed that the inactivation of ZIKV was due to the amotosalen and UVA treatment and not to the compound absorption device that removed residual amotosalen.
When testing the blood samples of asymptomatic blood donors from French Polynesia by RT-PCR, we measured ZIKV RNA loads ranging from 3.40 to 6.91 log copies/mL (mean, 4.85 log copies/mL). Equivalent ZIKV RNA loads, ranging from 930 to 728,800 copies/mL (i.e., 2.97 to 5.86 log copies/mL; mean, 4.40 log copies/mL) have been previously found from blood samples of 17 patients infected during the ZIKV outbreak in Yap Island in 2007.29 In our experiments, amotosalen and UVA light treatment induced a reduction in ZIKV RNA loads of 10.25 log copies/mL on average, that is, at least 104 times higher than the mean viral RNA loads and at least 103 times higher than the upper viral RNA loads measured in blood samples of infected patients and asymptomatic blood donors detected positive respectively during Yap's and French Polynesia's epidemics. These results suggest that the amotosalen and UVA light treatment may be used to inactivate ZIKV in blood products collected in ZIKV epidemic areas.
French Polynesia is a highly endemic area for DENV and has also recently experienced two large outbreaks of ZIKV and CHIKV. Amotosalen and UVA light treatment has been already shown to inactivate DENV and CHIKV in FFP.25, 26 Our study demonstrates that this photochemical process also inactivates ZIKV in plasma and induces a decrease of viral RNA loads higher than those found in ZIKV-infected French Polynesian blood donors. Based on our results, the amotosalen and UVA light inactivation process appears suitable to reduce the risk of plasma transfusion-transmitted ZIKV infections. This procedure is of particular interest in areas, such as French Polynesia or Brazil, in which several arboviruses are cocirculating.
ACKNOWLEDGMENTS
We are grateful to Philippe Desprès (Institut Pasteur, Paris, France) for providing flavivirus antibodies for immunofluorescence and titration assays. We thank Allison Imrie (University of Western Australia, Perth, Australia) who provided the titration protocol.
CONFLICT OF INTEREST
JG is an employee and owns stock of Cerus Corporation. The other authors have disclosed no conflicts of interest.




