Costs and outcomes after cardiac surgery in patients refusing transfusion compared with those who do not: a case-matched study
Supported by departmental funding.
Abstract
BACKGROUND
Although numerous studies have demonstrated the feasibility of cardiac surgery for blood refusal patients, few studies match to controls, and fewer examine cost. This historical cohort study aims to compare costs and outcomes after cardiac surgery in Jehovah's Witness patients who refuse blood transfusion with a group of matched patients accepting transfusion.
STUDY DESIGN AND METHODS
A retrospective database review was performed to find all patients having cardiac surgery who refused blood products from January 2005 to July 2012 at Duke University Medical Center. These 45 patients were closely matched 1:2 with controls who accepted transfusion based on characteristics likely to influence transfusion. Cost from day of surgery to hospital discharge and other outcome data (length of stay [LOS], discharge hemoglobin [Hb], acute kidney injury) were analyzed retrospectively.
RESULTS
Forty-five Witnesses having cardiac surgery were temporally matched to two controls having the same surgery. Median euroSCORE was the same in both groups (6.0, p = 0.9981). In the matched-pairs comparison of cost, there was no significant difference in total cost for Witnesses and controls. There was no difference in intensive care unit LOS (median, 1 day, both groups) or total LOS (median, 9 days for Witnesses vs. 7 days for controls). Mean Hb at discharge was higher in Witnesses than in controls (11.7 g/dL vs. 9.8 g/dL, p < 0.001). Thirty-day mortality was zero in both groups.
CONCLUSION
Utilizing applicable blood conservation measures, cardiac surgery may be performed with similar outcomes and cost from day of surgery to discharge compared to controls in select patients without blood transfusion.
Numerous retrospective studies have shown an association of transfusion of blood products and postoperative morbidity and mortality.1-9 Several randomized controlled trials have shown no benefit of a liberal transfusion strategy compared with a restrictive strategy in surgical patients,10, 11 although one prospective, randomized trial in patients with gastrointestinal bleeding demonstrated worse outcomes with liberal transfusion thresholds.12 Nonetheless, although it has not been clearly proven that transfusion results in adverse outcomes, Bradford Hill criteria strongly suggest causation.3 Moreover, preoperative anemia has also been associated with adverse outcomes and is a strong predictor of transfusion of allogeneic red blood cells (RBCs); blood loss and liberal transfusion triggers are the two other major predictors of perioperative transfusion.13
Among patients presenting for cardiac surgery, anemia is common14 and is the single most important predictor of perioperative erythrocyte transfusion.15 Furthermore, this patient population receives a disproportionate share of transfusions.16 Patient blood management (PBM) has recently been described as a proactive method to minimize the likelihood of having to resort to transfusion of RBCs as an emergency treatment of anemia. Three of the main pillars of PBM are to identify and treat anemia and iron deficiency preoperatively, to minimize perioperative RBC loss, and to use restrictive transfusion triggers.17
Jehovah's Witness (hereafter Witnesses) patients having cardiac surgery present a unique challenge to the perioperative team, as these patients often do not accept transfusion of whole blood, RBCs, platelets (PLTs), and plasma. The feasibility of cardiac surgery in Witnesses without transfusion was described decades ago18-20 and more recently techniques to optimize these patients preoperatively,21 intraoperatively,22 or both23 have been described. Because of the inability to use many blood components in the perioperative period with Witnesses, the principles of PBM are essential to safely perform cardiac surgery in this patient population.
Studies comparing outcomes among Witnesses having cardiac surgery to patients who accept blood have been published recently and suggest equivalent or improved outcomes.24, 25 Pattakos and colleagues26 reported that Witnesses matched to a group of patients who received transfusions had fewer complications, shorter length of stay (LOS), better 1-year survival, and similar 20-year survival. Frank and coworkers27 used a risk-adjusted, propensity score–matched model to compare patients refusing transfusion with controls for all inpatients, not only surgical patients. They found lower mortality in the bloodless group and lower total and direct hospital costs, which were attributed to the surgical subgroup. There are no published studies to date examining hospital cost in Witnesses compared to controls for major cardiac surgery. The aim of this study was to compare outcomes between Witnesses having cardiac surgery and matched controls, with the primary outcome being hospital cost from day of surgery to discharge.
MATERIALS AND METHODS
After institutional review board approval was obtained, a retrospective review of the Duke Center for Blood Conservation (CBC) database was performed to find all patients refusing blood transfusion (whole blood, RBCs, PLTs, plasma) and having cardiac surgery (coronary artery bypass graft [CABG] and/or valve repair/replacement) from January 2005 to June 2012 at Duke University Medical Center. Each CBC patient (Witness) who refused transfusion was matched with two control patients who did not refuse transfusion based on a search of an institutional database. Through a meticulous and systematic iterative filtering of more than 5000 potential matches, each Witness was matched to two control patients based on characteristics likely to influence transfusion. Control patients had the same procedure (including the same number of bypass grafts for patients who had CABG surgery) with the same surgeon in the same time period (January 2005-June 2012 for Witnesses and February 2005-December 2011 for controls). Controls had the same prior sternotomy (one Witness, two controls) and dialysis or renal failure (two Witnesses, four controls) status and the same preoperative hemoglobin (Hb) level (to the nearest 0.1 g/dL) and were closely matched on age, sex, height, and BMI, as previous studies have found these to be the preoperative risk factors for transfusion.15 To account for other comorbidities, they were closely matched on ASA score. This patient-focused process produced a set of controls matched individually on investigator-specified characteristics more closely than propensity matching likely would have achieved. Additionally, the euroSCORE risk index for cardiac surgery mortality was calculated for all patients (additive score, original version).28
Although individualized to each patient, methods of blood conservation in the Witness group included preoperative optimization of Hb with erythropoietin (EPO)-stimulating agents and iron (oral or intravenous [IV]), intraoperative use of cell salvage and acute normovolemic hemodilution, and minimizing blood draws including use of pediatric phlebotomy tubes. A more detailed description of the blood conservations techniques used in this study has previously been published.23
Detailed patient-level cost accounting data were collected for all study patients, from the day of surgery to the day of discharge. Cost data reflect the cost to the institution, as extracted from a cost-accounting system that uses relative value weights and general ledger costs to estimate the fixed, variable, and total costs of services provided. Cost items were grouped first into subgroups and then into four major cost groups based on type of item (clinical care outside of the operating room, diagnosis, supplies, and operative costs). Clinical care outside of the operating room included charges for ward and intensive care unit (ICU) care; electrophysiology lab; emergency department; preoperative holding area; and ancillary services such as respiratory, occupation, and physical therapy. Diagnosis included charges for preoperative testing including electrocardiogram, cardiac catheterization, magnetic resonance imaging, nuclear medicine, and chest x-rays. Supplies included charges from the pharmacy, lab charges, and blood bank or transfusion services. Operative costs included anesthesia and perfusion services, operating room time, and operating room supplies. A few cost items not fitting into any of these four categories (miscellaneous charges—family medicine clinic, rheumatology clinic, oncology clinic, general or transplant surgery clinic) were excluded from the analysis. Excluded miscellaneous costs accounted for only 0.8% of total cost. Total costs and the costs for each of the four major cost groups were summed for each patient from the day of surgery to the day of discharge.
The costs for each Witness were subtracted from the costs for each of the two corresponding matched controls to give two paired cost differences per Witness. In addition to the 1:2 paired tests, confirmatory tests were done with 1:1 matching using the closer of the two matches for each Witness. Because of the skewed nature of the data, the sign test was chosen and conducted as the most appropriate test of significance for these paired differences. Since this test is insensitive to extremes in the data, we confirmed the robustness of our conclusion with t tests after an attempt at transformation for normality, trimmed means, and Winsorized means. The sample size of 88 pairs would have an 80% power to detect a mean difference in total cost of $6853. This estimate for the nonparametric test is based on an adjustment to a paired t test with a 0.050 two-sided significance level and with observed standard deviation (SD).29
Other outcomes in the matched pairs, including LOS, discharge Hb, acute kidney injury (AKI; defined as a 50% increase of serum creatinine or a ≥0.3 increase in serum creatinine), and 30-day mortality were compared using signed-rank tests of significant difference or McNemar's tests for categorical outcomes such as AKI. A Fisher's exact test was used to compare rates of infection, where the rate was zero in one group. Infections were defined as any infectious process (wound infection, pneumonia, urinary tract infection, etc.) between surgery and hospital discharge. p values presented are from the matched tests, comparing the differences between pairs; descriptive means are presented from the two separate groups. Unpaired tests of descriptive characteristics of the two groups were made with the Wilcoxon rank-sum test. Computer software (SAS, Version 9.3, SAS Institute, Cary, NC) was used for all data assembly and analysis.
RESULTS
During the study period, there were 45 patients who had cardiac surgery and refused transfusion matched to 90 control patients. The groups were well matched according to the criteria described under Materials and Methods. These variables as well as other descriptive characteristics are shown in Table 1. Calculated euroSCORE values were very similar between Witnesses and their matched controls: the median difference between Witnesses and controls was zero (mean difference, Witness-matched control = −0.10, p = 0.6747, signed-rank test).
| Parameter | Control (n = 90)a | Witness (n = 45)a | p value | Test |
|---|---|---|---|---|
| euroSCORE | 6.0 (4.0-8.0) | 6.0 (4.0-8.0) | 0.9981 | Rank sum |
| Age (years) | 64 (58-72) | 66 (58-72) | 0.9182 | Rank sum |
| BMI | 29.1 (26.9-32.0) | 30.6 (26.6-32.7) | 0.5 | Rank sum |
| Height (cm) | 170.1 (163-178) | 171 (161-178) | 0.9609 | Rank sum |
| Number of grafts done | 2 (0-3) | 2 (0-3) | 0.9981 | Rank sum |
| Number of valves done | 0 (0-1) | 0 (0-1) | 0.9089 | Rank sum |
| Preoperative creatinine | 1.0 (0.9-1.2) | 1.0 (0.8-1.3) | 0.9512 | Rank sum |
| Female | 43 (47.8) | 22 (48.9) | 0.9031 | Chi-square |
| ASA | ||||
| 2 | 1 (1.1) | 1 (2.2) | ||
| 3 | 32 (35.6) | 12 (26.7) | 0.5346 | Chi-square |
| 4 | 57 (63.3) | 32 (71.1) | ||
| Surgery type | ||||
| CABG | 46 (51.1) | 23 (51.1) | ||
| CABG + valve | 8 (8.9) | 4 (8.9) | 1 | Chi-square |
| Valve | 36 (40) | 18 (40) |
- a Data are reported as median (IQR) or number (%).
Cost data were unavailable for one Witness. Therefore, the cost data analysis was done on the 44 Witnesses for whom cost data were available and their 88 matched controls. Cost items were grouped into four major cost groups based on type of item: clinical care outside of the operating room, diagnosis, supplies, and operative costs. In the matched-pairs comparison of cost, mean total costs ($35,306 for controls vs. $31,152 for Witnesses) as well as cost for each subcategory were higher for controls than for Witnesses (Fig. 1), although significance was only reached for the subcategories of diagnosis and supplies (Table 2). In Table 2, a positive difference indicates that costs were higher for the control group and a negative difference indicates that costs were higher in the Witness group. A box plot showing cost differences for each subcategory is in Fig. 2. In this figure, two extremely high outliers (both control patients, one for clinical care not in the operating room, one for supplies) were dropped from the boxplots to present a reasonable scale, but these records were retained in all analyses. A summary of individual cost items data for Witnesses and controls is available in Appendix S1 (available as supporting information in the online version of this paper).
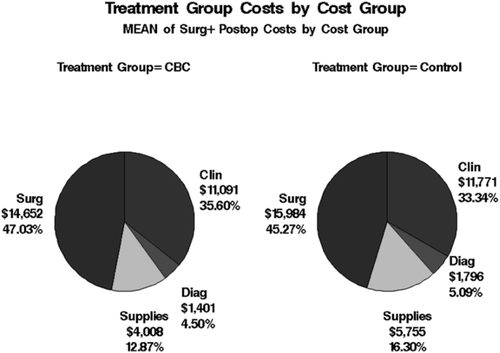
Total costs by group. Clin = clinical care outside of the operating room; Diag = diagnosis; Surg = operative costs. CBC = Center for Blood Conservation patients (Witnesses).
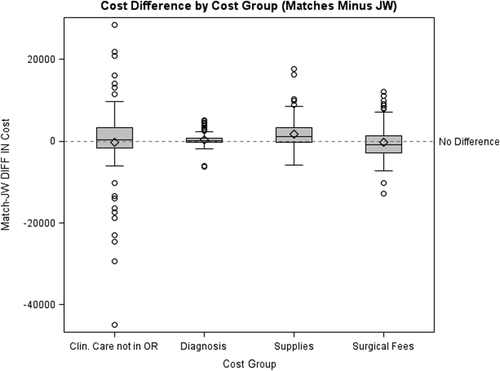
Box plot showing cost differences for the each subcategory between Witnesses and controls: mean (◇), median (—), interquartile range, and outliers (○).
| Difference in cost summary by cost group | 95% CL | p value | ||||||
|---|---|---|---|---|---|---|---|---|
| Cost group | P25 | Median | Mean | SD | P75 | Lower | Upper | Sign test |
| Total costs | −3154 | 1123 | 4153 | 21191 | 8295 | −325 | 3962 | 0.2408 |
| Clinical care not in operating room | −1518 | 405 | 680 | 12874 | 3429 | −32 | 1798 | 0.1093 |
| Diagnosis | −299 | 192 | 395 | 1689 | 780 | 20 | 365 | 0.0246 |
| Supplies | −346 | 1047 | 1747 | 3856 | 3305 | 619 | 1413 | 0.0004 |
| Operative costs | −2762 | −794 | 1332 | 14772 | 1622 | −1441 | 88 | 0.0693 |
- a Positive difference = control is higher; negative difference = Witnesses higher; 95% CL = 95% confidence limits on the median free from distributional assumptions of normality.
There was no difference in cross-clamp time (mean, 79.2 min for Witnesses, 65.2 min for controls, p = 0.0695) or total operating room time (mean, 396.4 min for Witnesses, 395.5 min for controls, p = 0.8825) between the two groups. The paired comparison between Witnesses and controls found that total hospital LOS was not different between Witnesses and controls (Table 3). Witnesses had a median total LOS (from hospital admission to discharge) of 9 days (interquartile range [IQR], 6-14 days) versus a median of 7 days (IQR, 5-12 days) for controls. Witnesses were admitted before surgery longer than controls (median, 3 days vs. 1 day), but the time from day of surgery to hospital discharge was 1.1 day shorter on average in Witnesses (median, 5 days for Witnesses vs. 6 days for controls). There was no difference in ICU LOS in the paired comparison (Table 3).
| LOS | Mean | SD | Min | P25 | Median | P75 | Maximum | Sign p test |
|---|---|---|---|---|---|---|---|---|
| Days admitted before surgery | 1.7 | 6.4 | −24 | −1 | 0.5 | 6 | 17 | 0.0444 |
| Days admitted postprocedure | −1.1 | 5.36 | −22 | −2 | −1 | 1 | 14 | 0.0187 |
| ICU LOS | −0.07 | 1.74 | −7 | 0 | 0 | 1 | 4 | 0.8827 |
| Total LOS | 0.6 | 9.2 | −32 | −2 | 1 | 5 | 29 | 0.1193 |
- a Witnesses minus control: positive means Witnesses longer.
Mean Hb at discharge was higher in Witnesses compared with matched controls (11.7 g/dL vs. 9.8 g/dL, p < 0.001) and Witnesses had a higher mean Hb at the start of surgery compared with controls (13.9 g/dL vs. 12.3 g/dL; mean difference 1.6, p < 0.0001, SD = 1.62, 95% confidence limits 1.32-2.0). The preoperative Hb, which was measured when the patient initially presented for workup before surgery, was the same for Witnesses and controls, since this was a matching criterion (Hb 12.9 ± 1.4 g/dL). However, day of surgery Hb, which was measured before induction of anesthesia, was higher for Witnesses due to preoperative Hb optimization. None of the Witnesses received transfusion of allogeneic RBCs, while 61 control patients (67.8%) received at least one transfusion of RBCs (p < 0.0001). Control patients received a median of 2 units of RBCs (IQR, 0-4 units). Data on chest tube drainage or other measures of intraoperative or postoperative blood loss were not available.
AKI was not significantly different in the two groups (73.3% in controls vs. 77.8% in Witnesses, p = 0.5749). One patient in the control group had a stroke preoperatively, but there were not enough data on this outcome to draw any meaningful comparison between the two groups. Zero Witnesses and seven controls (7.8%) had an infectious complication during their index hospitalization, which was not significant (Fisher's exact p = 0.0949). Thirty-day mortality was zero in both groups.
DISCUSSION
In this retrospective study matching Witnesses who do not accept transfusion to controls who accept transfusion, there was no significant difference shown in total cost from day of surgery to discharge between the two groups. The cost subgroups of diagnosis and supplies were significantly less in the Witness cohort compared with the control group, perhaps due in part to less postoperative laboratory testing and decreased blood bank costs. Witnesses had a longer time from admission to surgery, likely reflecting the time required to optimize preoperative anemia. However, they had a shorter time from surgery to hospital discharge, and no significant difference in total or ICU LOS, suggesting that the time lost to optimize patients before surgery is regained by a faster postoperative recovery. There were no infectious complications in the Witness group during the index hospitalization while there was a 7.8% rate of infectious complications in the control group during this period (p = 0.0949). Although this result did not achieve significance, previous studies have shown an association between transfusion and infection,3, 8 and this may have been a contributing factor to Witnesses having a shorter postoperative stay. There was no difference in the rates of AKI between the two groups, despite previous studies showing as association between transfusion and AKI.2, 30 The majority of the Witnesses in this study underwent acute normovolemic hemodilution in the operating room, which included administration of colloid starches, before the Food and Drug Administration's black box warning for increased risk of mortality and renal injury in the critically ill patient.31 All of the Witnesses received starches intraoperatively, compared to only 20 of the 90 control patients. It may be that any worsening of renal function from transfusion in the control group was matched with worsening renal function from the use of starches in the Witnesses.
Previous studies have shown that cardiac surgery can be performed in Witnesses with comparable outcomes to patients who accept transfusion.24-26 However, this study is unique in that it shows that these surgeries can be performed in Witnesses without increasing total cost relative to control patients. A significant limitation of the cost analysis is that it does not include the cost associated with preoperative optimization of Witnesses, which included additional laboratory tests and EPO for those patients who were anemic preoperatively. Preoperative cost data were unavailable for both Witnesses and controls and therefore not included in the cost analysis.
In an attempt to account for this limitation, we determined the institutional cost of common laboratory tests and medications in the preoperative optimization of Witnesses. The preoperative workup of a Witness consists of two visits to the Duke CBC where a complete blood count and iron studies (serum ferritin, reticulocyte count, serum iron, and iron saturation) are obtained. Those who are diagnosed with anemia of chronic disease buy oral iron (at their own expense) and receive up to four injections of EPO. Forty of the Witnesses in this study received at least one injection of EPO. There is no patient charge for the visit to the CBC. While there are overhead expenses associated with the CBC, because this center performs many other functions in the institution apart from preoperative workup of Witnesses, we are unable to estimate the institutional cost of an outpatient visit to the CBC. Total institutional cost for laboratory tests is $24.24 and the cost for four EPO injections is $1820 for a total institutional cost of $1844 for a Witness who needed four EPO treatments. Not all Witnesses required four treatments, and even if the full $1844 were added to every Witness, the total institutional cost would still not be significantly different from the controls (median difference, Witness $721 higher; mean difference, Witness $2309 lower; p = 0.5943). This does not include all charges related to preoperative optimization of Witnesses, such as cost of infusion center visits. Twelve of the 45 Witnesses received IV iron in conjunction with a dose of EPO, either as an outpatient, at an outside hospital, or during a previous hospitalization. Also several Witnesses with unstable angina were hospitalized preoperatively on a heparin infusion while their Hb was optimized with EPO and iron, accounting for the difference between day of admission to day of surgery among Witnesses and controls. Control patients with anemia in this situation typically proceeded to urgent CABG without optimization of anemia. The optimization protocol used in this study was recently described in more detail by McCartney and colleagues.23 Nonetheless, despite the significant limitation of lacking preoperative cost data, we feel that demonstrating that operative and postoperative recovery costs are similar for Witnesses and controls is of value.
Each Witness was closely matched with two control patients who had the same cardiac surgery with the same surgeon in the same time period in an effort to minimize bias. Including both CABG and valve repair or replacement surgeries does limit the specificity of the analysis, but including both groups gives a larger and more meaningful population. A hospital initiative to decrease transfusion rates was introduced several years before the study period and resulted in the formation of the CBC. During the 7.5-year study period, however, blood management practice was consistent, with the exception of the discontinuation of aprotinin in favor of aminocaproic acid in 2008 after safety concerns were raised and sale of aprotinin was suspended. Since each Witness was temporally matched to his or her controls, the comparison should remain valid even despite subtle changes in practice over time. However, as with other studies examining outcomes among Witnesses having cardiac surgery, this is a retrospective study and has all the attendant limitations associated with observational research.
Additionally, since Witnesses refuse transfusion, the care they receive is somewhat different throughout the perioperative period. Despite this, there was no significant difference in aortic cross-clamp or operating room time. This suggests that optimal surgical technique including obtaining adequate hemostasis was the same for both groups and reinforces our conclusion that the operating room costs are no different. Also, there may be surgical selection bias in that the Witnesses accepted for surgery are probably expected to have better outcomes than other patients. However, both groups were remarkably similar in terms of ASA score and euroSCORE, suggesting similar risk factors and comorbidities. In the study interval, one Witness was not offered CABG surgery due to his comorbidities (advanced age, reduced left ventricular function, significant mitral insufficiency, pulmonary hypertension, and thrombocytopenia). Six other Witnesses were not offered surgery immediately because there was no indication to operate when they were seen in clinic or they needed further testing to further evaluate comorbidities; all six of these patients were lost to follow-up and it is unknown if they had surgery at another center. One Witness was offered surgery pending Hb optimization, but died of non–cardiac-related causes before surgery.
One notable difference in this study compared with that recently published by Pattakos and coworkers26 is that their comparison of Witnesses to non-Witnesses was only among the 56% of non-Witnesses who were actually transfused, not just a comparison group of non-Witnesses regardless of transfusion status as in the current study (67.8% of the control group was transfused RBCs). Having the control group contain only transfused patients will tend to overestimate the adverse effects associated with the acceptance of transfusion in the non-Witness population.
It would be interesting to know whether patients discharged with a higher Hb, as with the Witness patients in this study, are less likely to be readmitted to the hospital following discharge after surgery. However, as a tertiary referral center, especially for our blood refusal patients, many patients travel a great distance for their surgery and are therefore unlikely to return to this institution if readmission is necessary. Therefore, readmission data to our institution alone could not provide a valid conclusion. Shehata and coworkers32 recently found that a Hb concentration between 8 and 10 g/dL after CABG made 30-day readmission no more likely than a discharge Hb concentration of 10 to 12 g/dL. Since the preoperative optimization of Witnesses often involves treatment with EPO and IV iron, it is unknown whether that would have any impact of readmission after surgery. In contrast to the study on anemia and readmission in CABG patients, several retrospective studies in medical patients have shown that anemia at discharge is associated with readmission.33-35 Alternatively, the higher postprocedure Hb in the Witnesses may have been a contributing factor to their shorter recovery time from day of surgery to discharge compared to the controls.
Overall, based on the results in this study and those of other investigators, cardiac surgery may be performed safely and without additional cost from day of surgery to discharge in select patients without blood transfusion, provided that preoperative anemia is treated and that a multidisciplinary team experienced in blood conservation techniques manages these patients.
ACKNOWLEDGMENTS
The authors acknowledge Betsy Hale, BS, for so meticulously retrieving institutional cost data from its labyrinth. We also thank John Stover, NP, for his assistance in caring for and helping to develop treatment protocols for our blood refusal patients.
CONFLICT OF INTEREST
RSR is a co-investigator on a pilot research project at UT Southwestern that has received funding from AMAG, the manufacturer of IV iron used in the pilot project. All other authors have disclosed no conflicts of interest.
REFERENCES
ABBREVIATIONS
-
- AKI
-
- acute kidney injury
-
- CABG
-
- coronary artery bypass graft
-
- CBC
-
- Center for Blood Conservation
-
- ICU
-
- intensive care unit
-
- IQR
-
- interquartile range
-
- LOS
-
- length of stay
-
- PBM
-
- patient blood management




