Fever in focus: Symptoms, diagnoses and treatment of febrile children in Ghana—A longitudinal hospital study
Jürgen May and Ralf Krumkamp contributed equally to this manuscript.
Sustainable Development Goal: Good Health and Wellbeing
Abstract
Background
Healthcare resources are often limited in areas of sub-Saharan Africa. This makes accurate and timely diagnoses challenging and delays treatment of childhood febrile illness. We explored longitudinal characteristics related to symptoms, diagnosis and treatment of hospitalised febrile children in a rural area of Ghana highly endemic for malaria.
Methods
Febrile children under 15 years, admitted to the study hospital paediatric ward, were recruited to the study and clinical data were collected throughout hospitalisation. Descriptive statistics were reported for all cases; for longitudinal analyses, a subset of visits with limited missing data was used.
Results
There were 801 hospitalised children included in longitudinal analyses. Malaria (n = 581, 73%) and sepsis (n = 373, 47%) were the most prevalent suspected diagnoses on admission. One-third of malaria suspected diagnoses (n = 192, 33%) were changed on the discharge diagnosis, compared to 84% (n = 315) of sepsis suspected diagnoses. Among malaria-only discharge diagnoses, 98% (n/N = 202/207) received an antimalarial and 33% (n/N = 69/207) an antibiotic; among discharge diagnoses without malaria, 28% (n/N = 108/389) received an antimalarial and 83% (n/N = 324/389) an antibiotic.
Conclusions
Suspected diagnoses were largely based on clinical presentation and were frequently changed; changed diagnoses were associated with lingering symptoms, underscoring the need for faster and more accurate diagnostics. Medications were over-prescribed regardless of diagnosis stability, possibly because of a lack of confidence in suspected diagnoses. Thus, better diagnostic tools are needed for childhood febrile illnesses to enhance the accuracy of and confidence in diagnoses, and to cut down unjustified medication use, reducing the risk of antimicrobial and malaria resistance.
BACKGROUND
Fever is one of the most common symptoms in children presenting to health clinics or hospitals in low- and middle-income countries and can be an indicator of many different ailments. In sub-Saharan Africa, one common cause is infection with Plasmodium falciparum, which caused 234 million cases of malaria in the World Health Organization (WHO) Africa region in 2021 [1]. Among non-malarial fevers, bacterial infections comprise a large proportion and other pathogens, particularly viruses, are also present but often remain undiagnosed [2-4].
In formal healthcare settings in malaria endemic regions, a febrile child is usually tested with a malaria rapid diagnostic test (RDT) and by microscopy and, if positive, treated according to WHO guidelines [5]. If the test is negative, capacity to accurately diagnose non-malarial infections varies greatly between facilities and can be a challenge in resource-constrained settings, particularly for illnesses that present with symptoms similar to malaria [6].
In regions with high malaria prevalence, diagnoses of febrile illnesses in children are challenging because differential diagnoses are difficult to confirm with limited diagnostic resources. Therefore, it is common practice and also recommended by WHO to treat febrile children where severe malaria is suspected with both antimalarials and antibiotics [5]. However, this option comes at the cost of antibiotic overuse, which burdens resource-poor healthcare systems and brings a risk of fostering antimicrobial resistance [7].
A better understanding of changes in symptoms, diagnoses, and treatment over time in hospitalised children would facilitate targeting of obstacles to timely and accurate diagnoses. We used data from hospitalised febrile children in a malaria-endemic area of Ghana to explore longitudinal characteristics of hospital stay.
METHODS
Study site
The data were collected between November 2013 and December 2015 at the Agogo Presbyterian Hospital (APH) in Agogo, Ghana. This research was part of a larger study focusing on causes of febrile illness in children, published elsewhere [8]. APH is a 350-bed district hospital located in the Asante Akim North District in the Ashanti Region. The climate is tropical with two rainy seasons, from April to July and September to November. The rainy seasons are associated with peaks in the incidence of malaria, which is holoendemic in the area [1, 9].
Children aged >30 days and < 15 years with a tympanic temperature of ≥38°C admitted to the APH paediatric ward were recruited. On admission, demographic and clinical data were collected. A suspected diagnosis was recorded, which included conditions the physician was considering on the differential diagnosis and was based mostly on clinical presentation. A final diagnosis was recorded at discharge and had more basis in laboratory results; both diagnoses were made by the treating physician.
Febrile children were tested for malaria with an RDT when available. Blood film microscopy was conducted by the hospital laboratory for identification of malaria parasites after a positive RDT result or when the RDT was unavailable. Microscopy results informed the discharge diagnoses. Suspected diagnoses for sepsis were based on clinical presentation and a blood sample was taken from all children for bacteriological analysis; microbiological methods for all diagnoses are described elsewhere [8]. Positive bacteriological results were available after 2–3 days and negative results after 7 days. Results informed discharge diagnoses, but a clinical discharge diagnosis of sepsis was also possible when no bacteria were identified.
Diagnoses were classified for analysis into the following categories: malaria, sepsis, meningitis, gastroenteritis, urinary tract infection (UTI), lower respiratory tract infection (LRTI), other infection (defined as any diagnosis caused by an infectious pathogen other than those above), and other non-infection illness (defined as any other ailment with a non-infectious origin). Cases of typhoid fever were grouped together with sepsis diagnoses. Multiple diagnoses were possible. Case reviews were conducted on days 1, 2, 3, 5 and 7 of the hospital stay, or up until discharge. During case reviews, the child's symptoms and treatment were recorded and blood samples were collected for malaria parasite microscopy to be read by study staff after study closure; results were not available to the treating physicians at the time. Treatment during hospitalisation was categorised from reported antibiotic and antimalarial prescription.
Data analysis
All patients with a hospital stay of at least 24 h were included in the descriptive statistics. Cases with more than 50% missing data among longitudinal treatment variables or <2 days hospital stay were excluded from longitudinal analyses. Median and interquartile range (IQR) were calculated for continuous variables and frequencies and percentages for categorical variables. Observations with missing values for other variables were excluded from the respective metric calculation, thus the denominator in calculations differs. Sankey diagrams were drawn to show patient-level changes in diagnoses from admission to discharge. We calculated the ‘sensitivity’ and ‘specificity’ with 95% confidence intervals (CIs) for all diagnoses; despite the lack of a gold standard, the term ‘sensitivity’ represents in this context the percent of discharge diagnoses suspected at admission and ‘specificity’ is the percent of suspected diagnoses included on the discharge diagnosis. We performed data analyses using R (version 4.1.2), RStudio, and the networkD3 package for Sankey diagrams [10-12].
Ethical considerations
This study and the informed consent procedures were approved by the Committee on Human Research, Publications and Ethics, School of Medical Science, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana (CHRPE/AP/427/13) and the Ethics Committee of the Ärztekammer Hamburg, Germany (PV4592). Prior to enrolment, written informed consent was obtained from parents or guardians of participants.
RESULTS
Overview
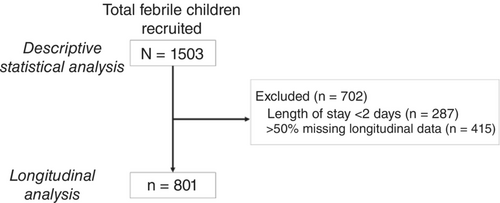
A total of 1503 admissions to APH paediatric ward fulfilled the inclusion criteria and those children were recruited (Figure 1). The median age was 2 years (IQR: 1–4); 79% (n/N = 1181/1503) were under 5 years old (Table 1). The median temperature on admission was 39.0°C (IQR: 38.5–39.6°). The most frequent symptoms on admission were vomiting (41%, n/N = 621/1502) and coughing (28%, n/N = 427/1501). Malaria and sepsis were the most common diagnoses on admission (78%, n/N = 1172/1503; 42%, n/N = 625/1503, respectively). In retrospective slide readings, malaria parasites were found in 59% (n/N = 885/1503) of patients on admission.
| Total | <1 year | 1 year | 2–4 years | ≥5 years | |
|---|---|---|---|---|---|
| Number of children | 1503 (100.0%) | 263/1503 (17.5%) | 342/1503 (22.8%) | 576/1503 (38.3%) | 322/1503 (21.4%) |
| Age (years) | 2 (1–4) | 0 (0–0) | 1 (1–1) | 3 (2–3) | 7 (5–9) |
| Sex (male) | 829/1503 (55.2%) | 151/263 (57.4%) | 181/342 (52.9%) | 331/576 (57.5%) | 166/322 (51.6%) |
| Length of stay (days) | 2 (2–4) | 3 (2–5) | 3 (2–5) | 2 (2–4) | 2 (1–5) |
| Temperature (C) | 39.0 (38.5–39.6) | 38.9 (38.3–39.3) | 39.1 (38.6–39.5) | 39.2 (38.6–39.7) | 39.0 (38.5–39.7) |
| Parasites present in blood | 885/1503 (58.9%) | 88/263 (33.5%) | 199/342 (58.2%) | 394/576 (68.4%) | 204/322 (63.4%) |
| Positive blood culture | 47/1431 (3.3%) | 4/241 (1.7%) | 10/318 (3.1%) | 16/561 (2.9%) | 17/311 (5.5%) |
| Self-reported medication use in 24 h before admission | |||||
| Antipyretic | 1086/1485 (73.1%) | 186/259 (71.8%) | 259/339 (76.4%) | 423/570 (74.2%) | 218/317 (68.8%) |
| Antibiotic | 65/1483 (4.4%) | 11/260 (4.2%) | 20/338 (5.9%) | 21/570 (3.7%) | 13/315 (4.1%) |
| Antimalarial | 164/1482 (11.1%) | 21/259 (8.1%) | 32/337 (9.5%) | 65/570 (11.4%) | 46/316 (14.6%) |
| Suspected diagnoses | |||||
| Malaria | 1172/1503 (78.0%) | 175/263 (66.5%) | 269/342 (78.7%) | 482/576 (83.7%) | 246/322 (76.4%) |
| Sepsis | 625/1503 (41.6%) | 104/263 (39.5%) | 133/342 (38.9%) | 229/576 (39.8%) | 159/322 (49.4%) |
| LRTI | 153/1503 (10.2%) | 54/263 (20.5%) | 40/342 (11.7%) | 39/576 (6.8%) | 20/322 (6.2%) |
| Gastrointestinal infection | 143/1503 (9.5%) | 50/263 (19.0%) | 50/342 (14.6%) | 27/576 (4.7%) | 16/322 (5.0%) |
| UTI | 75/1503 (5.0%) | 8/263 (3.0%) | 14/342 (4.1%) | 31/576 (5.4%) | 22/322 (6.8%) |
| Meningitis | 58/1503 (3.9%) | 9/263 (3.4%) | 8/342 (2.3%) | 35/576 (6.1%) | 6/322 (1.9%) |
| Other infection | 301/1503 (20.0%) | 71/263 (27.0%) | 65/342 (19.0%) | 116/576 (20.1%) | 49/322 (15.2%) |
| Other non-infection | 327/1503 (21.8%) | 55/263 (20.9%) | 85/342 (24.9%) | 131/576 (22.7%) | 56/322 (17.4%) |
| Discharge diagnoses | |||||
| Malaria | 870/1503 (57.9%) | 86/263 (32.7%) | 199/342 (58.2%) | 391/576 (67.9%) | 194/322 (60.2%) |
| Severe malaria | 552/870 (63.4%) | 47/86 (54.7%) | 127/199 (63.8%) | 258/391 (66.0%) | 120/194 (61.9%) |
| Sepsis | 144/1503 (9.6%) | 18/263 (6.8%) | 47/342 (13.7%) | 40/576 (6.9%) | 39/322 (12.1%) |
| LRTI | 164/1503 (10.9%) | 62/263 (23.6%) | 40/342 (11.7%) | 44/576 (7.6%) | 18/322 (5.6%) |
| Gastrointestinal infection | 111/1503 (7.4%) | 42/263 (16.0%) | 37/342 (10.8%) | 24/576 (4.2%) | 8/322 (2.5%) |
| UTI | 100/1503 (6.7%) | 14/263 (5.3%) | 17/342 (5.0%) | 37/576 (6.4%) | 32/322 (9.9%) |
| Meningitis | 13/1503 (0.9%) | 2/263 (0.8%) | 1/342 (0.3%) | 8/576 (1.4%) | 2/322 (0.6%) |
| Other infection | 392/1503 (26.1%) | 99/263 (37.6%) | 75/342 (21.9%) | 143/576 (24.8%) | 75/322 (23.3%) |
| Other non-infection | 316/1503 (21.0%) | 49/263 (18.6%) | 94/342 (27.5%) | 121/576 (21.0%) | 52/322 (16.1%) |
- Abbreviations: LRTI, lower respiratory tract infection; UTI, urinary tract infection.
- a Frequency and percentage are shown for categorical variables and median and interquartile range (IQR) for continuous variables. Denominator for continuous variables is equal to the total cases for that group, except where otherwise indicated.
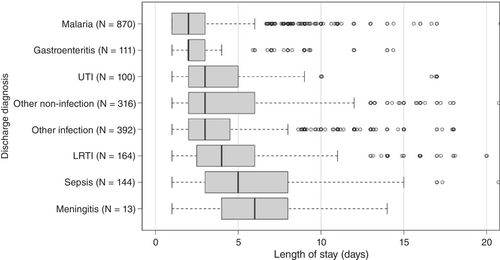
Close to half of cases stayed longer than 2 days (48%, n/N = 728/1503). Figure 2 shows the length of hospitalisation by discharge diagnosis. The shortest hospital stays were among children with a malaria discharge diagnosis (median: 2 days; IQR: 1–3 days); the longest hospital stays were among children with sepsis (median: 5 days; IQR: 3–8 days) or meningitis discharge diagnoses (median: 6 days; IQR: 4–8 days).
Forty seven percent of patients (n/N = 702/1503) were missing >50% of longitudinal treatment data or had a length of stay <2 days. Thus, 801 (53%) cases were used for longitudinal analyses. The frequency of vomiting reported on admission was higher among excluded cases compared to included cases (47% vs. 36%, Table S1), as was the frequency of malaria discharge diagnoses (65% vs. 51%).
Longitudinal symptoms and diagnoses
The distribution of symptoms changed over the course of hospital stays: the proportion with a fever of at least 38°C decreased from 100% (n/N = 801/801) at admission to 19% (n/N = 151/785) on day 1 and continued decreasing thereafter (Figure 3). Coughing increased in prevalence from 30% (n/N = 238/801) at admission to 47% (n/N = 332/703) on day 1 before declining slightly to 31% (n/N = 29/94) on day 7. Vomiting began at a higher prevalence than coughing (36%, n/N = 290/801) but decreased after that; all other symptoms comprised a relatively small proportion on admission and decreased further over time. Twelve percent of patients (n/N = 94/801) reported symptoms on their last review day prior to discharge.
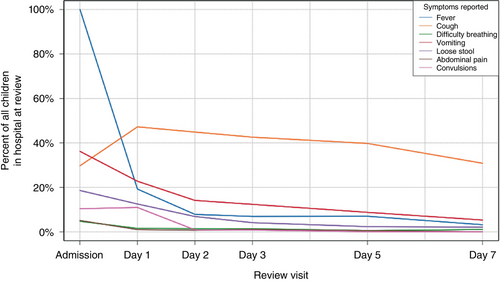
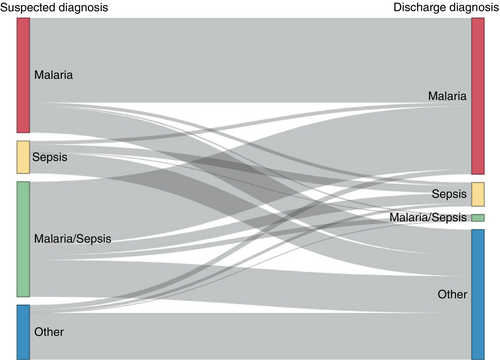
Figure 4 shows the evolution of malaria and sepsis diagnoses from admission to discharge. Of the 801 cases, 581 (73%) children presented with suspected malaria on admission; one-third of these (33%, n/N = 192/581) did not have malaria on the discharge diagnosis. The proportion of children with positive malaria slides increased from 67% (n/N = 389/581) of suspected malaria diagnoses to 90% (n/N = 372/412) of malaria discharge diagnoses. RDT usage among suspected malaria cases before admission was estimated to be between 35% and 50% (Agogo Presbyterian Hospital, personal communication). Sepsis was also frequent, listed as a suspected diagnosis for 373 (47%) of admissions, but 84% of these (n/N = 315/373) were not confirmed as sepsis on the discharge diagnosis. The proportion of children with positive bacteriology results increased from 3% (n/N = 21/625) of suspected sepsis diagnoses to 16% (n/N = 23/144) of sepsis discharge diagnoses.
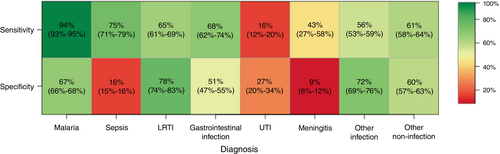
The proportion of discharge diagnoses already suspected on admission (‘sensitivity’) was higher for malaria (94%, n/N = 389/412; 95% confidence interval (CI): 93%–95%) than for other diagnoses (Figure 5). This proportion was also relatively high for sepsis discharge diagnoses (75%, n/N = 58/77; 95% CI: 71%–79%). Sensitivity was lowest among UTI (16%, n/N = 9/57; 95% CI: 12%–20%) discharge diagnoses. The proportion of suspected diagnoses that were also included on the discharge diagnosis (‘specificity’) was high for LRTI (78%, n/N = 73/93; 95% CI: 74%–83%) and low for UTI (27%, n/N = 9/33; 95% CI: 20%–34%), sepsis (16%, n/N = 58/373; 95% CI: 15%–16%) and meningitis diagnoses (9%, n/N = 3/33; 95% CI: 6%–12%).
The prevalence of any symptoms on day 1 was 7% higher among children with a changed diagnosis (i.e., whose discharge diagnosis was not suspected at admission) (72%, n/N = 207/286) compared to those with an unchanged diagnosis (65%, n/N = 323/499); this difference was 12% on day 2 (58%, n/N = 147/255 vs. 46%, n/N = 183/397). After day 2, the difference in the prevalence of symptoms was negligible. The median length of stay (IQR) was 3 [2–5] among children with a constant diagnosis and 3 [2–6]among children with a changed diagnosis.
Treatment
Antibiotic treatment was reported on at least one review day for 64% (n/N = 515/801) of children; antimalarial use was reported for 63% (n/N = 502/801). One third of children received both antimicrobials during their hospital stays (34%, n/N = 271/801) and only 7% (n/N = 55/801) received neither. Of children with a malaria-only discharge diagnosis, 98% (n/N = 202/207) were given an antimalarial and 33% (n/N = 69/207) were given an antibiotic; among those with no malaria on the discharge diagnosis, 28% (n/N = 108/389) received an antimalarial and 83% (n/N = 324/389) received an antibiotic.
For 22% (n/N = 161/746) of cases receiving medication, treatment was switched from one medication to another (e.g., antibiotics to antimalarials, both to antibiotics only), whereas 78% had a constant treatment regimen (n/N = 585/746). Changed diagnoses and switched treatments did not correspond well: the prevalence of changed diagnoses was only slightly higher among changed treatment regimens (41%, n/N = 66/161) compared with constant treatment regimens (35%, n/N = 205/585). Among children without malaria on the discharge diagnosis, 94% (n/N = 150/160) of those with a changed diagnosis and 96% (n/N = 174/182) of those with a constant diagnosis received an antibiotic. Among this same group, an antimalarial was given to 33% (n/N = 52/160) of those with a changed diagnosis and 31% (n/N = 56/182) of those with a constant diagnosis.
DISCUSSION
Our study aimed to characterise changes in symptoms, treatment and diagnoses in hospitalised febrile children in a malaria-endemic setting. Other studies have captured the prevalence of admission and discharge diagnoses [13, 14], but our study included an analysis of longitudinal changes at an individual level. In our data, we observed that many diagnoses changed during hospital stay. This might be explained by the difference in diagnostic methods: suspected diagnoses on admission were based on clinical presentation of symptoms and basic laboratory tests until more specific diagnostic results were available, which informed discharge diagnoses. Lingering symptoms, which were more prevalent among children with changed diagnoses, might also have motivated physicians to change the diagnosis. Hospital-acquired infections, even malaria, could also have played a role, but these were not detected or frequently suspected.
The highest sensitivity among diagnoses was estimated for malaria, likely in part because of the availability of the malaria RDT to confirm the suspected diagnosis. Furthermore, malaria discharge diagnoses were highly concordant with positive retrospective slide readings, indicating a high-quality discharge diagnosis for malaria. Incorrect suspected malaria diagnoses were likely those based on clinical presentation when an RDT was not available, and further discrepancies between suspected diagnosis and slide reading were possibly attributable to physician non-adherence to RDT results [15, 16]. Sepsis had a relatively high sensitivity because of the employment of sepsis as a blanket diagnosis at admission until blood culture results were available; this was likely motivated by the overlapping symptoms with malaria and the serious consequences of a delayed sepsis diagnosis. Specificity of sepsis diagnoses was accordingly much lower than that for malaria.
The estimated specificity for meningitis was low and its sensitivity was moderate, indicating the tendency to over-diagnose the infection but also demonstrating the presence of meningitis cases that were not identified on admission. The low sensitivity and specificity of UTI diagnoses indicate the especially challenging nature of the diagnosis. These findings emphasise a potential area for improvement in the diagnostic protocol for both infections [17].
In-hospital medication prescription was comparable to other studies [18, 19]. Over-prescription results in drug wastage, higher expenses, potential exposure to drug toxicity, neglect of the true source of fever and brings a risk of antimicrobial resistance [7]. However, withholding treatment in young children could in some cases also bring risk of morbidity and mortality [20]. Over-prescription specifically with antimalarials among children without malaria was present in our study, as also reported elsewhere [21, 22]. However, in our data, prevalence of antimalarial over-prescription was comparable between children with changed (33%) and constant diagnoses (31%), suggesting that this is not driven primarily by changes in the diagnosis but is rather a separate issue, perhaps resulting from non-adherence to RDT results because of lack of confidence in the results.
Similarly, antibiotic prescription among children without a malaria discharge diagnosis was comparable between those with a changed (94%) and constant diagnosis (96%), suggesting an almost empiric prescription of antibiotics for nearly all children without indicators for malaria; a similar pattern has also been described in other studies [18, 23]. Our study was not designed to identify the true cause of fever for each patient, so it is difficult to determine whether each antibiotic prescription was justified, but based on estimates of viral causes of fever in the region [2-4, 24], it is likely that in many of these cases antibiotics were over-prescribed. Empiric treatment with antibiotics remains the best approach to minimise the severity of disease when sepsis or bacterial pneumonia is a possibility [25-27], but overuse of antibiotics poses a risk of the development of resistances [7, 25]. This is even more important outside the hospital setting, therefore recent guidelines for pharmacists and primary healthcare workers recommend against the prescription of antibiotics without clear indication [26].
Interestingly, changed treatment regimens did not correspond well with changed diagnoses; instead, a culture of over-prescription of both antibiotics and antimalarials is present in the data, perhaps because of a lack of confidence in suspected diagnoses. The capacity to improve diagnoses is limited by the speed and availability of diagnostic tools, for instance in the delay of blood culture results. Affordable point-of-care diagnostic tools have the power to increase precision of diagnoses and to reduce unnecessary medication use [28]. The increased affordability and availability of RDTs for malaria enables the departure from presumptive treatment for malaria [29]. In our own study, malaria diagnoses had higher sensitivity and specificity and were treated less frequently with antibiotics compared to non-malarial diagnoses; this is, most likely, successfully attributable to the availability of the malaria RDT for informing diagnoses and treatment decisions. The use of host biomarkers to distinguish between bacterial and non-bacterial infections has also become a focus of recent research, although it is challenging [30-32]. Research in this area could facilitate the development of point-of-care tools that could guide diagnoses and treatment and reduce antibiotic use in resource-challenged settings [33].
This study was limited in its missing data; the proposal for the collection of longitudinal data was made after the start of the study, so a large part of the missingness among these variables was concentrated in the first patients recruited. We addressed this by separately analysing children with more complete data for longitudinal analyses (Table S1). The higher frequency of vomiting on admission and malaria discharge diagnoses among excluded cases (consisting of all cases with a length of stay <2 days) compared to included cases could be driven by the shorter length of stay among malaria diagnoses, which are frequently accompanied by vomiting, or by short gastroenteritis. The terms ‘sensitivity’ and ‘specificity’ were chosen for their interpretability in regard to our methods in comparing suspected and discharge diagnoses. This necessitated the assumption of the clinician-based discharge diagnosis as a ‘gold standard’ diagnosis for the patients, which could be misleading in the absence of a classical gold standard. However, the decreasing prevalence of LRTI and GI infections with increasing age is in agreement with findings from other studies [3, 24], which is encouraging in respect to the accuracy of the discharge diagnoses. It is possible that the true source of fever was not always correctly identified in the discharge diagnosis, especially among malaria cases with an undiagnosed co-infection; therefore, our results relating to discharge diagnoses must be interpreted carefully.
CONCLUSION
We examined data from hospital visits of febrile children in Agogo, Ghana to explore hospital etiologies, symptoms and diagnoses and hospital treatment. Our results provided a unique perspective of the longitudinal data collected. Malaria diagnosis was reliable and treatment effective with a short hospital stay. However, overuse of antibiotics, especially for suspected sepsis, is still a serious problem, which is difficult to improve. Better point-of-care diagnostics such as the use of biomarkers are needed to expedite diagnoses, improve accuracy and reduce unnecessary medication use.
ACKNOWLEDGEMENTS
BH, DE, RK, NS, DD, OMA, BK, AJ, YAS, EOD and JM designed and carried out the study with further assistance in data collection from FOB, clinical work from Theresa Rettig, Harry Owusu Boateng, Luise Ammer, Stefanie Steierberg and Wiebke Herr and laboratory analysis from Hassan Al-Emran, KGB, CWA, HH, Kwabea Oppong, Doris Winter, Michael Nagel, Tabea Binger, Anke Thielebein, Isabel Eckerle and Daniel Cadar. Open Access funding enabled and organized by Projekt DEAL. [Correction added on 31 January 2024, after first online publication: Projekt DEAL funding statement has been added.]
FUNDING INFORMATION
The study was supported by federal funds from the German Center for Infection Research (DZIF) (funding number TTU 03.804). LHR and RK also received funding from DZIF (funding number TTU 03.708).
CONFLICT OF INTEREST STATEMENT
All authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
Raw data are not publicly available to respect patient privacy; de-identified data and analysis code are available for download from the open-access data repository Zenodo at https://doi.org/10.5281/zenodo.8220138.




