The Second Zambian National Tuberculosis Drug Resistance survey – a comparison of conventional and molecular methods
Abstract
enObjective
The prevalence of MDR-TB in Zambia was estimated to be 1.8% in 2001. A second drug resistance survey was conducted in 2008 to determine trends; the use of the Genotype MTBDRplus assay was applied to compare results to the gold standard.
Method
A two-stage cluster sampling, with health facilities as primary sampling units. Processed sputum specimens were inoculated on solid media for culture; heat-inactivated bacterial suspensions from sputum samples were tested on a commercial line probe assay for the identification of rifampicin and isoniazid resistance.
Results
A total of 917 patients with TB were enrolled and 883 (96.3%) analysed. A total of 574 (65%) had LJ results and 824 (93.3%) had results from MTBDRplus assay. The median age was 32, and 63.3% were males. MDR-TB according to LJ-based DST was 1.1% (CI 0.1–2.4) whereas according to MDTBDRplus assay was 1.6% (CI 0.6–2.6). Isoniazid monoresistance in new cases was 2.4% (CI 0.613–4.26) based on LJ results and 5.0% (CI 3.2–6.7) based on the MTBDRplus; in retreatment cases, it was 4.4% (CI 0.3–8.6) and 2.40% (CI <0.1–5.1) on LJ and MTBDRplus, respectively. Rifampicin monoresistance in new cases was 0.1% (CI <0.1–0.4) based on LJ and 0.6% (CI 0.01–1.1) based on the MTBDRplus; in retreatment cases, it was 0% (CI 0–3.8) and 1.8% (CI <0.1–4.0) on LJ and MTBDRplus, respectively. There were no XDR-TB cases found and no association between MDR-TB and HIV.
Conclusion
There was no increase in MDR-TB prevalence in Zambia from 2001 to 2008; results from the two methods were similar. Molecular methods were quicker and simpler to use.
Abstract
frObjectif
La prévalence de la TB-MDR en Zambie a été estimée à 1,8% en 2001. Une deuxième surveillance de la résistance aux médicaments a été menée en 2008 afin de déterminer les tendances; le test Genotype MTBDRplus a été utilisé pour comparer les résultats à ceux de la référence standard.
Méthode
Echantillonnage en grappes à deux étages, avec les établissements de santé comme unités primaires d’échantillonnage. Les échantillons d'expectoration traités ont été inoculés sur un milieu solide pour la culture, les suspensions bactériennes des échantillons de crachats inactivés par la chaleur ont été testées avec une sonde linéaire commerciale pour l'identification de la résistance à la rifampicine et à l'isoniazide.
Résultats
Un total de 917 patients TB ont été inscrits et 883 (96,3%) ont été analysés. 574 (65%) avaient des résultats par la culture sur LJ et 824 (93,3%) avaient des résultats avec le test MTBDRplus. L’âge médian était de 32 ans, 63,3% étaient des hommes. La TB-MDR sur base des DST effectués sur LJ était de 1,1% (IC: 0,1 à 2,4) et de 1,6% (IC: 0,6 à 2,6) d'après le test MDTBDRplus). La mono-résistance à l'isoniazide chez les nouveaux cas était de 2,4% (IC: 0,613 à 4,26) selon les résultats sur LJ et 5,0% (IC: 3,2 à 6,7) selon le test MTBDRplus; chez les cas de retraitement, elle était de 4,4% (IC: 0,3 à 8,6) et 2,40% (IC: <0,1 à 5,1) sur LJ et par MTBDRplus, respectivement. La mono-résistance à la rifampicine chez les nouveaux cas était de 0,1% (IC: <0,1 à 0,4) selon les résultats sur LJ et 0,6% (IC: 0,01 à 1,1) selon le test MTBDRplus; chez les cas de retraitement, elle était de 0% (IC: 0 à 3,8) et 1,8% (IC: <0,1à 4,0) sur LJ et par MTBDRplus, respectivement. Aucun cas de TB-UR et aucune association entre la TB-MDR et le VIH n'on été trouvés.
Conclusion
Il n'y a pas eu d'augmentation de la prévalence de la TB-MDR en Zambie entre 2001 et 2008; les résultats des deux méthodes étaient similaires. Les méthodes moléculaires étaient plus rapides et plus simples à utiliser.
Abstract
esObjetivo
Se calculó que la prevalencia de tuberculosis multirresistente (TB-MR) en Zambia era 1.8% en el 2001. Se realizó un segundo estudio de resistencia a medicamentos en el 2008 para determinar tendencias; la prueba molecular Genotype MTBDRplus se utilizó para comparar los resultados con el patrón oro.
Método
Muestreo de conglomerados en dos etapas, con los centros sanitarios como unidades primarias de muestreo. Las muestras de esputo procesadas fueron inoculadas en medio de cultivo sólido: las suspensiones bacterianas inactivadas mediante calor, provenientes de las muestras de esputo, fueron analizadas utilizando pruebas comerciales estándar para determinar resistencias a rifampicina e isoniazida.
Resultado
De un total de 917 pacientes con TB en el estudio, 883 (96.3%) fueron analizados. 574 (65%) tenían resultados en Lowenstein-Jensen (LJ) y 824 (93.3%) tenían resultados de la prueba MTBDRplus. La edad media era de 32 años, y un 63.3% eran hombres. La TB-MR según la prueba LJ era del 1.1% (IC 0.1-2.4) mientras que según el ensayo MDTBDRplus era del 1.6% (CI 0.6-2.6). La mono-resistencia a isoniazida entre nuevos casos era del 2.4% (IC 0.613-4.26) basándose en los resultados de LJ y 5.0% (IC 3.2-6.7) basándose en el MTBDRplus; en casos con retratamiento, era del 4.4% (IC 0.3-8.6) y 2.4% (IC <0.1-5.1) según LJ y MTBDRplus, respectivamente. La mono-resistencia a rifampicina entre nuevos casos era del 0.1% (IC <0.1-0.4) basándose en LJ y 0.6% (IC 0.01-1.1) basándose en el MTBDRplus; en casos en retratamiento, era del 0% (IC 0-3.8) y 1.8% (IC <0.1-4.0) en LJ y MTBDRplus, respectivamente. No se encontraron casos de TB extremadamente resistente (TB-XDR) y no existía asociación entre –TB-MR y el VIH.
Conclusión
No había un aumento en la prevalencia de TB-MR en Zambia entre 2001 y 2008; los resultados de los dos métodos eran similares. Los métodos moleculares eran más rápidos y más fáciles de utilizar.
Introduction
Drug-resistant tuberculosis (DR-TB) and in particular multidrug-resistant tuberculosis (MDR-TB) is becoming widespread, causing a substantial obstacle to TB control in a number of countries. WHO estimates that there were 480 000 cases of MDR-TB in 2013 and that mortality due to MDR-TB amounted to 210 000 lives lost 1. While there are data from hospital-based studies and clinical settings with inherent risk of selection bias 2, surveillance data on DR-TB in sub-Saharan Africa are limited, as very few countries have the capacity to sustain routine surveillance; and as only a limited number of countries have repeatedly conducted drug resistance surveys to understand the actual trends in disease burden 1, 3. Some of the obstacles to implementing drug resistance surveillance and surveys in sub-Saharan Africa are the complicated logistics involved; in particular, the need for sputum samples to arrive in a reference laboratory within 2–3 days. Due to long distances, and often poor transportation and courier systems, this is difficult 4-6. However, molecular methods of drug resistance testing potentially offer a solution, as they do not require viable Mycobacterium tuberculosis (M.tb) bacilli 1, 7.
Zambia has one of the highest TB burdens in the African Region, with recent notification rates of about 314 per 100 000 population 1, 8. The HIV pandemic has been an important contributor to the increase in the TB cases that have been observed in the past two decades, with approximately 70% of all notified TB cases being co-infected with HIV in Zambia, but even more in areas of high co-endemicity such as in South Africa 9, 10. In 2001, a national survey of drug resistance was carried out following the WHO recommended methodology of clustered sampling and culture on Löwenstein Jensen (LJ) medium 11. Prevalence of multidrug resistance TB was estimated to be 1.8% (95% CI 0.8–3.5) in a nationally representative sample of smear-positive tuberculosis patients including both new and retreatment cases in 2001 12, 13.
In 2008, a repeat survey was conducted which aimed at determining whether drug resistance has remained the same. Conventional culture-based method; Löwenstein Jensen was used as the gold standard. Additional objectives were to apply the use of a rapid molecular method in a drug resistance survey (DRS), namely the Genotype MTBDRplus assay that can be performed directly on sputum, and to compare the results. Other objectives were to determine the association of HIV with MDR-TB in a population-based sample and to determine rates of extensively drug-resistant tuberculosis (XDR-TB).
Methods
The survey was designed according to the standard WHO recommended protocol 11 with a clustered sampling from the entire country. A target sample size of 1000 new patients was calculated to provide 80% power to detect as statistically significant a difference if the estimated prevalence of MDR-TB had risen to 5%, assuming a design effect due to the cluster design of 2 and adding 10% for expected losses.
Sampling method
The recruitment period was 12 months (January through December 2008). The weighted cluster sampling method was used, which allowed centres with large numbers of notification cases to be proportionally represented. A two-stage cluster sampling was performed in which the primary sampling units were health facilities (outpatient health centres or hospitals) that were sampled with probability proportional to size (PPS). The PPS was defined based on the number of notified new TB cases in the year 2006, and the secondary sampling units were individual patients that were sampled by taking the first consecutive 20 new patients and all the re-treatment patients who were diagnosed during the data collection period of 12 months. Consecutive patients with tuberculosis whose sputum smear was positive for acid-fast bacilli (AFB) by Ziehl-Neelsen (ZN) staining were included in the study. History of previous TB treatment, TB disease and demographic information were collected by structured interviews with patients by trained interviewers who were either physicians or clinical officers. A standardised questionnaire was used for the interviews. History of previous TB treatment was also validated against the hospital TB case registry. Forty-four heath facilities had a cluster of 20 patients each while 3 health facilities had 2 clusters per facility of 20 patients each; thus adding up to a total of 50 clusters from 47 health facilities (centres). Patients under 15 years of age who met the inclusion criteria were also recruited into the study.
Sputum preparation and culture
Two sputum samples, a spot sample and an early morning sample, were collected from each patient into 50 ml screw-cap centrifuge tubes (Falcon tubes, Becton Dickinson, USA) before commencement of treatment. A blood sample for HIV testing was also collected from consenting patients. Sputum samples were stored at the respective centres at 4 °C and later transported to the national reference laboratory in Lusaka. The two samples from an individual were then pooled and processed applying the N-acetyl L-cysteine (NaOH)-NALC method using the Becton Dickinson MycoPrep™ kit as per manufacturer instructions. After centrifugation, the sediments were reconstituted to 2.5 ml using phosphate buffer. Concentrated specimens were stained to confirm the presence of acid-fast bacilli (AFB) using the auramine phenol staining method and read using a fluorescence microscope.
The processed specimens were inoculated on solid media LJ (home-made) and incubated at 37 °C. The inoculated volume was 0.2 ml. The LJ slant contained 0.75% glycerol. Two millilitre of decontaminated sputum was also stored in a 2.2-ml micro centrifuge tube at −20 °C.
The LJ culture was checked weekly for growth. Tubes which exhibited growth on LJ were examined for potential mycobacterial growth. ZN staining was made from the cultures to confirm whether it was Mycobacterium tuberculosis complex. Pure cultures were subcultured on home-made LJ slant for DST. Mixed cultures were re-decontaminated and re-inoculated.
Isolate identification
Cultures were identified as presumptive M. tuberculosis complex (MTC) according to colony morphology and microscopic appearance and where confirmed as MTC by the capilia TB test. In order to reduce the time to identification, a chromatographic test (Capilia TB, TAUNS Laboratories Inc, Shizuoka, Japan) was used instead of the usual biochemical tests. Pure cultures were stored in 2.2-ml microcentrifuge tubes for archiving and DNA extraction. The archived and DNA samples were stored at −20 °C.
First-line Drug susceptibility
Drug susceptibility testing (DST) was performed on all M. tuberculosis isolates using the indirect proportional method. The drug concentrations used in the LJ system were streptomycin 4 μg/ml, isoniazid 0.2 μg/ml, rifampicin 40 μg/ml and ethambutol 2 μg/ml. According to our laboratory guidelines, pyrazinamide is not routinely tested.
Genotyping
Heat-inactivated bacterial suspension from the sputum sample stored for this purpose was tested on a commercial line probe assay system for the identification of rifampicin and isoniazid resistance (GenoType MTBDRplus, Hain Lifescience GmbH, Nehren, Germany). The lysate from the sputum was obtained by heating the decontaminated sputum at 95 °C in a water bath for 20 min, followed by sonication for 15 min. The samples were then centrifuged, and 5 μl of the supernatant was used in the PCR.
The principle of this assay is the polymerase chain reaction (PCR) amplification of relevant regions of genes including the katG and inhA promoter region for isoniazid resistance and rpoB genes for rifampicin resistance, followed by reverse-phase hybridisation to probes immobilised on a solid-phase membrane.
Drug susceptibility testing for second-line anti-TB drugs
All the isolates found to be MDR by genotypic method (GenoType MTBDRplus) were tested for resistance to second-line drugs using the GenoType MTBDRsl (Hain Lifesciences, Nehren, Germany) based on mutations or non-binding wild-type probes for the gyrA gene (fluoroquinolones), the rrs gene (aminoglycosides/cyclic peptides) and the embB gene (ethambutol).
HIV testing
A blood sample was collected for HIV testing after counselling, and consent had been obtained from the patients. Patient confidentiality of results was maintained at all stages. HIV testing was performed using two rapid tests, Determine HIV-1/2 (Abbot, Tokyo, Japan) and Unigold Recombinant HIV (Trinity Biotech, Wicklow, Ireland). Samples were first tested on Determine and those that were non-reactive were reported as negative. Positive samples were then confirmed with Unigold.
Statistical analysis
All data obtained in the laboratory was entered into a Microsoft Access database at the National Reference Laboratory and at the serology laboratory. The demographic and molecular data were double-entered into databases. Stata Version 12.1 was used for statistical analysis. Comparison of categorical variables was performed through the chi-square test. Univariable and multivariable analyses were performed by logistic regression. Significant testing was performed at 5% confidence level. The design effect was accounted for in the analysis by calculating robust standard errors using the method of Rao and Scott as implemented in the Stata ‘svy’ commands.
Ethical issues
Ethical clearance was obtained from the University of Zambia Research Ethics Committee, and approval from the Ministry of Health. Written informed consent was obtained before interviewing and collecting sputum samples. The DST results were communicated to the national TB programme for follow-up and treatment where applicable.
Results
A total of 917 sputum smear-positive patients with TB were enrolled in the survey out of a total sample size of 1000. There were 883 (96.3%) sputum samples submitted and analysed, including 772 new (82%, or 77.2% of the target sample size) and 156 previously treated patients (18%), with 0.5% treatment history unknown. A total of 574 (65%) had results from the LJ culture method because 23.6% (208/883) failed to grow, 9.6% (85/883) were contaminated and 1.8% (16/883) were missing results. A total of 824 (93.3%) had results from the genotype MTBDRplus, whereas 5.7% either failed or was uninterpretable and 1% (9/883) were missing (Figure 1). The median participant age was 32 years (range 6–77). As shown in Table 1, of the 883 participants, 63.6% were male and 36.4% female. A total of 1.5% (13/883) were children under the age of 15. 43.1% tested HIV positive, 43.3% HIV negative and 13% had no HIV test result. Based on the LJ-based DST results, 6 of the 574 samples showed MDR-TB whereas 13 of 824 showed MDR-TB according to the MTBDRplus assay. There were no children under the age of 15 with MDR-TB according to DST based on neither LJ nor the MTBDRplus assay (Table 1).
| Collected | DST LJ Results | MDR n(%) | MTBDRplus results | MDR n(%) | |
|---|---|---|---|---|---|
| Age (years) | n = 883 | n = 574 | n = 6 | n = 824 | n = 13 |
| 0–14 | 13 (1.47) | 10 (1.7) | 0 (0) | 9 (1.1) | 0 |
| 15–24 | 163 (18.5) | 115 (20) | 1 (16.7) | 149 (18.1) | 2 (15.4) |
| 25–34 | 355 (40.2) | 225 (39) | 3 (50.0) | 335 (40.6) | 4 (30.8) |
| 35–44 | 205 (23.2) | 126 (22) | 2 (33.3) | 191 (23.2) | 5 (38.5) |
| ≥45 | 136 (15.4) | 91 (16) | 0 (0) | 129 (15.6) | 2 (15.4) |
| Unknown | 11 (1.2) | 7 (1) | 0 (0) | 11 (1.3) | 0 (0) |
| Sex | |||||
| Female | 320 (36) | 209 (36) | 5 (83.3) | 300 (36.4) | 7 (53.8) |
| Male | 558 (63) | 362 (63) | 1 (16.7) | 521 (63.2) | 6 (46.2) |
| Unknown | 5 (1) | 3 (1) | 3 (0.36) | 0 (0) | |
| HIV status | |||||
| Positve | 381 (43.1) | 231 (40) | 2 (3.33) | 361 (43.8) | 7 (53.8) |
| Negative | 383 (43.3) | 268 (47) | 3 (50.0) | 356 (43.2) | 4 (30.8) |
| Unknown | 119 (13.5) | 75 (13) | 1 (16.7) | 107 (13.0) | 2 (15.4) |
| Retreatment | |||||
| No | 722 (82) | 474 (83) | 0 | 672 (81.5) | 2 (15.4) |
| Yes | 156 (18) | 96 (17) | 6 (100) | 148 (18.0) | 11 (84.6) |
| Unknown | 5 (0.5) | 4 (0.7) | 4 (0.48) | 0 (0) | |
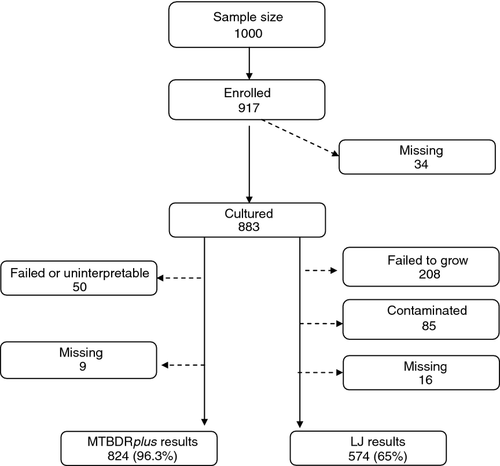
According to the LJ-based DST results (all proportions weighted and confidence intervals adjusted for cluster design), any resistance in new cases was 40/474 (8.1%; CI 5.1–11.1) and in previously treated (retreatment) cases 16/96 (17.2%; CI 7.9–26.4). Any resistance to rifampicin was 0.14% (CI <0.1–0.4) in new cases and 6.52% (CI <0.1–13.1) in previously treated cases, whereas any resistance to isoniazid in new cases was 4.5% (CI 2.1–6.9) and 11.9% in retreatments (CI 4.6–19.2). The MDR-TB cases based on LJ DST results were 0 (CI; 0–0.4) in new cases and 6.5% (CI <0.1–13.1) in previously treated cases; therefore, the overall MDR-TB due to this method was 1.1% (CI <0.1–2.4). Monoresistance to Isoniazid in new cases was 2.4% (CI 0.6–4.3) and 4.5% (CI 0.3–8.6) in retreatment cases, whereas rifampicin monoresistance was 0.14% (CI <0.1–0.4) in new cases and 0% (CI; 0–0.4) in retreatment cases (Table 2).
| Types of resistant patterns in new and retreatment cases (LJ DST results) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pattern of resistance | New cases | % | 95% CI | Retreatment cases | % | 95% CI | All cases | % | 95% CI |
| Total patients | 474 | 100 | 96 | 100 | 570 | 100 | |||
| Susceptible to all | 434 | 91.6 | 88.7 to 93.9 | 80 | 83.3 | 74.4 to 90.2 | 514 | 90.2 | 87.6 to 92.6 |
| Any resistance | 40 | 8.1 | 5.1 to 11.1 | 16 | 17.2 | 7.9 to 26.4 | 56 | 9.7 | 6.6 to 12.7 |
| Any resistance to | |||||||||
| RMP | 1 | 0.1 | <0.1 to 0.4 | 6 | 6.5 | <0.1 to 13.1 | 7 | 1.3 | 0.2 to 2.5 |
| INH | 22 | 4.5 | 2.2 to 6.9 | 12 | 11.9 | 4.5 to 19.2 | 34 | 5.8 | 3.5 to 8.1 |
| EMB | 1 | 0.3 | <0.3 to 0.8 | 1 | 1.0 | <1.0 to 3.0 | 2 | 0.4 | <0.2 to 0.9 |
| SM | 27 | 5.6 | 3.3 to 7.8 | 7 | 8.0 | 2.1 to 13.9 | 34 | 6.0 | 3.7 to 8.2 |
| H+R resistance (MDR-TB) | |||||||||
| INH+RMP Only | 0 | 0.0 | 0 to 0.8 | 4 | 4.2 | 1.2 to 10.3 | 4 | 0.7 | 0.04 to 1.3 |
| INH+RMP+EMB | 0 | 0.0 | 0 to 0.8 | 0 | 0.0 | 0 to 3.8 | 0 | 0.0 | 0.004 to 1.0 |
| INH+RMP+SM | 0 | 0.0 | 0 to 0.8 | 1 | 1.0 | 0.3 to 5.7 | 1 | 0.2 | 0.04 to 1.3 |
| INH+RMP+EMB+SM | 0 | 0.0 | 0 to 0.8 | 1 | 1.0 | 0.3 to 5.7 | 1 | 0.2 | 0.004 to 1.0 |
| Total MDR | 0 | 0.0 | 6 | 6.5 | <0.1 to 13.1 | 6 | 1.1 | <0.1 to 2.4 | |
| INH+EMB | 0 | 0.0 | 0 to 0.8 | 0 | 0.0 | 0 to 3.8 | 0 | 0.0 | 0.004 to 1.0 |
| INH+SM | 10 | 2.1 | 1.0 to 3.8 | 1 | 1.0 | 0.3 to 5.7 | 11 | 1.9 | 1.2 to 3.9 |
| INH+EMB+SM | 0 | 0.0 | 0 to 0.8 | 0 | 0.0 | 0 to 3.8 | 0 | 0.0 | 0.004 to 1.0 |
| Total INH + other reistance | 10 | 2.1 | 1.0 to 3.8 | 1 | 1.0 | 0.3 to 5.7 | 11 | 1.9 | 1.5 to 4.3 |
| RMP+EMB | 0 | 0.0 | 0 to 0.8 | 0 | 0.0 | 0 to 3.8 | 0 | 0.0 | 0.004 to 1.0 |
| RMP+SM | 0 | 0.0 | 0 to 0.8 | 0 | 0.0 | 0 to 3.8 | 0 | 0.0 | 0.04 to 1.3 |
| RMP+EMB+SM | 0 | 0.0 | 0 to 0.8 | 0 | 0.0 | 0 to 3.8 | 0 | 0.0 | 0.004 to 1.0 |
| Total RMP + other reistance | 0 | 0.0 | 0 to 3.8 | 0 | 0.0 | 0.2 to 1.8 | |||
| EMB+SM | 0 | 0.0 | 0 to 0.8 | 0 | 0.0 | 0 to 3.8 | 0 | 0.0 | 0.5 to 2.5 |
| Mono resistance | |||||||||
| RMP | 1 | 0.1 | <0.1 to 0.4 | 0 | 0.0 | 0 to 3.8 | 1 | 0.1 | <0.1 to 0.3 |
| INH | 12 | 2.4 | 0.6 to 4.3 | 5 | 4.5 | 0.3 to 8.6 | 17 | 2.8 | 1.1 to 4.4 |
| EMB | 1 | 0.2 | 0.005 to 1.2 | 0 | 0.0 | 0 to 3.8 | 1 | 0.2 | 0.004 to 1.0 |
| SM | 17 | 3.6 | 2.10 to 5.7 | 4 | 4.2 | 1.2 to 10.3 | 21 | 3.7 | 2.3 to 5.6 |
| Types of resistant patterns in new and retreatment cases (MTBDRplus results) | |||||||||
| Total patients | 672 | 100 | 148 | 100 | 820 | 100 | |||
| Susceptible to all | 631 | 93.9 | 91.8 to 95.6 | 129 | 87.2 | 80.7 to 92.1 | 760 | 92.7 | 90.7 to 94.4 |
| Any drug resistance | 41 | 5.8 | 3.9 to 7.7 | 19 | 10.8 | 5.3 to 16.4 | 60 | 6.8 | 4.9 to 8.7 |
| Any INH resistance | 37 | 5.3 | 3.4 to 7.1 | 16 | 9.0 | 3.9 to 14.1 | 53 | 6.0 | 4.2 to 7.8 |
| Any RMP resistance | 6 | 0.9 | 0.2 to 1.5 | 14 | 0.8 | 3.4 to 13.5 | 20 | 2.4 | 1.2 to 3.6 |
| MDR | 2 | 0.3 | <0.1 to 0.7 | 11 | 6.6 | 2.0 to 11.2 | 13 | 1.6 | 0.6 to 2.6 |
| INH mono resistance | 35 | 5.0 | 3.2 to 6.7 | 5 | 2.4 | <0.1 to 5.1 | 40 | 4.4 | 2.9 to 6.0 |
| RMP mono resistance | 4 | 0.6 | 0.01 to 1.1 | 3 | 1.9 | <0.1 to 4.0 | 7 | 0.8 | 0.2 to 1.4 |
- Correction added on 24 September 2015, after first online publication: Some values in Table 2 under the “Types of resistant patterns in new and retreatment cases (MTBDRplus results)” section were previously wrong and these have now been corrected and marked in bold.
However, based on the MTBDRplus assay using similar calculations, MDR-TB in new cases was found to be 0.3% (CI <0.1–0.7) and 6.7% (CI; 2.1–11.2) in previously treated cases. Overall, MDR-TB according to the MTBDRplus assay was therefore 1.6% (CI; 0.6–2.6). Isoniazid monoresistance in new cases according to the MTBDRplus assay was 5.0% (CI; 3.2–6.7) and 2.4% (CI <0.1–5.1) in retreatments whereas rifampicin monoresistance was 0.6% (CI <0.1–1.1) in new cases and 1.8% (CI <0.1–4.0) in retreatment cases (Table 2).
Comparing the performance of the MTBDRplus assay to the LJ results for MDR-TB cases, the MDTDRplus assay identified 13 MDR-TB cases against the LJ which identified 6 MDR-TB cases. Of these, the MDTDRplus correctly identified 5 cases according to the LJ and missed 1 MDR-TB case that was positive. As the LJ is currently the gold standard, the sensitivity of the MTBDRplus assay in detecting MDR-TB was therefore 83.3% and the specificity was 98.5% (Table 3).
| MTBDRplus | LJ Tests | |||
|---|---|---|---|---|
| Positive | Negative | Missing | Total | |
| MDR TB | ||||
| Positive | 5 | 2 | 6 | 13 |
| Negative | 1 | 541 | 278 | 820 |
| Missing | 0 | 28 | 22 | 50 |
| Total | 6 | 571 | 306 | 883 |
| INH | ||||
| Positive | 24 | 13 | 16 | 53 |
| Negative | 8 | 499 | 264 | 771 |
| Missing | 2 | 30 | 27 | 59 |
| Total | 34 | 542 | 307 | 883 |
| RIF | ||||
| Positive | 6 | 4 | 10 | 20 |
| Negative | 1 | 537 | 275 | 813 |
| Missing | 0 | 28 | 22 | 50 |
| Total | 7 | 569 | 307 | 883 |
| Resistance type | Sensitivity | Specificity |
|---|---|---|
| MDR-TB | 0.83 | 0.99 |
| INH | 0.75 | 0.97 |
| RIF | 0.86 | 0.99 |
Regarding isoniazid, 34 isolates were resistant on LJ, and of these, 8 were missed by MTBDRplus. However, of the 53 isolates that were resistant on MTBDRplus, 13 were missed by LJ, that is, the true positives were 34 as opposed to 53 identified by the MTBDRplus assay. Therefore, the assay correctly identified as positive 24 isolates of 34, and thus, the sensitivity was calculated to be 75.0% and the specificity was 97.4% (Table 3). Of the 13 that were missed by LJ, 7 had KatG mutations and 4 had inhA mutations, and 2 were presumably non-wild type. Overall, 36 isolates had katG mutations, 8 had inhA mutations and 1 had both. The LJ-missed isoniazid resistance seems more often inhA (as expected, low-grade resistance).
For rifampicin resistance, there were 7 isolates that were resistant on LJ, and of these, 1 was missed by the MTBDRplus assay. However, of the 20 isolates that were resistant on LineProbe Assay testing, 4 were missed by LJ (Table 3).
There was no association observed between HIV and MDR-TB in this survey (Table 4). Moreover, there were no isolates that were resistant to any of the second-line drugs that were tested; hence, no XDR-TB was identified.
| LJ based | MTBDRplus | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Age group | ||||||
| 0–24 | 1 | 1 | ||||
| 25–100 | 3.31 | 0.297–36.8 | 0.323 | 1.883 | 0.353–10.04 | 0.45 |
| Sex | ||||||
| Female | 1 | 1 | ||||
| Male | 0.23 | 0.0277–1.89 | 0.167 | 2.254 | 0.558–9.09 | 0.247 |
| HIV status | ||||||
| Positive | 1 | 1 | ||||
| Negative | 1.72 | 0.316–9.31 | 0.524 | 1.903 | 0.612–5.91 | 0.259 |
| TB type | ||||||
| New | 1 | 1 | ||||
| Retreatment | 1 | – | – | 23.41 | 4.70–116 | 0.0001 |
Discussion
The levels of MDR-TB remained low over the period of approximately eight years, from 2001 when the prevalence of MDR-TB was 1.2% in new cases and 1.8% in previously treated patients 12; compared to 2008 when it was 0 and 6.5% in new and previously treated patients, respectively, according to the gold standard. The low levels of MDR-TB were attained despite the challenges of TB control in terms of huge numbers of cases, weak health infrastructure, high HIV incidence rates of about 14.7% and inadequate resources 8. This low prevalence observed in new cases in our study is comparable to other findings of studies conducted in sub-Sahara Africa 14, 15. In recent years, there have been concerns and anxieties about MDR-TB in sub-Sahara Africa, especially due to the high burden of both TB and HIV in the region and the emergence of XDR-TB in South Africa which led to high mortality in HIV co-infected patients 16-18. However, our study shows that there was no evidence of the significant rise in MDR-TB over the period 2001 to 2008 in Zambia. The implementation of a good DOTS programme at all the levels of care over the said decade that ensured good treatment outcomes of patients could have played a role in sustaining these low levels 8, 10. This underscores the fact that good implementation of a national TB control programme could prevent escalating MDR-TB in addition to reducing transmission in communities 19-21. Also, the fact that six-month treatment regimens containing rifampicin in the continuation phase were only introduced in 2008 may have played some role in keeping numbers of DR-TB cases low. The availability of anti-TB drugs in the private sector in Zambia is limited, and most providers adhere to national guidelines; therefore, this could also contribute to the low prevalence as the possibility of substandard drugs circulating in the private sector is minimal. Another reason could be that the availability of adequate quality assured anti-TB drugs that are provided by the NTP free of charge to most private sector facilities and all the public sector facilities have contributed to maintaining good treatment outcomes. Rifampicin resistance in any combination other than MDR-TB was also low; and as expected, so was rifampicin monoresistance, which is in concordance with other findings 4, 22. The introduction of fixed dose combinations may have played a role in minimising the amplification of resistant strains; this may be the case as was shown by Monedero and Caminero that fixed dose combination reduces the risk of development of MDR-TB in patients taking them 23.
This study highlights the advantages to using MTBDRplus in conducting national drug resistance surveys including the fact that there is no need to culture samples in order to wait for growth; the samples can be heat-treated locally to increase safety. In addition, a bigger sample size can be managed easily; and this study showed a very good agreement with LJ, which is the current gold standard. In this study, MDR-TB detected by the MTBDRplus assay did not differ significantly from that which was detected through the LJ method; for instance, the results of 0 and 0.3% prevalence of MDR-TB in new cases for the LJ DST and MDTBDRplus assay and 6.5% and 6.7% in previously treated cases, respectively (Table 2). However, using LJ led to loss of a lot of samples (Figure 1) with only 65% analysed as compared to 96.3% of the samples analysed with the MTBDRplus assay. Therefore, molecular methods may be preferred over conventional DST. As the MTBDRplus assay has been endorsed by WHO as a recommended tool for diagnosis of drug-resistant TB 24, it offers a good alternative to the conventional methods in performing nationwide drug resistance surveys as shown in this study. The disadvantage of the assay is that currently, it is recommended to only detect rifampicin and some isoniazid resistance 25. Therefore, such surveys would not be informative on other types of resistance. Additionally, the MTBDRplus assay exhibited high sensitivity and specificity compared to LJ methods and conformed to various evaluation studies in different settings 26-28. The use of the assay has mainly been limited to hospital settings and to routine surveillance; however, we were able to show that it would be useful in population-based surveys in a low-resource setting. Although some cost-effectiveness studies have been carried out in using the MTBDRplus assay in routine surveillance 29, more studies are required to ascertain the cost implication when it has to be applied in population-based drug resistance surveys. There is also need to ensure quality assurance needs are fully attended to 25.
The high HIV prevalence rates in sub-Sahara Africa have stimulated debate about HIV as risk factor of MDR-TB; yet in this study, we have confirmed the findings of most population-based studies that HIV is not a risk factor for MDR-TB in our setting like in many others in the region 5, 30-33. There was no XDR-TB seen among 883 samples from across the country. However, the MTBDRsl assay has limitations in terms of its sensitivity to fluoroquinolones which is in the order of 0.87 and that 0.82 for the injectable second-line anti-TB drugs 34. In addition, the availability of second-line anti-TB drugs in Zambia is very limited, and the public sector only started using them for programmatic management of drug-resistant TB in 2010.
There is need to strengthen the laboratory network and begin to apply the new molecular technologies to strengthen routine surveillance of drug-resistant tuberculosis in order to ascertain trends 35. Nonetheless, we recommend that another special drug resistance survey should be conducted in Zambia to determine trends, especially considering that this survey was conducted 7 years ago and that since 2008, there have been changes in TB control in Zambia, such as the introduction of the 6-month treatment regimen using rifampicin containing fixed-dose-combination drugs throughout the treatment period, hence requiring improved and ongoing surveillance; the implementation of isoniazid preventive therapy (IPT) in people living with HIV/AIDS; and also the increased use of second-line anti-TB drugs in both the public and private sectors although this is not yet well documented. Although the prevalence of MD(X)R-TB was low in this particular study, it translates into more than 600 cases per year which is plenty of patients with MDR-TB that need to be treated for a low-resource setting, and therefore, the government should ensure that a very strong system for programmatic management of drug resistance TB programme is developed and then scaled-up. However, for effective TB control, emphasis still needs to be firmly on finding and curing sensitive cases as quickly and efficiently as possible 21.
We recommend that TB control programmes should invest in the use of molecular testing in routine surveillance and that future surveys should be conducted using molecular testing as we have shown that it is feasible to do so.




