Refractory Angiosarcoma of the Breast with VEGFR2 Upregulation Successfully Treated with Sunitinib
Secondary angiosarcoma of the breast is a rare, but well-described complication of radiation therapy for primary breast carcinoma. Currently, it appears refractory to most systemic chemotherapy though rare responses to taxanes exist 1, 2. Overall, patient prognosis is poor 3.
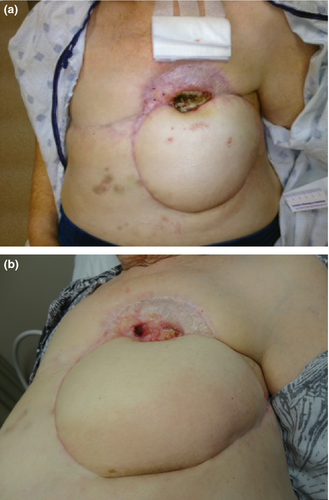
A 78-year-old female underwent left breast conservation and axillary node dissection in 1999 for invasive ductal carcinoma followed by whole breast radiation therapy. Eleven years later, she noted multiple small nodules in the medial aspect of the left breast. Biopsy of the nodules revealed angiosarcoma of the breast. A metastatic follow-up showed no evidence of distant disease and a modified radical mastectomy performed. Histopathology confirmed the presence of angiosarcoma with all margins negative. The patient completed a course of postmastectomy irradiation with dose limitation by previous radiation for her original breast conserving procedure. This was administered concurrently with adjuvant chemotherapy (Taxol and Adriamycin). The patient did not tolerate the chemotherapy but finished the course of radiation therapy. Ten months later, angiosarcoma nodules recurred along the mastectomy scar. Chemotherapy with single agent carboplatinum was ineffective. Six months later, she underwent a wide resection of the skin and soft tissue of the left chest wall with multiple cutaneous nodules and positive deep margin (the pectoral muscle/ribs) was noted. In the interim, she underwent a split thickness skin graft to cover the large defect. Within 6 months, recurrent disease appeared in the graft and surrounding soft tissue. With no documented distant metastases, the patient again underwent a resection of recurrent tumors, chest wall and two ribs requiring TRAM flap coverage. A Caris profile was requested. She remained free of local recurrence for only 4 months, when multiple and rapidly growing subcutaneous nodules became evident about the chest wall and flap (Fig. 1A). The Caris gene expression assay performed earlier demonstrated a potential therapeutic benefit of sunitinib due to the upregulation of VEGFR2. Sunitinib was then started at 50 mg daily and within 2 months the patient had a complete resolution of all her tumors (Fig. 1B). Fifteen months after the enactment of sunitinib, the patient is disease free, on a maintenance dose of 25 mg daily of sunitinib.
Sunitinib is a novel, potent multitargeted receptor tyrosine kinase inhibitor approved for treatment of metastatic renal cancer, imatinib-resistant gastrointestinal stromal tumor, and pancreatic neuroendocrine tumors 4. Recent reports indicate a potential therapeutic benefit of sunitinib in patients with angiosarcomas 5-7. Ours is the first report of a complete and durable response to sunitinib in a patient with chemo-resistant angiosarcoma of the breast. Sunitinib was administered on the basis of gene expression assay (whole genome RNA microarray, Illumina, San Diego, CA) demonstrating upregulation of VEGFR2. Furthermore, we retrospectively studied 16 additional cases of mammary angiosarcomas profiled at Caris Life Sciences (Phoenix, AZ) using RNA microarray, protein expression, gene copy analysis, and sequencing. These assays identified various gene/protein alterations indicating a potential response to various targeted and conventional treatments (Table 1).The possible utility of sunitinib as a targeted drug, based on alterations in VEGFR2, c-KIT, PDGFRA and PDGFRB 4, was observed in 44% of the cases (7/16). These findings concur with previous studies revealing VEGFR overexpression in angiosarcomas (3). Also, 11 of 16 cases (69%) overexpressed topoisomerase 2α protein indicating a potential therapeutic benefit of anthracyclines. Similarly, angiosarcomas exhibiting Topoisomerase 1α overexpression (7/16, 44%) may be targeted with Topo 1 inhibitors. Low thymidylate synthase levels are predictors of response to fluoropyrimidines were observed in five cases.
| Angiosarcoma | Alterations associated with sunitinib benefitb | Other markers with potential drug benefit (IHC) | Biomarkers associated with potential lack of benefit (IHC) |
|---|---|---|---|
| Case#1a | VEGFR2↑ | MGMT↓, TS↓, EPHA2↑b, SRC↑b |
Topo2α↓, Her2↓ Topo1α↓, RRM1↑ ER/PR/AR↓ |
| Case#2 | VEGFR2↑ | EPHA2↑b, SRC↑b, Topo2α↑ | Topo1α↓, Her2↓, RRM1↑ ER/PR/AR↓, TS↑ |
| Case#3 | PDGFRA↑ (IHC), c-Kit↑ (IHC) | SPARC↑ | Topo1α↓, Her2↓, RRM1↑ ER/PR/AR↓, MGMT↑, Topo2α↓ |
| Case#4 | VEGFR2↑ | Topo2α↑ | TS↑, ER/PR/AR↓, Her2↓ |
| Case#5 | PDGFRB↑, PDGFR↑ (IHC) | Topo2α↑ | ER/PR/AR↓, Her2↓ |
| Case#6 | c-Kit↑ (IHC) | Topo1α↑ | TS↑, RRM1↑, ER/PR/AR↓ Her2↓ |
| Case#7 | KRAS mutation (A146P)c | MGMT↓, Topo2α↑, Topo1α↑ | ER/PR/AR↓, Her2↓ |
| Case#8 | VEGFR2↑, PDGFR↑ (IHC) | TS↓ | Topo2α↓, MGMT↑ ER/PR/AR↓, Her2↓ |
| Case#9 |
None (c-KIT wild type)c |
MGMT↓, Topo1α↑ | TS↑, TLE3↓, ER/PR/AR Her2↓ |
| Case#10 | No relevant genes tested | PTEN↓ | ER/PR/AR↓, Her2↓, TLE3↓ |
| Case#11 |
None (c-KIT wild type)c |
Topo2α↑, TLE3↑ TUBB3↓ |
ER/PR/AR↓, Her2↓, TS↑ Topo1α↓ |
| Case#12 | No relevant genes tested | TS↓, Topo2α↑ | ER/PR/AR↓, Her2↓ Topo1α↓, MGMT↑, |
| Case#13 | Nonec |
Topo2α↑, Topo1α↑ TS↓ |
RRM1↑, MGMT1↑, ER↓ Her2↓ |
| Case#14 | VEGFR2↑ |
Topo2α↑, Topo1α↑ EPHA2↑b |
RRM1↑, TS↑, Her2↓ |
| Case#15 | Nonec | TUBB3↓, Topo2α↑ | TS↑, Topo1α↓, RRM1↑, MGMT1↑, Her2↓ |
| Case#16 | Nonec | Topo2α↑, Topo1α↑ MGMT↓, SPARC↑ | TUBB3↑, TS↑, Her2↓ |
| Case#17 | Nonec | TLE3↑, Topo2α↑ Topo1α↑, RRM1↓ | TS↑, MGMT↑, Her2↓ |
- a Described case.
- b Microarray assay.
- c By Sanger or Next-generation sequencing.
- ↑, upregulation/positivity; ↓, downregulation/low expression; AR, Androgen receptor; Topo1 &2, Topoisomerases 1 and 2; ER, Estrogen receptor; PR, Progesterone receptor; MGMT, O(6)-methylguanine-methyltransferase; TS, Thymidylate synthase; TLE3, Transducin-like enhancer of split 3; RRM1, Ribonucleotide reductase M1; SPARC, Osteonectin; TUBB3, Tubulin beta-3 chain; PDGFR, Platelet derived growth factor receptor; VEGFR, Vascular endothelial growth factor receptor; EPHA2, Ephrin type-A receptor 2; IHC, Immunohistochemistry.
Our study indicates that angiosarcomas of the breast are characterized by alterations of several angiogenic factors and tyrosine kinase receptors whose activity can be targeted by sunitinib. In addition, the benefits of multiplatform molecular profiling include information on tumor characteristics that could affect decision on treatments with conventional chemotherapy.




