Menopause, Menstrual Cycle, and Skin Barrier Function
Vedrana Karan Rakić and Milana Ivkov-Simić should be considered joint senior authors.
ABSTRACT
Background
There are a limited number of studies describing the impact of the menstrual cycle and postmenopause on the skin barrier function, and existing research data are conflicting. The aim of our research was to investigate the impact of the menstrual cycle and postmenopause on the epidermal barrier function and its main biophysical parameters—transepidermal water loss (TEWL) and skin hydration (SH).
Materials and Methods
Eighty-one female participants were included in the study, aged 18–65 years, of which 36 in the reproductive period (average age 27.06 ± 5.60 years) and 45 in postmenopause (average age 56.56 ± 4.37 years). TEWL and SH were measured during the ovulatory and mid-luteal phases in participants in the reproductive period, and on two occasions, 7 days apart, in posmenopausal participants.
Results
The mean TEWL value was significantly higher in the mid-luteal phase (TEWL 2; 9.92 ± 1.37) compared to the ovulatory phase (TEWL 1; 8.87 ± 1.59). However, no significant difference in TEWL was observed between the two groups of participants. The mean SH value was significantly higher in the ovulatory phase (SH 1; 40.55 ± 7.80) compared to the mid-luteal phase (SH 2; 36.27 ± 7.42). Moreover, SH in the ovulatory phase was significantly higher in comparison to the postmenopausal group (40.55:36.27; p = 0.009).
Conclusion
Our study indicates a more functional epidermal barrier during the ovulatory phase, as evidenced by higher TEWL values and lower SH values compared to the mid-luteal phase. However, the differences between the two participant groups remain intriguing, as no significant difference in TEWL was observed between them, despite significantly higher SH values in the ovulatory phase compared to the postmenopausal group.
1 Introduction
The function of the epidermal barrier of the skin, and its main biophysical parameters—transepidermal water loss (TEWL) and skin hydration (SH)—is still an active and prominent area of research [1-3]. Despite decades of research, the measurement of these parameters continues to demonstrate variability in results [1, 2, 4]. There are a limited number of studies describing the impact of the menstrual cycle and postmenopause on the epidermal barrier function, and its main indicators—TEWL and SH—and existing research data are conflicting.
In the epidermis, there is a water gradient, with the moisture content of the stratum corneum (SC) being lower than that of the deeper dermal layers [5-7]. As a result, there is passive diffusion of water from the inner layers towards SC [8]. The majority of the water evaporates from the skin surface, and this insensible loss of water, which occurs due to evaporation in the absence of active sweating, is referred to as TEWL [9]. However, a fraction of the water is retained within SC during water diffusion, representing the water content of SC and directly reflecting SH. In healthy skin, water concentration in SC is 15%–25%, whereas a lower percentage can indicate dry and dehydrated skin [5, 10]. A healthy skin with an intact epidermal barrier is characterized by a low TEWL level and normal hydration of the upper layers of the epidermis, whereas in skin conditions with impaired epidermal barrier (e.g., atopic dermatitis, keratinization disorders etc.) there is an increase in ТEWL combined with a decrease in SH [11]. As a result, various symptoms can occur, such as skin dryness, a lower irritation threshold, and pruritus, all of which can affect quality of life [1].
TEWL and SH values are influenced by endogenous, exogenous, environmental, and measurement/instrumentation factors [1]. The endogenous factors include age, gender, ethnicity, anatomical region of the body, skin temperature, sweating, skin health, and circadian rhythm [1, 2, 12]. Exogenous factors include skin washing and wet work, solvent/surfactants, occlusion, skin damage, smoking, and caffeine [1, 2, 12]. Environmental and measurement factors include air convection and movement, ambient air temperature, relative air humidity, direct light, and season [1, 13, 14].
Among the endogenous factors, female sex hormones, estrogen and progesterone, play a significant role and continue to pose challenges for researchers [14, 15]. Estrogen receptors have been identified in the skin, and estrogen is associated with numerous beneficial effects [16]. These include collagen synthesis, increased skin thickness, enhanced dermal water content through hyaluronic acid production, improved wound healing, melanogenesis, stimulation of keratinocyte differentiation, regulation of cell proliferation, and strengthened barrier function [16-18]. In contrast, the effects of progesterone are less well-defined. However, it is believed to exhibit anti-inflammatory and immunosuppressive properties, prevent collagen breakdown in the dermis (thus slowing skin aging), increase vascularity, and enhance sebum production [16, 17].
The cyclical fluctuations of female sex hormones during the menstrual cycle can lead to changes in skin structure and function, potentially impacting TEWL and SH [19]. Postmenopause adds another layer of complexity due to the significant decline in estrogen levels [20]. In this context, our research aimed to investigate the effects of the menstrual cycle and postmenopause on epidermal barrier function, offering insights into how hormonal changes might influence TEWL and SH measurements.
2 Materials and Methods
2.1 Participants
The research was performed at the Department of Physiology, Faculty of Medicine, University of Novi Sad. The research was previously approved by Ethics Committee of Faculty of Medicine, Novi Sad, University of Novi Sad (approval number: 01-39/244/1) and abided by the ethical guidelines of the Declaration of Helsinki. All participants signed an informed consent that acknowledged that participation in the study was voluntary and that withdrawal of consent would not result in any loss of benefit.
The inclusion criteria were as follows: 18–65 years of age; female; regular menstrual cycles lasting 25–31 days or diagnosed postmenopause; body mass index (BMI) 18.5–29.9 kg/m2; signed informed consent.
The exclusion criteria were as follows: irregular menstrual cycles or menstrual cycles lasting < 25 days or > 31 days; BMI ≥ 30 kg/m2; BMI < 18.5 kg/m2; personal history of allergies and/or inflammatory skin disease; clinically present acute and/or chronic skin disease; clinically present acute or chronic skin infection in the measurement region; clinically present acute infectious disease; skin pigmentation disorders in the measurement region; generalized hyperhidrosis; intense exposure to UV radiation 1 month prior to the start of the study; oral contraception or hormone replacement therapy (HRT); systemic retinoid therapy and/or systemic corticosteroid therapy within 6 months prior to the start of the study; application of topical therapy (e.g., corticosteroids, antihistamines, and immunomodulators) on the measurement region within 6 weeks prior to the start of the study; pregnancy; breastfeeding.
All participants underwent a detailed anamnesis interview and medical record review, followed by physical examination including full body skin exam, body weight (BW), and body height (BH) measurement. BW was measured using a medical scale (OMRON BF 214, Prizma Kragujevac) and BH using a stadiometer with an accuracy of 0.1 cm, after which BMI was calculated using the standardized formula BMI = BW/BH2 (kg/m2).
After applying the exclusion criteria, 81 female participants were included in the study, divided into two groups: (1) 36 participants in reproductive period (average age 27.06 ± 5.60 years, average BMI 22.03 ± 2.95 kg/m2); (2) 45 participants in postmenopause (average age 56.56 ± 4.37 years, average BMI 24.29 ± 5.05 kg/m2). The postmenopause was defined if 12 months of spontaneous amenorrhea had been observed based on a detailed anamnesis interview and medical record review. The most common skin type according to the Fitzpatrick scale was type II in both groups.
2.2 Protocol
The measurements were performed on two occasions. In participants in the reproductive period, the first measurement was obtained during the ovulatory phase, and the second during the mid-luteal phase of the menstrual cycle. Based on the length of the menstrual cycle, the beginning of the fertile days was determined (calendar method), after which the study participants used standardized urine test strips every day at the same time in order to determine the presence of luteinizing hormone (LH). A positive test, i.e., the presence of LH in the urine, indicates that ovulation will occur within 24 to 36 h, when there is maximum secretion of estrogen (ovulation phase) (Table 1) [21]. Within 24 h of confirmed LH presence in urine, the participants were tested for the first time. The second measurement was performed 7 days after ovulation, which corresponds to the mid-luteal phase when maximal progesterone secretion and secondary estrogen surge occur (Figure 1) [21].
| Variable (factor) | Reproductive (n = 36) | Postmenopause (n = 45) |
|---|---|---|
| Age (years) | 27.06 ± 5.60 | 56.56 ± 4.37 |
| BMI (kg/m2) | 22.03 ± 2.95 | 24.29 ± 5.05 |
| Cycle length (days) | 27.89 ± 1.37 | Not applicable |
| Menopause onset age (years) | Not applicable | 48.51 ± 5.27 |
| Postmenopause duration (years) | Not applicable | 8.04 ± 5.21 |
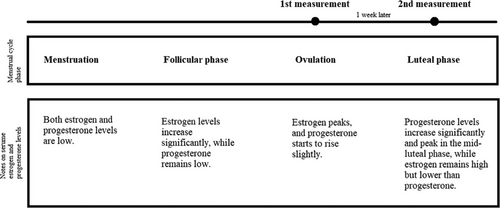
In the case of the participants in postmenopause, the measurements were also performed on two occasions, with an interval of 7 days between the two measurements.
On both occasions, the participants underwent TEWL and SH measurement and self-assessment of skin dryness intensity, pruritus intensity, and skin sensitivity. Both TEWL and SH were measured following current guidelines and protocols for in vivo measurement of these two parameters in clinical conditions [1, 9, 22]. TEWL was measured using Tewameter TM 300 (Courage + Khazaka electronic GmbH), which measures the gradient of water evaporation density from the skin indirectly using two pairs of sensors (for temperature and relative humidity) inside a hollow cylinder. This is an “open chamber” method that estimates TEWL continuously without affecting its microenvironment. Measured values express the evaporation rate in g/h/m2 [2]. SH was measured using Corneometer CM 825 (Courage + Khazaka electronic GmbH). The measurement is based on measuring the capacitance of the dielectric medium, specifically SC, i.e., the most superficial layer of the epidermis. The measured values are expressed in arbitrary units (au) [22].
Skin dryness intensity and skin itchiness intensity were self-assessed on a visual analog scale (VAS), whereas the skin sensitivity was assessed via the Sensitive Scale-10 (SS-10) questionnaire, which consists of a list of 10 symptoms that characterize sensitive skin. The participants had the task of quantifying nine symptoms within the last 3 days (stinging, burning, sensations of heat, tautness, itchiness, pain, skin discomfort, flushes, and redness) individually with a score from 0 (no intensity) to 10 (unbearable intensity) on a numerical scale, and the tenth—skin irritability on a VAS. The final score of SS-10 varied between 0 and 100.
2.3 Statistics
After data collection, appropriate statistical processing and analysis were conducted. Among the descriptive statistical methods, measures of central tendency were used (arithmetic mean with standard deviation or median with minimum and maximum value). To test the statistical significance of categorical variables, the χ2-test of independence or Fisher's exact probability test was used if in certain fields of the contingency table the frequency was less than or equal to 5. For testing statistical hypotheses, Chi-square test, Student's T-test, and Mann–Whitney test were used, as well as mixed analysis of variance (mixed between-within subjects ANOVA). Pearson's or Spearman's correlation test was used for correlation testing. SPSS, version 26 (IBM SPSS, Armonk, New York, USA) and Microsoft Excel 2019 (Microsoft, Redmond, Washington, USA) programs were used for statistical data processing. Alpha level values of p < 0.05 were considered statistically significant.
3 Results
The main characteristics of participants are summarized in Table 1. The average age of participants in the reproductive period was 27.06 ± 5.60 years, and the average BMI was 22.03 ± 2.95 kg/m2. The average age of participants in postmenopause was 56.56 ± 4.37 years, and the average BMI was 24.29 ± 5.05 kg/m2. The most common menstrual cycle length among participants in the reproductive period was 28 days, with cycles ranging from 25 to 30 days. The mean age when menopause occurred was 48.51 ± 5.27 years, and the mean postmenopause duration was 8.04 ± 5.21 years.
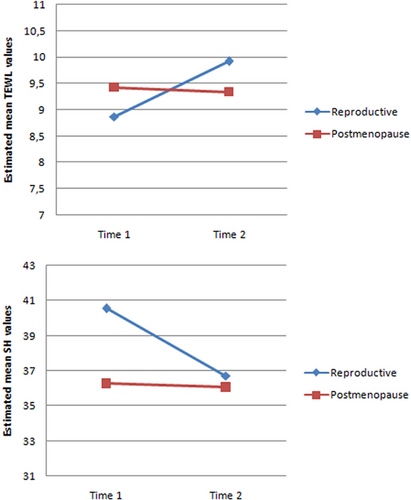
As presented in Table 2, there was a statistically significant difference in TEWL values between the ovulatory and luteal phases in the reproductive group (t = −5.461; p < 0.001). The mean TEWL value was significantly higher during the luteal phase (TEWL 2; 9.92 ± 1.37) compared to the ovulatory phase (TEWL 1; 8.87 ± 1.59). In the postmenopausal group, there was no statistically significant difference in TEWL values between the two successive measurements (t = 0.969; p = 0.338). In the reproductive group, there was also a statistically significant difference in SH values between the ovulatory and luteal phases (t = 3.011; p = 0.005). The mean SH value was significantly higher during the ovulatory phase (SH 1; 40.55 ± 7.80) compared to the luteal phase (SH 2; 36.27 ± 7.42). In the postmenopausal group, there was no statistically significant difference in SH values between the two successive measurements (t = 0.435; p = 0.666).
| Group | Variable (factor) | SD | SEM | p value | |
|---|---|---|---|---|---|
| Reproductive (n = 36) | TEWL 1 | 8.87 | 1.59 | 0.26 | |
| TEWL 2 | 9.92 | 1.37 | 0.23 | < 0.001 | |
| SH 1 | 36.27 | 6.01 | 0.90 | ||
| SH 2 | 36.72 | 7.42 | 1.24 | 0.005 | |
| Postmenopause (n = 45) | TEWL 1 | 9.43 | 2.13 | 0.32 | |
| TEWL 2 | 9.34 | 2.04 | 0.30 | 0.338 | |
| SH 1 | 36.27 | 6.01 | 0.90 | ||
| SH 2 | 36.06 | 6.15 | 0.92 | 0.666 |
Figure 2 shows a significant interaction between groups and time, indicating that the TEWL and SH values changed differently over time in each group. However, when examining TEWL differences between the two groups, no statistically significant difference was observed in either the first measurement (8.87:9.43; p = 0.191) or in the second measurement (9.92:9.34; p < 0.149). As for SH, a significant difference between the two groups was observed in the first measurement (40.55:36.27; p = 0.009), but not in the second measurement (36.72:36.06; p = 0.673).
The repeated measures analysis was used to evaluate differences between the parameter values from the two measurements are presented in Table 3. In the postmenopausal group, the mean value of skin dryness and skin itchiness was significantly higher in the second measurement, while the mean value of skin sensitivity was higher in the first measurement. On the other hand, in the reproductive group, no significant differences were observed when comparing the parameter values between the ovulatory and luteal phases. When comparing the groups in both measurements, the postmenopausal group showed a lower value for skin dryness (Dryness 1) in the first measurement (t = 2.833; p = 0.006; 3.51 ± 2.53:1.91 ± 2.52), and a lower value for skin sensitivity (SS 2) in the second measurement (t = 5.707; p < 0.001; 10.00 ± 8.31:1.80 ± 2.53). Conversely, a statistically higher value for the subjective feeling of skin itchiness was observed in the postmenopausal group compared to the reproductive group in both the first (Itch 1) (t = −6.675; p < 0.001; 1.62 ± 1.99:5.36 ± 3.04) and second measurements (Itch 2) (t = −5.747; p < 0.001; 1.86 ± 1.58:9.64 ± 8.92).
|
Reproductive (n = 36) |
Postmenopause (n = 45) |
||||
|---|---|---|---|---|---|
| SD | SD | p value | |||
| Dryness 1 | 3.51 | 2.53 | 1.91 | 2.52 | 0.006 |
| Dryness 2 | 3.52 | 2.34 | 4.70 | 2.99 | 0.057 |
| p value | 0.962 | < 0.001 | |||
| SS 1 | 10.75 | 12.47 | 10.87 | 9.97 | 0.962 |
| SS 2 | 10.00 | 8.31 | 1.80 | 2.53 | < 0.001 |
| p value | 0.601 | < 0.001 | |||
| Itchiness 1 | 1.62 | 1.99 | 5.36 | 3.04 | < 0.001 |
| Itchiness 2 | 1.86 | 1.58 | 9.64 | 8.92 | < 0.001 |
| p value | 0.378 | 0.001 | |||
Correlation analysis of TEWL, SH and subjective parameters in both groups (Table 4)revealed the following in the first measurement: a statistically significant negative correlation between SH and the subjective feeling of skin itchiness (r = −0.238; p = 0.033; small effect size), and positive correlations between skin sensitivity and both skin itchiness (r = 0.398; p < 0.001; medium effect size) and skin dryness (r = 0.483; p < 0.001; medium effect size). In the second measurement, there was a statistically significant negative correlation between skin dryness and SH (r = −0.441; p < 0.001; medium effect size), and a positive correlation between skin dryness and skin itchiness (r = 0.415; p < 0.001; medium effect size).
| TEWL 1 | SH 1 | SS 1 | Itchiness 1 | Dryness 1 | ||
|---|---|---|---|---|---|---|
| TEWL 1 | ra | 1 | −0.069 | −0.053 | 0.025 | −0.063 |
| Sig.b | 0.541 | 0.640 | 0.826 | 0.576 | ||
| SH 1 | R | 1 | 0.089 | −0.238* | −0.173 | |
| Sig. | 0.431 | 0.033 | 0.122 | |||
| SS 1 | r | 1 | 0.398** | 0.483** | ||
| Sig. | 0.000 | 0.000 | ||||
| Itchiness 1 | r | 1 | 0.150 | |||
| Sig. | 0.181 | |||||
| Dryness 1 | r | 1 | ||||
| Sig. | ||||||
| TEWL 2 | SH 2 | SS 2 | Itchiness 2 | Dryness 2 | ||
|---|---|---|---|---|---|---|
| TEWL 2 | r | 1 | −0.010 | 0.069 | −0.112 | −0.106 |
| Sig. | 0.929 | 0.542 | 0.319 | 0.346 | ||
| SH 2 | r | 1 | −0.008 | −0.025 | −0.441** | |
| Sig. | 0.941 | 0.827 | 0.000 | |||
| SS 2 | r | 1 | −0.129 | 0.167 | ||
| Sig. | 0.253 | 0.136 | ||||
| Itchiness 2 | r | 1 | 0.415** | |||
| Sig. | 0.000 | |||||
| Dryness 2 | r | 1 | ||||
| Sig. | ||||||
- Note: The symbols * and ** indicate statistical significance levels for correlations. A single asterisk (*) denotes p < 0.05, meaning there is less than a 5% chance the result is due to random variation. A double asterisk (**) denotes p < 0.01, indicating stronger significance with less than a 1% probability the result occurred by chance.
- a Pearson correlation coefficient.
- b Significance level.
4 Discussion
A limited number of studies describe the impact of the menstrual cycle and postmenopause on the function of the epidermal skin barrier and the existing data are conflicting. TEWL and SH are significant indicators of the epidermal biophysical properties, and their mutual relationship depends on the condition of the skin [13, 22]. Our study showed significantly higher TEWL in the mid-luteal phase compared to the ovulatory phase. As for SH mean values, they were significantly lower in the mid-luteal phase compared to the ovulatory phase. This is consistent with the data proving a correlation between changes in the skin barrier and changes in the level of the main estrogen-estradiol during the menstrual cycle [23-25]. It is considered that there is increased permeability of the skin barrier in the mid-luteal phase (between days 22 and 26 of the menstrual cycle), when there is minimal estrogen/progesterone secretion ratio, in comparison to the period of maximum estrogen secretion (around day 13 of the menstrual cycle) [24, 25]. Therefore, it is assumed that estradiol has a protective effect on the skin barrier, which is opposed by the effect of progesterone, although the precise mechanism of action of progesterone on the epidermal barrier has not been clarified [26-28]. This is in accordance with data that impaired barrier function or increased skin sensitivity occurs during the second phase of the menstrual cycle when both progesterone and estrogen are secreted, but also in menopause [26, 29, 30]. Contrary to our assumption, our study showed no significant differences in TEWL between postmenopausal participants and those in the reproductive period during both phases of the menstrual cycle. A study investigating the effects of menopause, comparing TEWL of several anatomical regions between young and middle-aged women and postmenopausal women, also showed no significant differences [31].
Regarding SH, a significant difference was observed in the first measurement between participants in the ovulatory phase of their reproductive period and those in postmenopause. This difference favored the ovulatory phase group, confirming the role of estrogen in maintaining adequate water content in SC. Low estrogen levels in postmenopause cause collagen atrophy and reduced water content [32, 33]. Research involving postmenopausal women has demonstrated that over 60% report experiencing a variety of skin issues during perimenopause and menopause. These issues include skin laxity, dryness, rhytids, diminished skin vigor, hyperpigmentation, exacerbation of dark circles, and reduced skin thickness [34-36]. HRT appears to have a notable effect on skin quality and provides cosmetic benefits, especially for women who have never used it [36]. HRT can alleviate menopausal symptoms and has a well-documented positive impact on dermal collagen density, thickness, and content quality [37]. Furthermore, transdermal HRT with estrogen has been shown to significantly increase the water-holding capacity of SC, suggesting that estrogen may play a role in its barrier function [27]. Postmenopausal women not taking HRT are more likely to have dry skin compared to women taking estrogen [27].
Our study showed no significant difference in the second measurement between the luteal phase of participants in reproductive period and the participants in postmenopause. A possible explanation could be the opposite effect of progesterone, raising the question of a potentially similar estrogen/progesterone ratio in the luteal phase and postmenopause.
In addition, hormonal pH changes associated with the menstrual cycle may be an important factor in the aggravation of itch as increasing pH is known to activate the proteinase-activated receptor-2, a well-known itch mediator [38]. The results of our study support this, since the values for the subjective feeling of skin itchiness and dryness were higher in the luteal phase in comparison to the ovulatory phase, although these differences were not statistically significant. Moreover, postmenopausal participants showed significantly higher values of the subjective feeling of skin itchiness compared to both phases of the menstrual cycle.
In the luteal phase, there was a significant negative correlation between skin dryness and SH, as well as a positive correlation between skin dryness and skin itchiness. This may correlate with research suggesting the onset and exacerbation of various dermatoses that occur at the peak levels of progesterone in the menstrual cycle. Dermatoses that exacerbate perimenstrually include acne, psoriasis, atopic eczema, irritant dermatitis, and erythema multiforme. Possible underlying mechanisms include reduced immune and barrier function resulting from cyclical fluctuations in estrogen and/or progesterone [32]. As for the postmenopausal group, statistically higher values of skin itchiness (unlike the skin sensitivity and dryness) were observed in comparison to reproductive group regardless of phase. This finding aligns with our expectations, given the data describing pruritus as frequently seen symptom in postmenopausal women, commonly associated with xerosis [37]. However, it should be noted that, contrary to our expectations, we observed statistically significant difference between the subjective parameter values between the two measurements in the postmenopausal group. This points to a potential source of bias in our study, as all symptoms were self-assessed and therefore may have depended on subjective perceptions and/or motivation to fulfill the given task.
The findings of our study should be interpreted in light of several additional limitations. Primarily, no direct measurements of plasma estrogen and progesterone were performed due to the limited budget. Direct assessment of these hormones, in conjunction with the calendar method and urinary LH kit for ovulation prediction, would reveal correlations between hormone levels and TEWL, as well as between hormone levels and SH. This would enhance the relevance and strengthen the conclusions of our findings.
The second limitation pertains to the relatively brief duration of our study, which relied on only two assessments. This hinders our ability to understand longitudinal changes of SH and TEWL, particularly those associated with different postmenopausal stages and physiological conditions. Population-based research indicates that mean estradiol levels remain stable until approximately 2 years before the final menstrual period, then decrease by 67% over the subsequent 4 years [39, 40]. Notably, significant hormonal shifts also occur during pregnancy, with rising estrogen and progesterone levels potentially influencing skin barrier function, water-binding capacity, and fluid retention [41, 42].
Furthermore, our study included only female participants who were not undergoing HRT or using hormonal contraceptives. This criterion ensured the absence of external hormonal influences. However, a control group of participants using HRT and/or contraceptives could strengthen the results, as transdermal HRT and estrogen-containing oral contraceptives have been shown to affect SH and TEWL [27, 37, 40, 43, 44]. Moreover, analyzing cutaneous manifestations of internal hormonal influences, such as endocrine disorders, could provide valuable insights into potential correlations between hormonal imbalances and skin barrier function [45, 46]. Although our primary goal was to focus specifically on female subjects and the impact of the menstrual cycle and postmenopause on TEWL and SH, including a male control group and examining sex-based skin differences would also strengthen the validity of the results and address existing questions regarding inconsistencies in TEWL and SH between sexes [47, 48].
5 Conclusion
Our study indicates that the epidermal barrier is more functional during the ovulatory phase, as evidenced by higher TEWL values and lower SH values compared to the mid-luteal phase. Interestingly, despite significantly higher SH values in the ovulatory phase compared to the postmenopausal group, no significant difference in TEWL was observed between the two groups.
In conclusion, our findings provide partial insight into the effects of menstrual cycle phases and postmenopause on TEWL and SH, which may help reduce measurement variability in future research on epidermal barrier function. Future studies incorporating factors such as sex hormones, postmenopausal hormone therapy, sex differences, and endocrine diseases could further minimize variability and enhance the validity of results.
Acknowledgments
The authors have nothing to report.
Ethics Statement
Study approval statement: This study protocol was reviewed and approved by Ethics Committee of Faculty of Medicine, Novi Sad, University of Novi Sad (approval number: 01–39/244/1).
Consent to participate statement: All subjects signed an informed consent that acknowledged that participation in the study was voluntary and that withdrawal of consent would not result in any loss of benefit.
Conflicts of Interest
The authors have no conflicts of interest to declare.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




