C10orf99 regulates angiogenesis by regulating VEGF and MMP2 in psoriasis
Abstract
Background
Psoriasis is a common chronic inflammatory skin disease characterized by epidermal hyperplasia, inflammatory cell infiltration and excessive neovascularization. It was previously found that C10orf99 regulates keratinocyte proliferation and inflammation in psoriasis. However, the role of C10orf99 on angiogenesis associated with psoriasis is unknown.
Methods
We used imiquimod (IMQ)-induced psoriasis mice and M5-induced cellular model of psoriasis to research the effects of C10orf99 knockdown on angiogenesis in psoriasis in vivo and in vitro.
Results
In this study, our data showed that C10orf99 knockdown ameliorated imiquimod (IMQ)-induced red plaques in mice and reduced the number of microvessels. Furthermore, we found such an effect was achieved by down-regulating the expression of vascular endothelial growth factor (VEGF) and metallomatrix proteinase 2 (MMP2). Consistently, C10orf99 knockdown significantly downgraded the expression of MMP2 and VEGF in cellular model of psoriasis.
Conclusion
These results suggested that C10orf99 could regulate angiogenesis in psoriasis and it might be a promising potential target of psoriasis.
1 INTRODUCTION
Psoriasis is a chronic autoimmune skin disease which seriously affects the quality of patients’ life.1, 2 The prevalence is approximately 2%−4% worldwide.1 Its typical histological features include the infiltration immune cells, epidermal hyperproliferation and aberrant angiogenesis.3 Angiogenesis plays a crucial role in the onset and development of psoriasis and anti-angiogenic therapy may be an valid approach to treat psoriasis.4-6
C10orf99 is a human antimicrobial peptide ubiquitously expressed in epithelial tissues.7, 8 We previously demonstrated that C10orf99 protein was significantly up-regulated in lesional skin samples of psoriatic patients and it could contribute to the development of psoriasis by promoting the keratinocyte proliferation via ERK1/2 and NF-κB pathway.9 In addition, C10orf99 has been reported to regulate inflammatory response of keratinocytes.10 However, the role of C10orf99 on angiogenesis associated with psoriasis is unknown.
In the current study, we investigated the effects of C10orf99 knockdown on IMQ-induced angiogenesis in mice and M5-induced cellular model of psoriasis.
2 MATERIALS AND METHODS
2.1 Cell culture
Human keratinocyte cell line HaCaT cells were cultured in DMEM containing 10% FBS and 1% penicillin-streptomycin in a 5% CO2 incubator at 37°C.
2.2 Induction of psoriatic model in vitro
HaCaT cells were stimulated with 10 ng/mL recombinant TNF-α, IL-17A, IL-22, IL-1α, and OSM (Peprotech, USA) in combination (named M5 cytokines cocktail) to establish the cellular model of psoriasis.
2.3 Lentivirus transduction
The oligonucleotides of shRNAs were listed in Table 1. Cells were incubated with 100 µL of the virus suspension (titer 1 × 108 TU/mL) for 48 h and puromycin (5 mg/mL) was used to screen the stable infected cells.
| Name | Sequences |
|---|---|
| siRNA-1 |
forward 5′-GACAUCAUGUGAGGCUCUGUATT-3′ reverse 5′-UACAGAGCCUCACAUGAUGUCTT-3′ |
| siRNA-2 |
forward:5′-CAUCUUCUCCACAGAAGGGAATT-3′ reverse: 5′-UUCCCUUCUGUGGAGAAGAUGTT-3′ |
| siRNA-3 |
forward: 5′- GCCAUCAACUUUCAGAGCUAUTT-3′ reverse: 5′- AUAGCUCUGAAAGUUGAUGGCTT-3′ |
| si-NC |
forward:5′-UUCUCCGAACGUGUCACGUTT-3′ reverse: 5′-ACGUGACACGUUCGGAGAATT-3′ |
2.4 RNA preparation and qRT-PCR analysis
Total RNA was extracted using TRIzol (CWBIO) according to the Manufacturer's protocol. qRT-PCR was carried out with SYBR qPCR Master Mix (Vazyme). The following primer sequences in Table 2 were used in the experiment.
| Gene name | Primer sequence (5′−3′) |
|---|---|
| GAPDH |
forward 5′-TCAACGGCACAGTCAAGG-3′ reverse 5′-TGAGCCCTTCCACGATG-3′ |
| VEGF |
forward 5′-AGACAGAACAAAGCCAGAAAATCAC-3′ reverse 5′-CACGTCTGCGGATCTTGGA-3′ |
| MMP-2 |
forward 5′-GCAGGAGACAAGTTCTGGAGATACA-3′ reverse 5′-ACGACGGCATCCAGGTTATCA-3′ |
2.5 Western blot
Western blot was performed as described previously.9 Primary antibody including anti-β-actin (TA-09), anti MMP-2 (YT2798), anti VEGF (Sc-7269) were used.
2.6 Experimental animals and study design
BALB/c female mice at 8 weeks of age were given free access to food and water. Mice were randomly separated into four groups (six mice per group): control group (Ctr), IMQ group (IMQ), IMQ + sh-NC group (IMQ+sh-NC), IMQ + shRNA group (IMQ+sh-C10orf99). Mice in control group were applied with vaseline cream and others were topically applied with 62.5 mg of IMQ cream (5%, Mingxin, Chengdu, China) on the dorsal shaved back for 6 days. Mice in IMQ + sh-NC group and mice in IMQ + sh-C10orf99 group were injected intradermally with the corresponding adenovirus particles as previously described.9 At the 7th day, mice were sacrificed and the back skin samples were collected for subsequent detections. This study was approved by the Animal Experimental Ethics Committee of Fujian Provincial Hospital.
2.7 Clinical erythema score
Erythema of mouse back skin was scored independently from 0 to 4: 0, none; 1, slight; 2, moderate; 3, severe; 4, very severe.
2.8 Histological evaluation and immunohistochemistry
Skin tissues were fixed in formalin immediately after removal. About 5 µm-thickness cross-sections were stained with H&E staining. For immunohistochemical staining, sections were incubated with specific primary antibodies against MMP-2 (YT2798), VEGF (Sc-7269) and CD31(YT0752). Then observed the number of microvessels under a microscope.
2.9 Statistical analysis
Data were expressed as mean ± SEM. All analysis were performed with GraphPad Prism 5.0. Comparisons between two groups were carried out by student's t test. P < 0.05 was considered significant.
3 RESULTS
3.1 C10orf99 knockdown ameliorated IMQ-induced psoriatic erythema in mice
Initially we evaluated the effect of C10orf99 knockdown on IMQ-induced psoriatic erythema in mice. As showed in Figure 1A, IMQ treatment induced erythematous plaques on mice back. Erythema score of the IMQ group increased compared with the control group (Figure 1B). The erythema score of the sh-C10orf99 group was significantly lower than the sh-NC group, demonstrating C10orf99 knockdown considerably attenuated IMQ-induced psoriatic erythema.
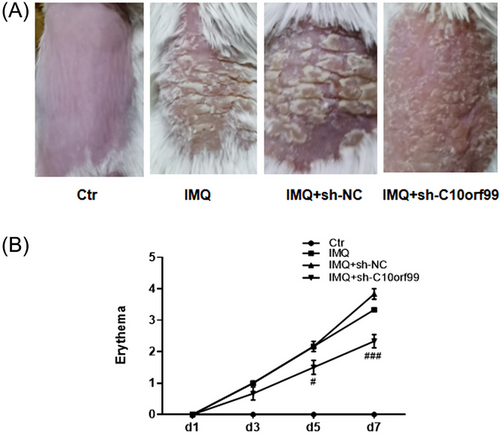
3.2 Effect of C10orf99 knockdown on angiogenesis in histopathological analysis
In Figure 2A, HE examination demonstrated that IMQ treatment induced a marked vessel formation as compared to the controls. We further evaluated CD31 expression and the microvessel density (MVD) by immunohistochemistry (Figure 2B). The MVD in the psoriatic mice was increased compared with that in the control group. Conversely, C10orf99 knockdown reduced the neovascularization and MVD in psoriatic mice (Figure 2B and C).
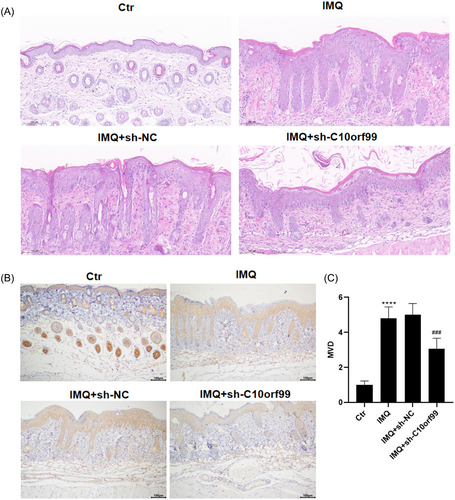
3.3 C10orf99 knockdown reduced IMQ-induced VEGF expression in the mouse skin
VEGF is an important proangiogenic factor in angiogenesis. The result of immunohistochemistry suggested that the expression of VEGF was significantly higher in the psoriatic mice and C10orf99 knockdown reduced IMQ-induced VEGF expression in mice (Figure 3A–C).
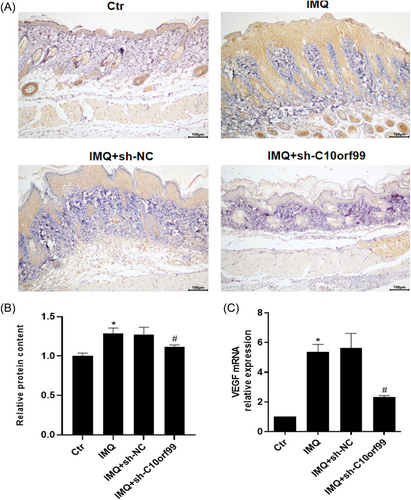
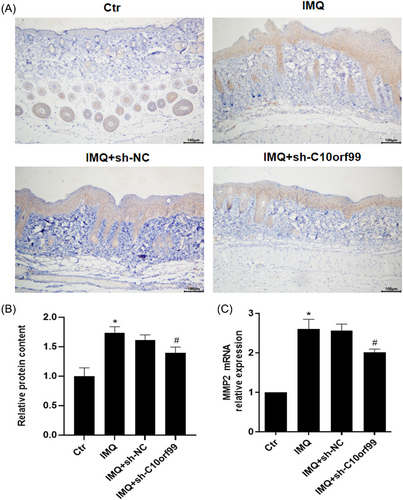
3.4 C10orf99 knockdown reduced IMQ-induced MMP2 expression in the mouse skin
MMP2 is involved in the angiogenic process. Therefore, we further evaluated the effect of C10orf99 knockdown on MMP-2 expression. Immunohistochemical analysis showed IMQ significantly induced MMP2 expression compared to control mice (Figure 4A and B). Meanwhile, C10orf99 knockdown significantly reduced MMP2 expression. This was also confirmed by MMP-2 mRNA expression by qRT-PCR (Figure 4C).
3.5 Effects of C10orf99 knockdown on the production of MMP2 and VEGF in M5-stimulated keratinocytes
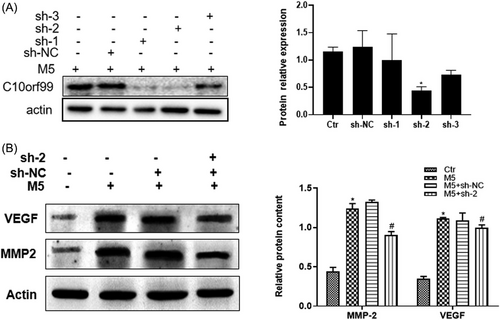
M5 cytokines cocktail was used to treat HaCaT cells to induce psoriasis inflammatory milieu. Firstly, HaCaT cells were transfected with lentivirus system to knockdown the expression of C10orf99. Western blot result showed the effective down-regulation of C10orf99 by shRNA-2 (Figure 5A). The western blot results showed M5 significantly induced the expression of VEGF and MMP2 in HaCaT cells, whereas C10orf99 knockdown decreased VEGF and MMP2 expression under conditions of M5 stimulation (Figure 5B).
4 DISCUSSION
In this study, we investigated the role of C10orf99 in psoriasis-associated angiogenesis. Our data showed that C10orf99 knockdown ameliorated IMQ-induced angiogenesis in psoriatic mice. Furthermore, C10orf99 knockdown significantly down-regulated levels of MMP2 and VEGF in psoriatic skin and M5-treated HaCaT cells in vitro.
Abnormal microangiogenesis of skin lesions is a major histological feature of psoriasis and it is closely related to the occurrence and development of psoriasis.3, 4, 9 C10orf99 has been noticed for its role in inflammation and cell proliferation.10-15 It has been reported that C10orf99 promoted angiogenesis in the process of wound healing.16 However, the relevance between C10orf99 and angiogenesis in psoriasis still remains unclear. In current study, we found that C10orf99 knockdown could significantly attenuate IMQ-induced psoriasis-associated angiogenesis in mice.
The angiogenesis in psoriasis is regulated by several angiogenic mediators, such as VEGF and MMPs.4, 5, 17, 18 VEGF is the most important proangiogenic factor and it's role in angiogenesis has been well known.19, 20 Studies showed VEGF is up-regulated in the psoriatic lesions and serum and VEGF expression is positively related to the severity of psoriasis.21-24 In our study, we confirmed the level of VEGF is elevated in mice psoriatic skin tissues. We found that C10orf99 knockdown could reduce IMQ-induced VEGF expression in mice. Moreover, C10orf99 knockdown suppressed the expression of MMP2 in skin of IMQ-treated mice.
It has been reported that keratinocyte is the major source of pro-angiogenic mediators in psoriasis.18 Thus, we established the psoriatic cell model with M5 and explore the effect of C10orf99 knockdown on the production of MMP2 and VEGF in vitro. Firstly, we found that M5 could significantly promote the expression of VEGF and MMP2 in HaCaT cells. Additionally, we found that C10orf99 knockdown suppressed the expression of VEGF expression and MMP2 expression in M5-stimulated HaCaT cells.
In summary, our findings demonstrated that C10orf99 contributed to the increased vascularization by regulating VEGF expression and MMP2 expression in psoriasis. Thus, C10orf99 is involved in the pathogenesis of psoriasis by regulating keratinocyte proliferation, inflammation and angiogenesis, and it may be a therapeutic target for clinical treatment of psoriasis. However, further investigations are required to elucidate the molecular mechanisms.
ACKNOWLEDGMENTS
This work was supported by Natural Science Foundation of Fujian province (2020J05262), National Natural Science Foundation of China (81903220) and joint funds for the innovation of science and technology of Fujian province (2020Y9022).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this article.




