Low-level laser activates Wnt/β-catenin signaling pathway-promoting hair follicle stem cell regeneration and wound healing: Upregulate the expression of key downstream gene Lef 1
Jiang BangHong, Wang YuKun and Shi Ao contributed equally to this work as co-first authors.
Abstract
Background
The objective of this study is to investigate the mechanism by which low-level laser stimulation promotes the proliferation of intraepithelial hair follicle stem cells (HFSCs) in wounds. This research aims to expand the applications of laser treatment, enhance wound repair methods, and establish a theoretical and experimental foundation for achieving accelerated wound healing.
Methods
The experimental approach involved irradiating a cell model with low-level laser to assess the proliferation of HFSCs and examine alterations in the expression of proteins related to the Wnt/β-catenin signaling pathway. A mouse back wound model was established to investigate the effects of low-level laser irradiation on wound healing rate, wound microenvironment, and the proliferation of HFSCs in relation to the Wnt/β-catenin signaling pathway.
Results
The research findings indicate that low-level laser light effectively activates the Wnt signaling pathway, leading to the increased accumulation of core protein β-catenin and the upregulation of key downstream gene Lef 1. Consequently, this regulatory mechanism facilitates various downstream biological effects, including the notable promotion of HFSC proliferation and differentiation into skin appendages and epithelial tissues. As a result, the process of wound healing is significantly accelerated.
Conclusion
Low levels of laser activates the Wnt signalling pathway, promotes the regeneration of hair follicle stem cells and accelerates wound healing.
1 INTRODUCTION
As the prevalence of soft tissue injuries resulting from external forces or inadequate patient nutrition continues to rise, there is a growing need for more effective interventions in the management of post-traumatic scar formation. Despite significant advancements in regenerative medicine, the inability of humans to regenerate skin remains a persistent challenge in the field of wound healing.1 Furthermore, the absence of epidermal-derived organs, including hair follicles and sweat glands, in scar tissue further complicates the process of wound repair. However, it should be noted that not all mammalian wounds in nature exhibit regression through scar healing.2, 3 In a study conducted in 2012, Seifert4 discovered that following skin avulsion in African spiny mice, hair follicle stem cells (HFSCs) exhibited the ability to facilitate the regeneration of epithelium and appendages, resulting in scar-free healing. This finding suggests the potential for scarless wound healing in humans. Subsequent research has increasingly demonstrated that hair follicles harbor stem cells capable of multidirectional differentiation, enabling them to divide and proliferate into various epidermal cells. This process effectively replenishes the shedding and loss of epithelial cells.5-9 Consequently, HFSCs have gained prominence as a promising source for regenerative repair of epithelial tissues in recent years. Investigating how to promote scar healing through follicular stem cell proliferation is of great importance.
The laser possesses several advantageous characteristics, including high brightness, high monochromaticity, strong directionality, and good coherence. Consequently, it has found extensive application in the medical field. The utilization of lasers in clinical medicine can be categorized into two types: high-level laser therapy and low-level laser therapy (LLLT). The latter involves the use of lasers with an output power in the milliwatts range, a wavelength range typically spanning from 600 to 1100 nm, an energy density ranging from 1 to 10 J/cm2, and a power density ranging from 3 to 90 mW/cm. It does not cause irreversible damage to biological tissue and does not cause a significant increase in local temperature while it can improve blood circulation, promote cell regeneration, hair growth, wound healing, tissue repair and inhibit fibrosis. Now LLLT has become a popular treatment in plastic and reconstructive surgery. Research studies indicate that when LLLT is used to treat non-scarring alopecia areata, the hair follicle structure remains intact in the skin and LLLT has the potential to stimulate dormant HFSCs, thereby facilitating hair regrowth.10-12 However, the impact of LLLT on HFSC activation in post-traumatic wounds, as well as the specific wavelength, energy range, and mechanism of action involved, are yet to be fully understood.13-15
Numerous studies have demonstrated the significant involvement of the Wnt/β-catenin signaling pathway in the control of self-renewal and differentiation of tissue stem cells. Subsequent investigations have revealed that targeted suppression of the CTNNBl gene in mouse skin tissues prevents the initiation of hair follicle morphogenesis by skin stem cells. However, these cells are capable of forming a “dermalcyst” exhibiting characteristics of epidermal cell differentiation, albeit lacking certain markers associated with keratinocytes in hair follicles. Additionally, previous studies have demonstrated that the specific and stable expression of β-catenin in epidermal cells of transgenic mice leads to the manifestation of hypertrichosis and the formation of pilomatricomas, which are benign tumors composed exclusively of hair matrix and hair cells. These observations strongly imply that the Wnt signaling pathway plays a crucial role in the induction of differentiation in skin stem cells, ultimately resulting in the development of tissue structures like hair follicles.16-18 Based on a synthesis of previous research, we posit that the proliferation and differentiation of HFSCs play a crucial role in the regeneration of skin appendages and epithelial tissues, facilitating the restoration of normal epithelium and hair growth in the healing of skin soft tissue trauma. This regenerative process is concomitant with the activation of the Wnt signaling pathway. Furthermore, LLLT promotes the proliferation of HFSCs through the regulation of Wnt signaling pathway overexpression. We conducted in vivo and in vitro experiments respectively, to elucidate the mechanism of low-level laser stimulation of follicular stem cell proliferation in trabecular epithelium by detecting the expression of β-catenin, a core protein of Wnt signaling pathway, Lef1, a key downstream gene, Cytokeratin-19 (CK19) and Ki67, a marker of follicular stem cells, in the presence and absence of low-level laser treatment.
2 MATERIALS AND METHODS
2.1 Laser treatment
The experiment uses a 670 nm LED array as LLLT source. (670 ± 10 nm GaAlAs LED array; Philips; The Netherlands). In the in vitro experiment, the cultured HFSCs were divided into four groups based on the presence or absence of low-level laser irradiation and DKK1 intervention(Dickkopf-related protein 1, Antagonism of Wnt/β-catenin signaling pathway activity by competitive binding of LRP5/6 receptors to Wnt proteins). These groups included the blank control group (Con group), the blank control + inhibitor group (Con + DKK1 group), the laser treatment group (LT group), and the laser treatment + inhibitor group (LT + DKK1 group). The concentration of DKK1 used was 400 ng/mL. In vivo experiments, animal models of trauma were constructed by designing skin wounds on the back of mice, and the mice were divided into a blank control group (Con group) and a laser treatment group (LT group) according to the presence or absence of low-level laser treatment. The laser treatment group placed a 670 nm LED array 2–3 cm directly above the cell or wound surface for 120 s at an irradiance of 60 mW/cm2 once a day. Each treatment administered a radiation exposure dose of 4 J/cm2. In the blank control group, the LED array was positioned above the wound of the mice for a duration of 120 s. Following a treatment period of 14 days, Western blot, HE staining, and Masson staining techniques were employed to assess the expression of the core protein β-catenin in the Wnt signaling pathway, the downstream key gene Lef1, the HFSCs marker CK19, and the cell proliferation marker Ki67.
2.2 Isolation and culture of HFSCs
The mice were submerged in a beaker containing 75% ethanol and subsequently transferred to a meticulously sterile workbench. Afterwards, the mice were positioned in a supine position and the intact skin surrounding the whiskers was excised. The excised skin tissues were then immersed in a solution of phosphate-buffered saline (PBS) containing double antibodies for a duration of 5 min. Individual hair follicles were carefully grasped from the dermal surface using micro tweezers, and the bulbous portion along with the upper hair shaft was excised. The hair follicle tissue was placed in a centrifuge tube, and subsequently, a digestion solution consisting of collagenase I, neutral protease II, and DMEM complete medium was added. The digestion process was carried out at a temperature of 37°C for a duration of 16-18 h. Following digestion, the centrifuge tube was retrieved, and the tissue was pipetted by means of repeated blowing until the liquid exhibited cloudiness. The digestion solution underwent filtration using 100 mesh and 200 mesh cell sieves, and the resulting filtrate was subjected to centrifugation at 300 g to retain the cellular precipitate. The cells were then resuspended in a complete medium for mouse hair follicle cells and inoculated onto Petri dishes pre-coated with collagen from mice tails. The medium was incubated at 37°C in a 5% CO2 incubator and replaced after 48 h.
2.3 Immunofluorescence staining
Immunofluorescence staining was conducted to assess the proliferation of HFSCs and examine the molecular expression of β-catenin, Lef1, CK-19, and Ki67. The cell monolayer was rinsed thrice with PBS, followed by fixation with 4% paraformaldehyde for 15 min and permeabilization with 0.5% TritonX 100 for 20 min at ambient temperature. Following the washing procedure, goat serum was introduced drop by drop onto the slide, which was subsequently closed and left at room temperature for a duration of 30 min. After aspirating the closed solution, the primary antibody (CK-19) was added and the slide was placed in a wet box for overnight incubation at a temperature of 4°C. Subsequently, the fluorescent secondary antibody (Cy3-labeled sheep anti-rabbit IgG) was introduced in a dark environment and incubated for a period of 1 h at a temperature of 37°C within the wet box. Following the dropwise restaining of the nuclei with DAPI and sealing, the images were captured through observation under a fluorescence microscope.
2.4 Western blot analysis
HFSCs were extracted using RIPA Lysis Buffer, which contained 1 mM PMS(Servicebio)The protein concentration was determined using a BCA Assay Kit (Servicebio). Protein from each sample was separated by SDS-PAGE and then transferred onto PVDF membranes. After eluting the transferred film with TBST, add the mixed ECL luminescent solution, put it into the chemiluminescence instrument, start chemiluminescence according to the preset program, and save the original image as TIFF format after the exposure is completed. The saved TIFF format was used to analyze the data with AIWBwellTM analysis software. The evaluation items include the original grayscale values read automatically by the software and the ratio of the grayscale value to the internal reference grayscale value, which represent the relative content of the sample.
2.5 Quantitative PCR
Total RNA of HFSCs was purified using RNAprep pure cell/Bacteria Kit (Wuhan Servicebio Technology CO., LTD), following the protocol prescribed by the manufacturer. RNA samples obtained from the control and treated cells were subjected to real-time (RT)-PCR analysis. The expression of the target genes was normalized against that of endogenous GAPDH and relative gene expression was calculated using theΔΔCT method.
2.6 Animal model establishment
Eight-week-old female C57 mice of SPF grade were positioned in the prone position and administered anesthesia using a chloral hydrate solution. The dorsal hair was removed using an electric shaver, extending from the back of the neck to the tail. A longitudinal path was created in the posterior midline, measuring 0.6 cm in diameter, to excise the skin and subcutaneous tissue up to the lumbodorsal fascia. The mice were fixed with medical gauze and elastic bandages. After modeling, penicillin injection was performed postoperatively. C57 female mice were kept according to the protocol approved by the Experimental Animal Center of Bengbu Medical College.
2.7 HE staining analysis
The mice were euthanized on days 10 and 14, and the traumatized skin soft tissues were embedded in paraffin and subsequently sectioned. Following dewaxing and rehydration, the tissue samples were subjected to staining with hematoxylin solution for a duration of 5 min, followed by a 5-s rinse with water. The specimens were then treated with a mixture of 1% hydrochloric acid and ethanol for 3 s. After a thorough water wash and bluing with 1% ammonia for 60 s, the samples were dehydrated using an ethanol gradient and stained with a 0.5% eosin alcohol solution for 5 min. Following rehydration using an ethanol gradient and achieving xylene transparency, the specimen was subsequently sealed with neutral gum. Subsequently, microscopic observation was conducted to evaluate the extent of new granulation tissue formation and the absence of angiogenesis.
2.8 Statistical analysis
All experimental data were presented as the mean ± standard deviation at least three independent experiments. Experimental results were analyzed using SPSS (Statistical Product and Service Solutions). The statistical significance of difference was determined by the Student's t-test. The value of p < 0.05 was considered statistically significant.
3 RESULTS
3.1 LLLT activates Wnt/β-catenin signaling pathway in cultured HFSCs and promotes HFSCs proliferation
To elucidate the effect and the molecular mechanism of LLLT on the Wnt/β-catenin signaling pathway in HFSCs, TSA immunofluorescence staining was conducted in the four groups of cultured HFSCs to examine the expression level of important signaling molecules including core proteins β-catenin and Lef1, and we further examined the expression of HFSCs marker CK-19 and cell proliferation marker Ki67. Based on the findings, the immunofluorescence intensities observed in the LT and LT+DKK1 groups were significantly greater compared to the Control and Control+DKK1 groups. Furthermore, subsequent administration of the Wnt signaling pathway inhibitor DKK1 resulted in significantly higher immunofluorescence intensities in the LT+DKK1 group compared to the Control group, and in the LT group compared to the LT+DKK1 group (Figure 1A, B). The semi-quantitative immunofluorescence analysis showed the same results (Figure 1C, D).
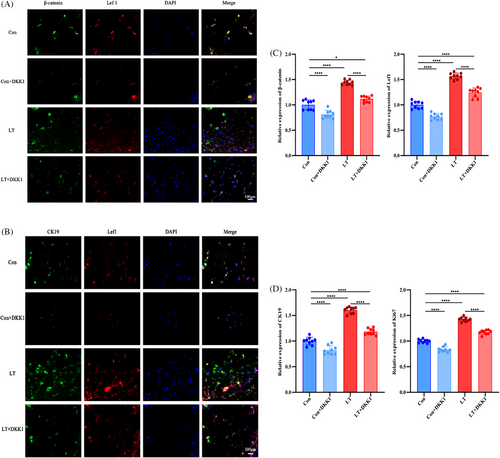
In order to provide additional evidence regarding the impact of LLLT on the activation of the Wnt/β-catenin signaling pathway and the promotion of HFSCs proliferation, four groups of cultured HFSCs were subjected to Western blot analysis to assess the expression levels of β-catenin, Lef 1, CK-19, and Ki67. The findings indicate that the expression levels of β-catenin, Lef1, CK19, and Ki67 were significantly elevated in the LT and LT+DKK1 groups compared to the Con and Con+DKK1 groups. Additionally, the protein expression level in the Con+DKK1 group was significantly reduced compared to the Con group, while the protein expression level in the LT+DKK1 group was significantly lower than the LT group (Figure 2A).
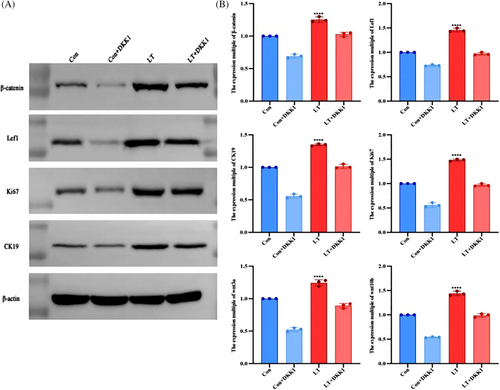
To further substantiate our hypothesis at the genetic level, we conducted fluorescent quantitative PCR experiments on four groups of cultured HFSCs to analyze the expression of four relevant proteins. The findings revealed a significant increase in gene expression ploidy in the LT and LT+DKK1 groups when subjected to low-level laser intervention, as compared to the Con and Con+DKK1 groups. Meanwhile, under the intervention of DKK1, the ploidy of gene expression was notably reduced in the Con+DKK1 group compared to the Con group, as well as in the LT+DKK1 group compared to the LT group. Additionally, we also extendedly examined the gene expression level of Wnt3a and Wnt10b, found that Wnt3a and Wnt10b gene expression level in the LT group was significantly higher than the Con group, which shows us that LLLT may activate the Wnt signaling pathway by increasing the concentration of Wnt3a and Wnt10b ligands (Figure 2B).
Our data demonstrate that LLLT can activate Wnt/β-Catenin signaling pathway, increase the expression level of signaling core proteinsβ-catenin and Lef1, and then promote HFSCs’ proliferation.
3.2 LLLT significantly accelerates wound healing and significantly promotes the proliferation of HFSCs
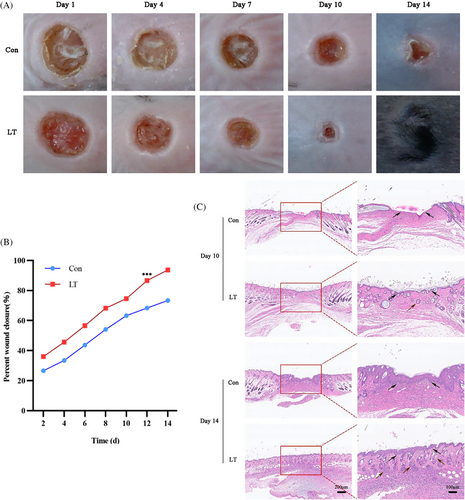
In order to evaluate the impact of LLLT on the process of wound healing in mice, a trauma animal model was developed by creating a skin wound on the dorsal region of the mice. Subsequently, the mice were categorized into two groups: a control group (Con group) and a laser treatment group (LT group), based on whether they received low-level laser treatment or not. The duration of the treatment period spanned 14 days. The wound healing progress of each group was observed at specific intervals postoperatively, namely 1, 4, 7, 10, and 14 days. The size of the wound area was determined using Image Pro Plus 6.0 (IPP6.0 Media Cybernetics) software. The formula was as follows: day n wound area percentage = (day 1 wound area—day n wound area)/day 1 wound are×100%. On days 10 and 14, the mice were euthanized, and the traumatized skin soft tissue was embedded in paraffin for subsequent histological analysis. The findings indicate that the rate of wound healing in the dorsal skin of mice subjected to low-level laser treatment was superior to that observed in the control group. Notably, complete wound closure was achieved by day 14, and throughout the healing process, the wound exhibited enhanced cleanliness, reduced exudation, and diminished infection (Figure 3A, B).
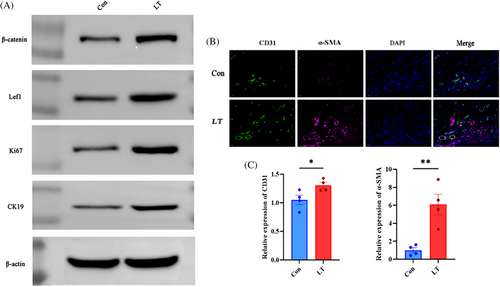
On the 10th day, wound tissue samples were collected from two groups of mice for the purpose of conducting a Western blot experiment. The results of the Western blot analysis indicated that the expression levels of β-catenin, Lef1, CK19, and Ki67 were significantly higher in the LP group compared to the Con group, thus confirming the previous findings (Figure 4A).
The histological results of HE staining demonstrated that by day 10, the wound surface of mice in the LT group had achieved substantial epithelialization, accompanied by a notable presence of newly formed blood vessels. Additionally, the results of Masson's staining indicated a significant augmentation in collagen production. Conversely, the wounds in the Con group mice exhibited incomplete epithelial coverage and a lower level of collagen synthesis. On the 15th day, the wounds of the mice in the LP group exhibited significant progress in healing, characterized by well-organized collagen reorganization, the presence of mature blood vessels, an intact structure, and substantial regeneration of hair follicles, sebaceous glands, and other skin appendages. Conversely, the wounds of the mice in the Con group did not achieve complete healing (Figure 3C).
On the 10th day, we used immunofluorescence staining and fluorescence semi-quantitative analysis by the TSA method on the wounds of mice in the LT and Con groups, respectively, to detect and analyze the vascular endothelial cell regeneration-related proteins,α-SMA and CD31, in order to assess the regeneration of endothelial cell tissues in the trabeculae of the two groups and to evaluate angiogenesis. Among them,α-SMA fluorescence was dark red and CD31 fluorescence was green. It was found that the fluorescence expression content of the wounds in the LT group was increased compared with that of the Con group, and the results of the semi-quantitative analysis of immunofluorescence illustrated the same results, which proved that more neovascularization was formed in the wounds of the LT group compared with that of the Con group, and the process of wound repair was more rapid (Figure 4B, C).
These results suggest low-level laser significantly activates Wnt signaling pathway, promotes proliferation of HFSCs, and significantly accelerates wound healing.
4 DISCUSSION
LLLT has been developed over the years as a complex therapeutic procedure tool and is used clinically for many different diseases. The biological effects LLLT at the cellular and molecular levels may include cell viability, proliferation rates, and DNA integrity and repair of damaged DNA. It was found that different intensities of LLLT treatment had different effects on cell process stimulation with different effects. Multiple studies have indicated that low levels of red or near-infrared light have the potential to stimulate mitochondrial activity, inhibit apoptosis, enhance cell turnover, recruitment, and proliferation, as well as regulate cellular metabolites. Research has demonstrated that LLLT may promote the alteration of cellular redox status and plays an important role in maintaining cellular activity and inducing photobiostimulation processes.19 LLLT has also been observed clinically to reduce inflammation and pain, and to accelerate the induction of wound healing under non-thermal effects.20 LLLT is widely used in biomedicine for the treatment of various skin injuries such as burns, surgical wounds, diabetic wounds and ulcers.21-23 The key biological effects are: increase in ATP production, increase in mitochondrial membrane potential and its activity, conversion of fibroblasts into myofibroblasts, promotion of cell proliferation, cell differentiation and collagen production.24 While numerous studies have provided evidence of the beneficial impact of LLLT in clinical treatment, limited research has been conducted to investigate the precise mechanisms and functions of LLLT in the context of wound healing.
The Wnt/β-catenin signaling pathway plays a key role in embryonic development, stem cell maintenance and intestinal tissue regeneration, and its aberrant activation is associated with the pathogenesis of several malignant tumors. Abnormally activated Wnt/β-catenin signaling is a key causative factor in several cancers.25 Vikash Singh et al. investigated the global role of IGF2BP1 protein in controlling the expression of target genes of the Wnt/β-catenin signaling pathway in colorectal cancer cells. The research found that tIGF2BP1 acts as an RNA-binding protein that binds to specific mRNA molecules and regulates gene expression by modulating their stability, localization and translation. For example, it can stabilize the mRNAs of βTrCP1 and GLI1, thereby upregulating their expression. Meanwhile, the IGF2BP1 acts as a transcriptional target of the Wnt/β-catenin pathway, which is commonly reactivated in non-transformed and CRC cells, modulating mRNA stability to regulate oncogene expression.26 Also Alexander Zemtsov et al. have proposed that patients with myotonic muscular dystrophy are deficient in MBLN-1 protein, which interferes with DNA splicing and affects RNA maturation, transport, and degradation, leads to a greater susceptibility to skin cancer in patients with MMD.27 D'Ambrosio et al. indicate a high risk of specific cancer types, such as thyroid, skin, and pancreatic cancers, among patients with evidence of myotonic dystrophy (DM). This may be related to abnormal activation of the Wnt/β-catenin signaling pathway and abnormalities in genes such as CTNNB1 and PLAG1. These studies further corroborate this result. This provides some theoretical basis for our understanding of the importance of the Wnt signaling pathway in promoting the proliferation and differentiation of skin stem cells, thereby promoting wound healing.
Presently, a considerable number of studies are dedicated to exploring the signaling pathways responsible for regulating the self-renewal and differentiation of tissue stem cells, with a growing body of evidence suggesting the involvement of the Wnt signaling pathway in this intricate process. The Wnt signaling pathway plays a crucial role in the growth and cyclic conversion of hair follicles. In the classical Wnt signaling, Wnt ligands interact with LRP5/6 co-receptors and Frizzled-type receptors, resulting in the inactivation of theβ-linked protein disruption complex. This in turn leads to the accumulation of β-linked proteins and their translocation into the nucleus.β-catenin, in association with Tcf/Lef transcription factors, regulates the activity of downstream target genes. DKK1, a potent antagonist of Wnt signaling, binds to LRP5/6 and prevents the binding of FzLrp, thereby specifically inhibiting the Wnt signaling pathway. In light of the aforementioned studies, an investigation was conducted to explore the correlation between the activation of the Wnt signaling pathway and the proliferation of HFSCs through the utilization of LLLT. Specifically, the experiment employed LED red light with a wavelength of 670 nm and DKK1 at a concentration of 400 nm/mL. The obtained experimental findings demonstrate that low levels of laser stimulation can effectively activate the Wnt signaling pathway, resulting in the upregulation of the core proteinβ-catenin and the downstream key gene Lef1 expression, ultimately leading to the promotion of HFSCs proliferation. As previously stated, our research indicate that the utilization of the 670 nm LED red light has the potential to enhance the proliferation of HFSCs by partially activating the Wnt signaling pathway. This finding highlights the significance of this therapeutic technique in the context of stem cell therapy. Furthermore, the evidence obtained from our current study strongly suggests a comprehensive association between LLLT, the Wnt signaling pathway, and the proliferation of HFSCs. However, further investigations are required to elucidate the precise relationship between LLLT, the Wnt signaling pathway, the proliferation of HFSCs, and wound healing in vivo.
In superficial wound healing where the skin appendages remain intact, re-epithelialization begins simultaneously from the hair follicle and wound edges, leading to rapid wound healing. During wound healing, HFSCs begin to differentiate into epidermal cells and migrate to the wound site to help replace the lost epidermis. During re-epithelialization, basal keratin-forming cells migrate from the wound edges and follicular stem cells migrate from the follicular isthmus and augmentation zone to the wounded area to close the wound. During normal skin dynamic equilibrium, stem cells in the basal layer continuously form a new epidermis by dividing, committing to terminal differentiation and migrating to the skin surface.28 HFSCs are located in the hair follicle augmentation zone and isthmus. In uninjured skin, stem cells in the bulge regenerate new hairs and cells in the isthmus renew the non-hairy part of the hair follicle. However, once a wound occurs, both stem cell populations respond to the injury in response to the injury. They begin to differentiate into epidermal cells and migrate to the wound site to help replace the lost epidermis.29
The precise molecular mechanisms underlying the promotion of wound healing by HFSCs are not yet fully elucidated. Research indicates that the Wnt signaling pathway may exert regulatory control over this phenomenon, as it plays a crucial role in organ development by governing cellular proliferation, differentiation, and migration. Activation of Wnt signaling occurs immediately following tissue injury and has been demonstrated to be involved in all stages of the healing process, including the regulation of inflammation, initiation of programmed cell death, and recruitment of a reservoir of stem cells within the wound site.
In the present study, a mouse dorsal trauma model was employed in an in vivo experiment to investigate the effects of 670 nm LED red light on the activation of the Wnt signaling pathway, proliferation of HFSCs, and acceleration of wound healing. The results obtained from this experiment demonstrated a significant acceleration in wound healing. Additionally, in an in vitro experiment, the same outcome was confirmed through Ki67 immunohistochemical staining. Therefore, we cautiously conclude that LLLT activates the Wnt signaling pathway, promotes β-catenin accumulation and upregulates downstream Lef 1 concentration, which in turn promotes HFSC proliferation, enhances collagen synthesis and angiogenesis by regulating the downstream activities of a series of signaling pathways. However, the specific mechanism of how LLLT activates the Wnt signaling pathway, whether through upregulation of Wnt-related ligand concentrations or other possible causes, and the specific pathway downstream of the Wnt signaling pathway that initiates the proliferation of HFSCs are still not fully understood and need to be further explored in the future. In addition, the optimal optical parameters for LLLT under different treatment protocols, such as wavelength, irradiance energy density and power density are also different, and further experiments are needed for therapeutic applications.
5 CONCLUSIONS
In summary, our study reveals that under low-level laser treatment, the Wnt signaling pathway is significantly activated during wound healing, pathway Nucleus β-catenin accumulates in the cytoplasm and translocates into the nucleus, which leads to the expression of the signaling downstream of key genes Lef1 increasing. Meanwhile, activation of the Lef 1 gene regulates a range of downstream biological effects, it promotes the proliferation and differentiation of HFSCs, enhances collagen synthesis and angiogenesis, it may also improve the resistance of wounds to infection ultimately exerting a pro-wound healing effect and accelerate wound healing.
ACKNOWLEDGMENTS
This work was supported by grants from Natural Science Projects at Bengbu Medical University (2023byfy008) and Natural Science Research Project of Anhui Educational Committee (2022AH051451; KJ2021A0772; KJ2019A0345). Appreciate the supporting by the supporting by Central Laboratory of The First Affiliated Hospital of Bengbu Medical University.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




