Correlation of non-invasive psycho-physiological and skin-physiological measures
Abstract
Introduction
Psychological stress alters epidermal barrier function. While intensive studies on the underlying mechanism have been performed in mice, human studies are limited. Non-invasive skin-physiology measures have not yet been directly linked to non-invasive psycho-physiological assessments.
Methods
Standard measures of (I) transepidermal water loss prior to and after experimental barrier perturbation via tape stripping, (II) skin surface pH, (III) electrodermal activity, and (IV) heart rate function were taken over a 24 h time period. To document perceived stress, a standardized stress self-assessment questionnaire, namely the Trierer Inventar zum chronischen Stress (TICS), was utilized.
Results
Twenty healthy, Caucasian (Fitzpatrick skin phototype I-II), female volunteers (21–32 years, mean age 27, SD = 3.67 years) were included in this study (random sample). Significant correlations were shown for 24 h delta transepidermal water loss changes, that is, barrier repair kinetics (sympathetic activity) and heart rate variability (parasympathetic activity). Further correlations were noted for electrodermal activity and skin surface pH. Perceived stress, as documented by the TICS questionnaire, did not correlate with psycho- and skin physiological parameters, respectively.
Conclusion
The presented approaches may provide a basis for non-invasive objective research on the correlation between psychological stressors and epidermal barrier function.
1 INTRODUCTION
The skin's outer surface acts as a physical, chemical, and antimicrobial defence system.1-3 During periods of psychological stress (PS) in both rodents and humans, integrity and regeneration of this permeability barrier are disturbed.4-8 In mice, PS-induced barrier perturbation could be reversed by co-administrated sedatives, such as chlorpromazine or diazepam8 PS stimulates endogenous production of glucocorticoids (GC) and correlates with barrier perturbation.7 Co-administration of the GC receptor antagonist, RU 486, blocked emergence of PS-induced abnormalities in barrier homeostasis. Subsequent studies directly demonstrated the negative consequences of GC on barrier function.9 PS activates the hypothalamic-pituitary-adrenal (HPA) axis10-13 and pituitary-derived ACTH stimulates adrenocortical production as well as secretion of GC, suppressing the immune system. Downregulation of epidermal antimicrobial peptides and increased severity of cutaneous skin infection accompanied by increased production of endogenous GC, which in turn inhibited epidermal lipid synthesis and decreased lamellar body secretion, have been linked to PS in rodents.14 PS due to sleep deprivation decreased epidermal cell proliferation, impaired epidermal differentiation, and decreased the density and size of corneodesmosomes, which was linked to its degradation. Barrier compromise was linked to decreased production and secretion of lamellar bodies, which in turn could be attributed to a decrease in de novo synthesis of epidermal lipids.6 The use of mice to test correlations between barrier function and PS is, however, limited due to of the fundamental differences in skin physiology and pathology between rodents and humans.10, 11
In humans, barrier regeneration correlates with perceived PS, that is, the higher perceived PS, the greater deterioration in barrier function.5 Acute PS alters barrier integrity and barrier recovery kinetics, accompanied by increases in plasma GC, norepinephrine, interleukin-1ß, tumour necrosis factor, and circulating natural killer cells.4 Furthermore, exacerbation of atopic dermatitis associated with PS has been linked to an upregulation of neuropeptide mediators in the peripheral nervous system.15, 16 In order to objectively determine PS, objective physiological measures related to the autonomic nervous system (ANS) activity have been established.17 Changes in electrodermal activity (EDA) and heart rate function are responses of the ANS.18-20 Heart rate variability (HRV) is mediated by ANS, which controls homeostasis in the body. The ANS comprises the sympathetic and the parasympathetic nervous system. Under normal conditions, an increase in heart rate is caused by increased activity of the sympathetic nervous system. The systematic change of heart rate in relation to respiration, a regular increase of heart rate with inspiration, and a decrease with exhalation was labelled as “Respiratory Sinus Arrhythmia” (RSA). RSA is mediated predominantly by parasympathetic influences. The higher the activity levels of the sympathetic nervous systems, the lower RSA (or, generally speaking, HRV). Vice versa, the higher the activity levels of the parasympathetic nervous systems, the higher HRV. Thus, sympathetic and parasympathetic nervous system function in contrast to the regulation of HR.21 Since the ANS controls heart rate, HRV is a measurement of how well these systems can perform this regulation.
Against this background, within the framework of the presented non-invasive, experimental, skin bioengineering study, skin bioengineering techniques22 were correlated with psychophysiological measures17-21 to provide a basis for research into the effects of psychological stressors, inhibitors, or both on epidermal barrier function.
2 MATERIALS AND METHODS
2.1 Trial design and participants
This study was a non-invasive, experimental, skin bioengineering trial. Ethic approval was obtained by the ethics committee at Osnabrück University, Osnabrück, Germany (procedure number 4/71040/0/6). The study complied with the Helsinki Declaration of 1975, and later revisions. Only skin-healthy, Caucasian (Fitzpatrick skin phototype I–II), female volunteers were included in the study. Informed consent was obtained from all participants prior to their inclusion in the study. Participants were chosen according to their willingness to volunteer (random sampling).
2.2 Skin physiology
Skin surface pH was measured with a flat glass electrode (Mettler-Toledo, Giessen, Germany) attached to a pH-meter (Skin-pH-Meter® PH905, Courage & Khazaka, Cologne, Germany). Barrier function was determined by measuring transepidermal water loss (TEWL; Tewameter® TM300, Courage & Khazaka, Cologne, Germany). For the evaluation of barrier integrity, tape stripping on the volar aspect of the forearm was necessary, for which a commercially available adhesive tape (3 M Blenderm surgical tape, 3 M Deutschland, Neuss, Germany) was used. The number of tape strips required to increase TEWL threefold reflected barrier integrity. The practical procedure of the stripping itself was performed according to Dickel et al.23 For most subjects, 20# to 128# tape strippings (mean tape stripping #49) were required to achieve this degree of barrier disruption. Recovery of barrier function was calculated as a percentage value. Change in TEWL during 24 h, from the immediate post-tape strip TEWL to the final TEWL, was divided by the change in TEWL from immediately before to immediately after the tape stripping.
2.3 Psychophysiology
EDA was recorded employing a MentalBioScreen K3 (Porta Bio Screen GmbH, Berlin, Germany). Two Electrodes were applied to the right–hand thenar/hypothenar sites with a 0.5 V constant voltage to obtain skin conductance (SC) using a sampling rate of 1 Hz and a 0.5 Hz filter over a 24 h time period. Signals were recorded on to a SD card and transferred after the recording period to a PC for analysis employing the VisualFeedback K3 Software.
Heart rate was recorded by means of the eMotion ECG sensor and HRV was analysed offline using the HRV Scanner software (BioSign, Ottenhofen, Germany). Electrocardiograms can be taken with various numbers of electrodes connected to the body. In the 2-lead electrocardiogram used in this study, electrodes were placed on the right subclavicular and in the area of the heart apex. Time domain measures of HRV include various statistics that are calculated from the time intervals between heart beats. Time intervals were determined by the R-R intervals, the period of time between peaks in the electrocardiograph (ECG). In addition to the objective psychophysiological measures, the participants filled in a standardized 24-item mood Trierer Inventar zum chronischen Stress (TICS) questionnaire.24
2.4 Experimental design
TEWL measurements were taken after the participants had rested/acclimatized for 30 min in an environment with a temperature of 20–22°C and relative humidity of 48%–50%, according to the guidelines given by the European Society of Contact Dermatitis.25 After experimental barrier perturbation via tape stripping and subsequent TEWL measurements, EDA and ECG electrodes were placed as described and left for 24 h recordings. Participants returned 24 h after barrier perturbation and rested again for 30 min in the above described environment. TEWL measures were taken and electrodes were removed.
2.5 Statistics
Statistical analysis was performed using SPSS software version 22.0 (SPSS, Chicago, IL, U.S.A.). The significance level was set at p ≤ 0.05. All measured and calculated parameters are reported as arithmetic mean and standard deviation (mean ± SD). Pearson correlations were calculated to quantify the relation between questionnaire-based stress level self-assessment and skin physiological and psycho-physiological measures.
3 RESULTS
Twenty female university students (21–32 years, mean age 27, SD = 3.67 years) from Osnabrück University, Osnabrück, Germany were included in this study. Correlation between HRV and delta TEWL after a 24 h time period was negative (correlation, Pearson = −0.557) during the diurnal phase, that is, the higher the HRV the lower was delta TEWL, reflecting the difference between TEWL immediately after initial barrier perturbation via tape stripping's and TEWL 24 h after barrier perturbation (p < 0.01, Figure 1). Correlation between HRV and delta TEWL after a 24 h time period was negative (correlation, Pearson = −365) during the nocturnal phase, that is, the higher the HRV the lower was delta TEWL, reflecting the difference between TEWL immediately after initial barrier perturbation via tape stripping's and TEWL 24 h after barrier perturbation (p < 0.05, Figure 1).

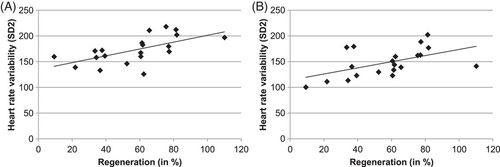
Correlation between HRV and percent barrier regeneration after a 24 h time period was positive (correlation, Pearson = 0.609) during the diurnal phase, that is, the lower the HRV the lower was the epidermal barrier regeneration 24 h after barrier perturbation via tape stripping (p < 0.01, Figure 2). Correlation between HRV and percent barrier regeneration after a 24 h time period was positive (correlation, Pearson = 0.519) during the nocturnal phase, that is, the lower the HRV the lower was the epidermal barrier regeneration 24 h after barrier perturbation via tape stripping (p < 0.01, Figure 2).
No correlation was revealed between the 24 h EDA measurements and barrier recovery kinetics, that is, either delta TEWL or percent barrier regeneration (data not shown). Correlation between EDA and skin surface pH prior to perturbation via tape stripping after a 24 h time period was negative (correlation, Pearson = −0.409), that is, the higher EDA the lower was the skin surface pH (p < 0.05, Figure 3). Moreover, 24 h after barrier abrogation this correlation was still negative (correlation, Pearson = −0.345), but not significant (p = 0.068).
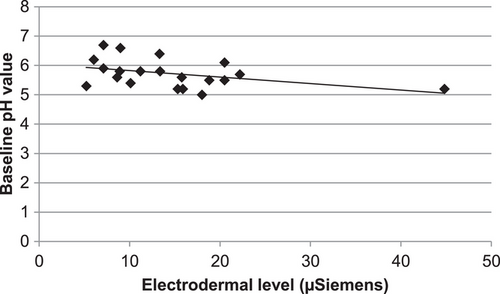
The skin surface pH did not correlate with HRV measures. No correlation could be obtained between the data retrieved from TICS questionnaires and either barrier recovery kinetic data or skin surface pH (data not shown). Neither correlated the results from the questionnaires with the EDA or HRV parameters (data not shown).
4 DISCUSSION
To the best of our knowledge, the present study is the first to correlate non-invasive skin physiology measures with non-invasive psychophysiological measures, that is, linking epidermal barrier recovery kinetics over a 24 h time period to the activity of the ANS, objectively reflected by HRV and EDA. In this study, 20 young, healthy female university students volunteered to participate at the end of a study year. The study year ends with examinations, which may be perceived as stressful by most, but not all students.26, 27 The higher the PS level during a diurnal or nocturnal phase, the higher the activity of the sympathetic nervous system, the lower the HRV, one of the objective stress measures employed in this study. Barrier recovery kinetics, presented here as delta TEWL or percent barrier regeneration 24 h after artificial perturbation, correlate with HRV; the lower the HRV, the lower barrier regeneration and the higher delta TEWL during the diurnal and nocturnal phase (p < 0.01 and p < 0.05).
In a currently conducted prospective observational study, Lyu et al. were able to show that PS—in this special case induced by poor sleep and severe anxiety—can harm skin barrier homeostasis in university students,28 which corroborates the findings of the presented experimental skin bioengineering study. Garg et al. revealed correlations between PS and epidermal permeability barrier function in university students during final examinations and after a winter vacation.5 A decline in barrier recovery kinetics after barrier disruption by tape stripping correlated with an increase in perceived PS during the higher stress occasions, as the TICS questionnaires did not correlate with either objective skin- or psychophysiological measures, respectively.4 The lack of correlation may reflect that under a period of moderate PS (end of a study year), perceived PS varies.27 Further, human studies have shown that experimental stress (e.g., an extreme interview) causes a delay in barrier repair after perturbation and increases plasma GC, norepinephrine, interleukin-1beta and interleukin-10, tumour necrosis factor-alpha, and increases circulating natural killer cell number and activity.4 Under PS, high secretion of pituitary-derived ACTH stimulates adrenocortical production and secretion of GC.10, 11 Inhibition of epidermal lipid synthesis and decreased lamellar body secretion has been linked to PS and high serum GC levels.14, 29 PS precipitates or exacerbates—or both—a large number of skin diseases, including atopic dermatitis and psoriasis,15, 30 which frequently are treated topically with GCs. Even short-term GC (namely Clobetasol) treatments exert abnormal stratum corneum integrity and a delayed barrier recovery.9 The vicious circle never ends.
In the presented study, EDA parameters did not correlate with barrier recovery kinetics. This method of measuring the electrical conductance of the palmar surface of the skin, varies with the activity of the sympathetic controlled sweat gland activity. If sympathetic activity of the ANS increases, sweat gland activity increases, which in turn increases skin conductance. Skin conductance may be used as an immediate measure of strong emotional and sympathetic responses, but will not well reflect the underlying ANS status over a 24 h time period as a continuous measurement of the HRV. Stratum corneum acidification has been linked to NH1, an Na(+)/H(+) antiporter,31 the filaggrin-histidine-urocanic acid pathway32 and the activity of secretory phospholipase,33 but so far not to the ANS. The skin's sympathetic nerve activity correlates with increased sweat gland activity, and under PS with an impaired cutaneous immune response, affecting atopic dermatitis and psoriasis,15, 30 however, a link between sympathetic nerve activity retrieved via EDA on the thenar/hypothenar region and the skin's surface pH on the volar aspect of the forward has not been found in the current literature. Here, a negative correlation between baseline EDA and skin surface pH was observed (p < 0.05). Hypothetically a (relative) high sympathetic nerve activity may activate the above-mentioned biochemical pathways, followed by a subsequent lower skin surface pH. While it remains to be elucidated whether the skin's surface pH is indeed linked to the ANS in humans, it seems obvious that barrier recovery kinetics are linked to the ANS, as reflected by continuous HRV recordings over 24 h. The presented findings may be a basis to use this non-invasive method for studies on ANS effectors and inhibitors and their effect on skin barrier function.
5 CONCLUSION
In the presented non-invasive, experimental, skin bioengineering trial, ssignificant correlations were shown for skin barrier repair kinetics (sympathetic activity) and heart rate variability (parasympathetic activity). Further correlations were noted for electrodermal activity and skin surface pH. The presented approach may provide a basis for non-invasive objective research on the correlation between psychological stressors and epidermal barrier function in the future.
ACKNOWLEDGMENTS
We thank Psyrecon Research & Consulting Institute for Applied Psychophysiological Research, Wuppertal, Germany for financially supporting this study.
CONFLICT OF INTEREST STATEMENT
R. Stürmer is managing director of Psyrecon Research & Consulting Institute for Applied Psychophysiological Research, Wuppertal, Germany. The other authors have nothing to declare.
ETHICAL STATEMENT
This study was conducted according to the principles laid out in the Helsinki Declaration of 1975, and later revisions. Ethic approval was obtained by the ethics committee at Osnabrück University, Osnabrück, Germany (procedure number 4/71040/0/6).
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




