A single-center, randomized, controlled study on the efficacy of niacinamide-containing body emollients combined with cleansing gel in the treatment of mild atopic dermatitis
Jun-Rong Zhu and Jie Wang contributed equally to this paper.
Abstract
Objective
To observe the effect of niacinamide-containing body emollients combined with a cleansing gel on the clinical symptoms of mild atopic dermatitis (AD) in adults.
Methods
From July 2022 to January 2023, adults with mild AD were enrolled at Huashan Hospital Affiliated to Fudan University using single-center, randomized and placebo-controlled methods. They were divided into three groups: the control group, treatment group 1 (T1) receiving niacinamide-containing body emollients alone, and treatment group 2 (T2) receiving emollients plus niacinamide-containing cleansing gel. All patients were orally administered 10 mg of ebastine tablets daily. AD severity (SCORAD score), peak pruritus numeric rating scale (PP-NRS), patient-oriented measure of eczema (POEM), dermatological quality of life index (DLQI) score, transepidermal water loss (TEWL), and stratum corneum water content (SCWC) were measured by the same dermatologist at days 0, 7, 14, and 28.
Results
A total of 122 patients were enrolled, including 38 in the control group, 42 in the T1 group and 42 in the T2 group. There were no obvious adverse reactions at the end of the study and the clinical scores and stratum corneum barrier of all the groups improved significantly relative to baseline. The SCORAD, PP-NRS, DLQI, TEWL and SCWC scores in T1 group (12.43 ± 3, 3.3 ± 0.9, 7.1 ± 2.33, 17.1 ± 9.12, 67.2 ± 21.46, seperately) and T2 group (11.17 ± 3.26, 3 ± 1.3, 6.5 ± 2.11, 16.3 ± 9.12, 69.4 ± 24.52, seperately) were significantly improved than the control group(15.1 ± 3.64, 4.3 ± 1.7, 9.5 ± 2.46, 21.2 ± 9.47, 52.7 ± 22.43, seperately) at the endpoint of the study, while compared the POEM scores, only T2 group showed the difference with control group (5.2 ± 1.4 vs. 6 ± 1.6). The epidermal barrier parameters of TEWL and SCWC in the T2 group (17.57 ± 5.24, 66.46 ± 21.38, seperately) were significantly better than that of the T1 (19.96 ± 4.45, 56.45 ± 20.48, seperately) and control group(21.89 ± 7.03, 51.56 ± 16.58, seperately) on the 14th day of follow-up.
Conclusion
The use of niacinamide-containing body emollients can significantly improve the clinical symptoms, quality of life, and skin barrier function in patients with mild AD. The addition of niacinamide-containing cleansing gel can also affect the clinical efficacy at certain time points.
1 INTRODUCTION
Atopic dermatitis (AD) is a common chronic, recurrent, inflammatory skin disease in dermatology, characterized by a chronic and persistent course, marked by severe itching, and substantially impacting patients’ quality of life.1 Depending on the severity, AD can be categorized as mild, moderate, or severe. Presently, there is no effective cure for AD, and the primary treatment goal is symptom alleviation, recurrence reduction, and the enhancement of patients’ quality of life.1 Guidelines for the diagnosis and treatment of AD in numerous countries have underscored the utility of topical emollients in restoring the compromised skin barrier function of AD patients. These emollients effectively reduce itching and the urge to scratch, resulting in clinical improvement.2-4 In addition, skin hygiene practices hold a crucial role in AD management. Cleansers, in particular, aid in the removal of bacteria from the stratum corneum and facilitate the elimination of crusts, rendering them a recommended component in AD treatment.3
Niacinamide, an amide derivative of vitamin B3, constitutes a hydrophilic endogenous substance renowned for its multifaceted benefits. It exhibits antipruritic, antimicrobial, photo-protective and skin-lightening effects when administered with adequate bioavailability.5 Several studies have substantiated the beneficial effects of niacinamide in enhancing the barrier function of the stratum corneum.5, 6 However, its application in the context of AD is relatively limited in the existing literature. In response to this knowledge gap, we conducted a single-center, randomized, controlled clinical investigation at Huashan Hospital affiliated to Fudan University. Our primary objective was to assess the potential improvement in clinical symptoms of AD and the enhancement of skin barrier function through the utilization of niacinamide-infused body emollients and cleansing gels.
2 MATERIALS AND METHODS
A single-center, randomized, controlled clinical study was conducted in Huashan Hospital affiliated to Fudan University from July 2022 to January 2023. This study was approved by the Ethics Committee of Huashan Hospital affiliated to Fudan University (ethics approval number: 2022–922). All patients were recruited from dermatology clinics and provided informed consent.
2.1 Patients eligibility criteria
Patients aged 18−65 years old with a confirmed diagnosed AD (fulfilled the diagnosis criteria of Hanifin and Rajka) and classified as having mild AD were eligible for inclusion in this study. Exclusion criteria included: (1) Those who are known to be allergic to the ingredients or excipients of the research medical skin care products; (2) Exhibited acute skin lesions such as exudation, erosion or blisters; (3) Those who are using topical anti-inflammatory, glucocorticoids, PDE4 inhibitors or biological agents; (4) Those who are suffering from psychiatric diseases, malignant tumors; (5) Patients with other skin diseases that may interfere with the observation and evaluation of this trial; (6) The patients may not be able to complete the study for any other reasons, or the investigator believes that he/she is not suitable to participate in the study.
2.2 Study design
Participants were randomly assigned to one of three groups using a random number table generated by SPSS statistical software and sealed in opaque envelopes. Upon enrollment of each patient, an envelope was opened, and the remainder of the random number obtained when divided by three determined their group: a remainder of 1 for T1, a remainder of 2 for T2, and a remainder of 0 for the control group.
As the guideline for AD recommended the use of antihistamines as an adjuvant therapy,2 all patients received 10 mg of ebastine tablets (manufactured by Industrias Farmaceuticas Almirall, Co., Ltd.) daily as a foundational treatment. The control group applied Emollient Cream No. 1 (produced by Huashan Hospital, China, main ingredients: stearic acid, white petrolatum, glycerin) twice daily. The T1 group applied niacinamide-containing body emollients (Bioderma Atoderm PP Baume, 200 mL, produced by Bioderma Cosmetics Trading Co., Ltd., France) twice daily, while the T2 group applied emollients after using niacinamide-containing cleansing gel (Atoderm Intensive Gel Moussant, 200 mL, produced by Bioderma Cosmetics Trading Co., Ltd., France). The weekly topical application amount was approximately 250 g. The pH values of the cleanser ranged between 5 and 6, and skin cleansing was performed gently and meticulously. Throughout the study, the T1 and control groups exclusively used water during bathing, refraining from the use of shower gels or soap products, and no other emollients were permitted across all groups. Clinical evaluations included the Scoring of Atopic Dermatitis (SCORAD) and Peak Pruritus Numeric Rating Scale(PP-NRS) for assessing clinical symptoms; the patient-oriented eczema measurement (POEM) and dermatological quality of life index (DLQI) were used for evaluate the impact of AD on patients'quality of life; the Corneometer® (Courage-Khazaka, Cologne,Germany) was employed to measure transepidermal water loss (TEWL) and stratum corneum water content (SCWC) as indicators of stratum corneum barrier funtion. These assessments were conducted at days 0, 7, 14, and 28.The study algorithm is illustrated in Figure 1.
2.3 Statistical analysis
Statistical analyses were processed by SPSS software (version 17.0) (SPSS, Inc., Chicago, IL, USA). All data were presented as mean ± standard deviation (SD). Differences in gender among groups were analysed using chi-square tests. Differences in age and SCORAD, PP-NRS, POEM, DLQI, TEWL, and SCWC between groups were assessed using t-tests. All analyses were two-sided with a signifificant level of P < 0.05.
3 RESULTS
3.1 Baseline characteristics
A total of 128 patients were enrolled in this study and 122 patients were included in the final analysis. Among them, 42 cases were in the T1 group, 42 cases in the T2 group, and 38 cases in the control group. The baseline characteristics are presented in Table 1. There were no significant differences in age, gender distribution, and baseline SCORAD score among the three groups (P > 0.05).
| T1 | T2 | Control group | |
|---|---|---|---|
| Patients no. | 42 | 42 | 38 |
| Gender no. | |||
| Male | 18 | 20 | 18 |
| Female | 24 | 22 | 20 |
| Mean age | 30.87 ± 11.24 | 32.74 ± 10.33 | 29.96 ± 9.84 |
| Baseline SCORAD | 18.86 ± 3.04 | 19.14 ± 3.36 | 19.26 ± 3.23 |
3.2 Changes in clinical scores
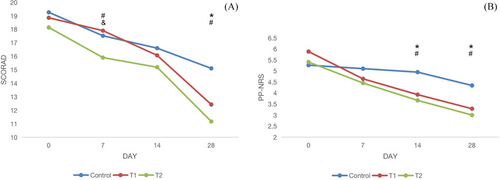
At days 0, 7, 14, and 28 of the study, the changes in SCORAD, PP-NRS scores among groups were shown in Table 2 and Figure 2. At the end of treatment, there were significant differences of these clinical scores between the treatment groups (T1 and T2) and control group (P < 0.05). At day 7, T2 group showed greater decline of SCORAD than the other groups (P < 0.05); while on day 14, the treatment groups (T1 and T2) showed greater decline of PP-NRS than control group (P < 0.05).
| T1 group | T2 group | Control group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 7 | Day 14 | Day 28 | Day 0 | Day 7 | Day 14 | Day 28 | Day 0 | Day 7 | Day 14 | Day 28 | |
| SCORAD | 18.86 ± 3.04 | 17.9 ± 4.04 | 16.07 ± 4.15 | 12.43 ± 3 | 19.14 ± 3.36 | 15.9 ± 3.05 | 15.19 ± 3.15 | 11.17 ± 3.26 | 19.26 ± 3.23 | 17.53 ± 4.09 | 16.6 ± 4.37 | 15.1 ± 3.64 |
| PP-NRS | 5.8 ± 1.5 | 4.6 ± 1.1 | 3.9 ± 1.4 | 3.3 ± 0.9 | 5.4 ± 2.4 | 4.5 ± 2.0 | 3.7 ± 1.5 | 3 ± 1.3 | 5.2 ± 2.3 | 5 ± 2.2 | 4.9 ± 1.8 | 4.3 ± 1.7 |
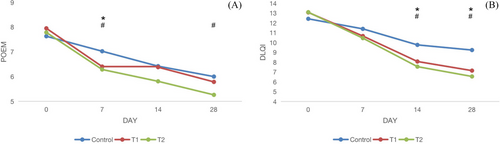
| POEM | 8 ± 2.1 | 6.4 ± 1.4 | 6.4 ± 2.1 | 5.8 ± 1.7 | 7.8 ± 2.3 | 6.3 ± 1.9 | 5.8 ± 1.7 | 5.2 ± 1.4 | 7.6 ± 2.2 | 7 ± 1.9 | 6.4 ± 1.8 | 6 ± 1.6 |
| DLQI | 12.9 ± 3.24 | 10.7 ± 2.98 | 8.1 ± 2.54 | 7.1 ± 2.33 | 13.2 ± 3.89 | 10.5 ± 3.41 | 7.5 ± 2.84 | 6.5 ± 2.11 | 12.5 ± 3.86 | 11.8 ± 2.81 | 9.7 ± 3.88 | 9.5 ± 2.46 |
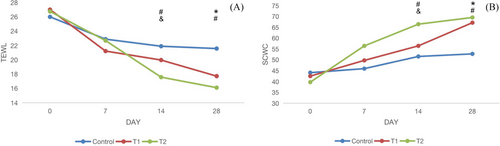
| TEWL | 27.4 ± 11.25 | 21.2 ± 9.87 | 19.96 ± 4.45 | 17.4 ± 4.82 | 26.8 ± 12.55 | 22.3 ± 11.85 | 17.57 ± 5.24 | 16.10 ± 7.30 | 25.7 ± 13.74 | 22.7 ± 11.75 | 21.89 ± 7.03 | 21.2 ± 9.47 |
| SCWC | 41.6 ± 18.45 | 49.2 ± 15.47 | 56.45 ± 20.48 | 67.2 ± 21.46 | 39.8 ± 15.42 | 56.7 ± 19.47 | 66.46 ± 21.38 | 69.4 ± 24.52 | 44.5 ± 16.83 | 45.7 ± 17.44 | 51.56 ± 16.58 | 52.7 ± 22.43 |
- Abbreviations: DLQI, dermatological quality of life index; POEM, patient-oriented measure of eczema; PP-NRS, peak pruritus numeric rating scale; SCORAD, scoring of atopic dermatitis; SCWC, stratum corneum water content; SD, standard deviation; T1, treatment group 1; T2, treatment group 2; TEWL, transepidermal water loss.
3.3 Changes in impact on quality of life
At days 0, 7, 14, and 28 of the study, the changes in POEM and DLQI scores among groups are shown in Table 2 and Figure 3. At day 7, both treatment groups (T1 and T2) showed greater decline of POEM, but only T2 group showed significant difference with control group at the end of treatment (P < 0.05). At day 14 and 28, both treatment groups (T1 and T2) showed greater decline of DLQI than control group (P < 0.05).
3.4 Changes in epidermal barrier function
At the 0th, 7th, 14th, and 28th days follow-up of the study, the changes in TEWL and SCWC score between groups were shown in Table 2 and Figure 4. At the end of treatment, there were significant differences in TEWL and SCWC between the treatment groups (T1 and T2) and the control group, and the improvement of TEWL and SCWC in the T2 group was significantly better than that of the T1 in the 14th day follow-up (P < 0.05).
3.5 Safety evaluation
No product-related adverse effects were identified throughout the study.
4 DISCUSSION
AD is clinically characterized by pruritus and dry skin.1 Immune abnormalities and skin barrier dysfunction are the primary pathogenesis of AD.1 The central focus of AD treatment is the restoration of the skin's barrier function, Current clinical management typically follows a “stepwise therapy” approach.2, 3 Patients with mild to moderate AD were applied with topical medications, while moderate to severe AD require systemic anti-inflammatory therapies, including traditional immunosuppressants, dupilumab, and Janus kinase (JAK) inhibitors.3, 7 Fundamental treatments such as appropriate bathing and the use of topical emollients are recommended for all AD patients. Emollients, when used judiciously, can enhance skin moisture, reduce inflammation, and restore the skin's barrier function.8 Recent studies have indicated that topical emollients administered to AD patients can ameliorate AD symptoms,4, 8 and they have also been shown to lower the risk of AD in infant.9
Skin cleansers serve multiple functions, including antiseptic and moisturizing. They contain ingredients like surfactants, moisturizers, and adhesives.10 Surfactants are mainly responsible for the cleansing effectbut may lead to dry and itchy after use. Studies have demonstrated a correlation between the skin microbiota of AD patients and disease severity, with Staphylococcus being the dominant skin flora in AD patients. Notably, approximately 90% of AD skin lesions are associated with Staphylococcus aureus infection.11 The use of weak acidic or pH-neutral liquid antibacterial cleaners can ameliorate the AD symptoms.12 Guidelines for AD treatment recommend the use of hypoallergenic, non-irritating cleansers, preferably with a pH close to the normal epidermal pH (approximately 6).3
In our study, all patients were administered ebastine tablets orally as a foundational treatment due to their pruritus complaints. The control group utilized Emollient Cream No. 1, a moisturizing product produced by Huashan Hospital, containing key components such as stearic acid, white petrolatum and glycerin, which offer basic moisturizing effects. We chose it as the control group because its components are similar to the basic components of the experimental group, which has a better control effect. Nicotinamide can stimulate the synthesis of ceramides, free fatty acids and cholesterol, thereby consolidate and repair the skin barrier.5, 13 Several studies have confirmed the beneficial effects of niacinamide in improving skin barrier function.5, 13 The cleansing gel we employed is a fragrance-free, preservative-free, pregnancy safe product, encompassing not only cleansing ingredients, but also moisturizing components like niacinamide, mannitol and xylitol, This combination cleanses the skin while simultaneously restoring the skin flora and protecting the skin barrier.
Our study investigated the enhancement of clinical symptoms, the impact on patients' life and skin barrier function in individuals with mild AD by using niacinamide-containing body emollients combined with cleansing gel. This study found that compared to the control group, patients using niacinamide-containing body emollients, with or without cleansing gel, had better improvement on clinical symptoms, impact on quality of life and skin barrier function by the fourth week. Several parameters showed significant differences as early as day 14, including PP-NRS and DLQI scores, with the POEM score displaying a significant difference as early as day 7, though it became statistically insignificant by day 14.
Patients using the cleansing gel demonstrated greater improvements than those using emollients alone. The combined group exhibited significant differences in SCORAD on the 7th day and in POEM on the 28th day of follow-up. In terms of skin barrier function, on the 14th day of follow-up, the cleanser group(T2) displayed superior improvement in TEWL and SCWC compared to the emollients only group (T1) . Although the difference between the two groups was not statistically significant on the 28th day, it still indicates that the combined group exhibited better clinical efficacy and skin barrier function at specific time points. Expanding the sample size or prolonging the study duration may yield more robust results.
It is worth noting that, a statistically significant difference between a group is not the same as a clinically important difference. Schram14 assessed the minimal clinically important difference (MCID) in SCORAD and POEM scores, suggested that the MCID in total SCORAD was 8.7, which is higher than the difference of T1 group (6.43), T2 group (7.97), and control group (4.16) in this study. Schram also suggested that a minimum change in total SCORAD of 4.1 was the optimal cutoff change that can predict change in IGA, which is lower than the difference we found in all the groups. The overall mean difference of the POEM was 3.4 points, which is higher than the difference of T1 group (2.2), T2 group (2.6), and control group (1.6), and an improvement of 1.5 points on the POEM was the optimal cutoff point, which is lower than the difference we found in all the groups. These results suggested that although basic treatments has a certain therapeutic effect, it still could not be expected greatly from a non-pharmacological intervention.
Throughout the study, no adverse effects related to the product application were reported. In conclusion, our study explored changes in clinical improvement, quality of life, skin barrier repair and safety in individuals with mild AD following the application of niacinamide-containing body emollients and cleansing gel. We observed that these two moisturizing and cleansing products can aid in AD treatment, with the combined use of niacinamide-containing body emollients and cleansing gel showing better therapeutic effects than emollients alone at specific time points. However, this study also has limitations, including its short duration, the inability to assess long-term product effects, and a small sample size. Moreover,the observed benefits might be attributed to ingredients other than niacinamide in these two products. Futher studies are imperative for a more comprehensive understanding in the future.
CONFLICTS OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Yes. All patients in this manuscript have given written informed consent for participation in the study and the use of their de-identified, anonymized, aggregated data and their case details for publication.
Open Research
DATA AVAILABILITY STATEMENT
Data available on request from the authors.




